Abstract
Toxicity potentiation of two monomers [bisphenol-A-glycidyldimethacrylate (BisGMA) and urethanedimethacrylate (UDMA)] as well as two comonomers [triethyleneglycoldimethacrylate (TEGDMA) and 2-hydroxyethylmethacrylate (HEMA)], each in combination with H2O2, was investigated on the viability on human gingival fibroblasts (HGF) and human pulpal fibroblasts (HPF). The applied concentration of H2O2 was 0.06 or 0.1 mmol/l, respectively, corresponding to the EC0 of H2O2 in HGF or HPF. The cell viability was assessed by the XTT test. From this test the half maximum effect concentrations (EC50) were calculated from fitted sigmoidale curves. EC50 values were (HGF; mmol/l; mean ± s.e.m.; n = 5): HEMA 11.9 ± 0.9, TEGDMA 3.7 ± 0.3, H2O2 0.36 ± 0.04, UDMA 0.27 ± 0.08, and BisGMA 0.11 ± 0.03. No significant (P < 0.05) differences in the EC50 values were observed when HGF was exposed to substances, as compared to HPF. No significant decrease of the EC50 values was found when HGF or HPF, respectively, was exposed to HEMA or BisGMA in addition with H2O2 up to the concentration of 0.1 mmol/l, as compared to those EC50 values of each compound without H2O2 addition. A significant decrease of the TEGDMA EC50 value from 3.7 to 2.1 or 0.4 mmol/l, respectively, was found when cells were exposed to TEGDMA in combination with H2O2 (0.06 or 0.1 mmol/l), as compared to that TEGDMA EC50 value without H2O2 addition. A significant decrease of the UDMA EC50 value from 0.27 to 0.11 or 0.08 mmol/l, respectively, was found when HGF or HPF was exposed to UDMA in combination with H2O2 (0.06 or 0.1 mmol/l), as compared to that UDMA EC50 value without H2O2 addition. The addition of H2O2 (0.06 or 0.1 mmol/l) resulted in a toxicity potentiation of TEGDMA and UDMA, but not of HEMA and BisGMA, on HGF or HPF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphenol-A-glycidyldimethacrylate (BisGMA) and urethanedimethacrylate (UDMA) are used as monomers to build the three-dimensional structure of the dental resin composites. The comonomer triethyleneglycoldimethacrylate (TEGDMA) is a common component of both bonding and resin composites. Its content varies from 10 to 30%. In some bonding resins TEGDMA is also used to reduce viscosity and enhance bond strength to dentin. The comonomer 2-hydroxyethylmethacrylate (HEMA) is a wetting agent that facilitates penetration of the hydrophobic components like TEGDMA and UDMA into hydrophilic environments like dentin (Nakabayashi and Takarada 1992).
Monomers/comonomers, additives, and polymerization products can be released from resin composites into the oral cavity (Ferracane and Condon 1990; Ferracane 1994) or can diffuse into the pulp space (Gerzina and Hume 1996). Due to its “hydrophilic” character, high amounts of monomers/comonomers, e.g., TEGDMA (Geurtsen and Leyhausen 2001), can leach into an aqueous environment, such as the oral cavity. Monomers/comonomers may cause adverse local and systemic effects like irritation to skin or eyes and gastrointestinal complaints (Mathias et al. 1979; Lonnroth and Shahnavaz 1997). In vitro studies revealed genotoxic, mutagenic, estrogenic, and teratogenic effects of composite components (Heil et al. 1996; Schweikl and Schmalz 1997; Kleinsasser et al. 2004; Schwengberg et al. 2005).
Important properties, e.g., wear resistance and susceptibility to discoloration, as well as biological features such as local and/or systemic cell/tissue compatibility of a composite are highly influenced by its monomer/polymer conversion. This degree of conversion varies between 35 and 77% (Rueggeberg and Caughman 1993; Spahl et al. 1998). Due to this comparably low degree of conversion, the biocompatibility of a composite resin may be impaired. Released monomers/comonomers from composite resins can also enter the intestine by swallowed saliva (Sasaki et al. 2005; Sofou et al. 2005), and after uptake the monomers/comonomers can be metabolized (Reichl et al. 2001). In previous animal experiments the uptake, distribution, metabolism, and excretion of HEMA and TEGDMA were investigated (Reichl et al. 2001). Cytotoxic responses to dental composite resins and their components have been described earlier and recently (Kehe et al. 2001; Al-Hiyasat et al. 2005; Cavalcanti et al. 2005; Terasaka et al. 2005).
Due to aesthetic reasons the wish to get white teeth is steadily increasing in human beings. Therefore dentists seek to discover solutions and techniques capable of bleaching vital teeth whose color has been altered. For example a thermocatalytic technique with hydrogen (Lai et al. 2003) and the nightguard vital technique have been indicated for bleaching vital teeth (Fugaro et al. 2004).
Several peroxides [e.g., carbamide peroxide, sodium perborate, and hydrogen peroxide (H2O2)] are used in the dental practice to bleach natural human teeth. For the application, the dentist makes a fitted tooth splint in which the bleaching paste can be put by the dentist or the user. “Office-bleaching” is when the dentist makes the bleaching procedure in the dental practice. H2O2 (30–35%) is used for “office-bleaching.”
The so-called “at-home” nightguard vital tooth bleaching has as well come into the focus of both the dentist and the patient’s interest (Hegedus et al. 1999). This method was first described by Haywood and Heymann (1989), and the technique allows for the patient’s application of bleaching agents outside the dental office. Here carbamide peroxide is mostly used as a precursor, which is slowly changed to the reactive compound H2O2 and urea. In the home-bleaching paste 10–35% carbamide peroxide is mostly used, which is equivalent to 3–12% H2O2. To get the best results the producers recommend letting the paste work on the teeth for about 10 h continuously (e.g., overnight) for 5 days. A series of repeated treatment is recommended. Carbamide peroxide, sodium perborate, and/or H2O2 can contact oral cells (e.g., gingival and/or pulpal fibroblasts) during and/or after the treatment and can also enter the intestine through the paste swallowed with the saliva.
Moreover damage to the (anti)-oxidative system and/or increased oxidative stress in cells can lead to endogenous increase of H2O2 and then some diseases can occur even in human beings (Salahudeen et al. 2000; Cuttle et al. 2001; Lee et al. 2001).
In the previous experiments it has been demonstrated that TEGDMA and UDMA were more toxic than HEMA in human gingival fibroblasts (HGF) and human pulpal fibroblasts (HPF) (Kehe et al. 2001; Al-Hiyasat et al. 2005; Cavalcanti et al. 2005; Terasaka et al. 2005). HGF and HPF are useful cells to compare and quantitate the toxicities of these substances, because in the human physiological situation these oral cells can be simultaneously (highly) exposed to both the released monomers/comonomers and H2O2, if bleaching is applied in patients with composite restored teeth.
Synergistic effects between resin components were described (Ratanasathien et al. 1995). In the previous experiments synergistic toxic effects had been demonstrated when rat kidney cells were exposed to TEGDMA in combination with H2O2 (Reichl et al. 2003). Scarce information is available about toxicity potentiation of composite components in combination with xenobiotics on human oral cells. Therefore the toxicity potentiation of the dental composite components TEGDMA, HEMA, UDMA, or BisGMA, each administered in combination with H2O2, was investigated on HGF and HPF.
Materials and methods
Chemicals
TEGDMA, HEMA, UDMA, and BisGMA were obtained from Degussa (Darmstadt, Germany). Triton X-100 and H2O2 were obtained from Merck (Darmstadt, Germany). HEMA was diluted with medium, TEGDMA, UDMA, and BisGMA were dissolved in DMSO (final monomer/comonomer concentration 30 mM) and diluted with medium (final concentration of DMSO 1%).
Cell culture
Human gingival fibroblasts (Primary HGF-1, American Type Culture Collection, passage number 7-17) and HPF (Primary HPF, passage number 7-17) were grown each on 75 or 175 cm2 cell culture flasks to approximately 70% confluence in a 5% CO2 atmosphere at 100% humidity and 37°C. Two sets of HPF obtained from different adults were cultured from biopsies of the attached healthy premolar and molar teeth according to the method described in the previous studies (Jones et al. 2005). The medium used was Quantum 333 with l-glutamine supplemented with 50 IU/ml penicillin and 50 μg/ml streptomycin. After the cells had reached confluence they were washed with Dulbecco’s phosphate-buffered saline (PBS), and incubated with trypsin/EDTA and seeded into a 96-well microtiter plate at a density of 2 × 104 cells/well in 200 μl of growth media, and then the cells were incubated for 24 h as described above. All the chemicals were purchased from PAA Laboratories GmbH, Cölbe, Germany.
Exposure
After removal of the medium, the adherent and confluent HGF and HPF were incubated with increasing concentrations of the following substances: HEMA (0.1–30 mM), TEGDMA (0.03–10 mM), BisGMA (0.01–0.3 mM), or UDMA (0.01–1 mM), respectively, diluted with Quantum 333 medium each. All the cells were incubated for further 24 h. The control cells received either medium only, or medium + DMSO (final DMSO concentration 1%). The cells treated with 1% Triton X-100 were used as negative control.
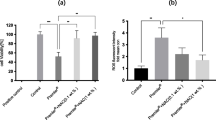
Human gingival fibroblasts and HPF were exposed to the monomers/comonomers as described above in combination with H2O2 (final H2O2 concentration 0.06 or 0.1 mmol/l, respectively). At these H2O2 levels no toxic effects in the XTT test were observed on HGF or HPF, which received H2O2 only (without monomers/comonomers). Therefore in this test system these H2O2 levels correspond to the EC0 of H2O2 in HGF (Fig. 1) or HPF. H2O2 was diluted with Quantum 333 medium.
XTT-based viability assay
Twenty thousand cells per well were seeded in 96-well tissue culture plates and precultured 48 h before assay. The proliferating cell monolayers were then washed (200 μl HBSS per well) and incubated in the absence or presence of composite components in HBSS without Phenol Red. Twenty-four hours later a mixture of XTT (sodium 3′-[1-[phenylamino-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzenesulphonic acid) labeling reagent (Scudiero et al. 1988) and electron-coupling reagent were added as recommended by the supplier (cell proliferation kit II; Boehringer Mannheim, Germany). The assay is based on the cleavage of the tetrazolium salt XTT to form an orange soluble formazan dye by mainly mitochondrial dehydrogenase activity in living cells. Formazan formation was quantified spectrophotometrically at 450 nm (reference wavelength 670 nm) using a microtiter plate reader (BioLumin 960; Molecular Dynamics). Five replicates of each concentration were used in each assay.
Measurement of glutathione
After exposure of HGF or HPF to substances (see Sect. ”Exposure”) glutathione contents of cell layers were measured according to the methods previously described by Walther et al. (2004).
Statistics
All the values were expressed as percentage of the maximum value and plotted on a concentration log-scale, and the range of maximum slope was detected. Half maximum effect substance concentration at the maximum slope was assessed as EC50 value. Calculations were performed using GraphPad Prism 4 software package (GraphPad Software Inc., San Diego, CA, USA). The data are presented as mean ± s.e.m. (n = 5). The statistical significance (P < 0.05) of the differences between the experimental groups was checked using the t-test, corrected according to Bonferroni–Holm (Forst 1985).
Results
No significant (P < 0.05) differences in the results between both sets of primary HPF were observed in this test system. No significant (P < 0.05) differences in the EC50 values were observed when HPF was exposed to substances as compared to HGF. No significant (P < 0.05) differences in the EC50 values were observed when the control cells received medium only, or medium + DMSO (data not shown).
The concentration curves have been made in relation to the control cells, which received medium + DMSO.
The EC50 values of tested compounds in HGF are presented as mean ± s.e.m. in Table 1. The EC50 value of H2O2 (without comonomer/monomer addition) was 0.36 mmol/l (Table 1; Fig. 1).
The EC50 values of HEMA, TEGDMA, UDMA, and BisGMA, each administered in combination with H2O2, are presented in Table 1. No significant (P < 0.05) decrease of the EC50 values was found when HGF was exposed to BisGMA or HEMA in addition with H2O2 (0.06 or 0.1 mmol/l, respectively), compared to those EC50 values of each compound without H2O2 addition. No significant (P < 0.05) differences in the EC50 values were observed when HPF was exposed to these substances as compared to HGF (data not shown).
A significant decrease of the TEGDMA EC50 value from 3.7 mmol/l (without H2O2) to 2.1 or 0.4 mmol/l, was found when HGF was exposed to TEGDMA, administered in combination with H2O2 (0.06 or 0.1 mmol/l, respectively; Table 1). No significant (P < 0.05) differences in the EC50 values were observed when HPF was exposed to these substances as compared to HGF (data not shown).
A significant decrease of the UDMA EC50 value from 0.27 mmol/l (without H2O2) to 0.11 or 0.08 mmol/l, was found when HGF was exposed to UDMA, administered in combination with H2O2 (0.06 or 0.1 mmol/l, respectively; Table 1). No significant (P < 0.05) differences in the EC50 values were observed when HPF was exposed to these substances as compared to HGF (data not shown).
Concentration curves of monomers/comonomers, administered in combination with H2O2, are presented in Fig. 2 (HEMA, TEGDMA) and in Fig. 3 (UDMA, BisGMA).
Glutathione content
A significant (P < 0.05) decrease in the glutathione content from 100% (control) to 31% was found, when HGF received a non-toxic dose of 1.0 mmol/l TEGDMA in combination with a non-toxic dose of 0.1 mmol/l H2O2 (Table 2).
A significant (P < 0.05) decrease in the glutathione content from 100% (control) to 41% was found, when HGF received a non-toxic dose of 0.1 mmol/l UDMA in combination with a non-toxic dose of 0.1 mmol/l H2O2 (Table 2).
No significant (P < 0.05) differences in the glutathione content were found when HPF was exposed to these substances as compared to HGF (data not shown).
Discussion
In the earlier studies the release, diffusion, and penetration of monomers/comonomers from composite and bonding resins through dentin were demonstrated (Bouillaguet et al. 1996; Geurtsen and Leyhausen 2001; Reichl et al. 2001; Mazzaoui et al. 2002; Datar et al. 2004). A release of monomers/comonomers into the oral cavity is due to many factors, e.g., abrasion and elutriation by saliva (Kedjarune et al. 1999). Monomers/comonomers can be released from composites and can contact oral cells.
The stable carbamide peroxide is a common source of H2O2 mostly used to bleach natural human teeth. During the treatment it is slowly changed to the reactive bleaching compound H2O2. All bleaching compounds are applied on the teeth in the oral cavity and thus can be incorporated from the user when saliva is swallowed and/or by uptake from the mucous oral cells, and/or by diffusion through the dentin. The amount of swallowed and/or incorporated bleaching compounds by the users during the treatment and thus the amount of these compounds entering the cells in the organism is unknown.
Under physiological situation (without bleaching procedure) the (anti)-oxidative enzymes catalase and glutathione peroxidase are able to maintain the concentration of H2O2 in cells on levels of about 10−7 mol/l H2O2. Otherwise damage of the (anti)-oxidative system and/or increased oxidative stress can lead to dramatic endogenous increase of H2O2 in the cells and thereby can lead to cell damage in the organism. Even specific symptoms can occur in human beings with the existence of a faulty (anti)-oxidative system and/or increased oxidative stress (Reichl et al. 2001; Cavalcanti et al. 2005; Sasaki et al. 2005). In previous experiments synergistic toxic effects had been demonstrated when rat kidney cells were exposed to TEGDMA in combination with H2O2 (Reichl et al. 2003).
In the following study the human oral cells HGF and HPF were used. No significant (P < 0.05) differences in all the results were found when HGF was exposed to these substances as compared to HPF. These results indicate that individual cell differences between HGF and HPF are only of minor relevance for this test system. When HGF was exposed to H2O2 only (without monomers/comonomers) the EC50 value was 0.36 ± 0.04 mmol/l (Fig. 1). For testing additive effects of monomers/comonomers in combination with H2O2 a non-toxic dose level of H2O2 must be used. The H2O2 concentrations of 0.06 or 0.1 mmol/l, respectively, were chosen for these experiments because at these H2O2 levels no toxic effects were observed in HGF or HPF in this test system. Therefore the H2O2 concentration levels of 0.06 or 0.1 mmol/l correspond to the EC0 value for H2O2 in these cells in this test system. A H2O2 concentration higher than 0.1 mmol/l is not advisable, because at 0.2 mmol/l it is not possible to distinguish between a possible monomer/comonomer synergistic and a toxic effect caused by H2O2 itself (see Fig. 1).
All monomers/comonomers inhibited the cell viability in a typical dose-dependent manner (Figs. 2, 3). A significant decrease of the EC50 value from 3.7 to 0.4 mmol/l was found when the cells were exposed to TEGDMA, administered in combination with the non-toxic H2O2 dose of 0.1 mmol/l, compared to that EC50 value without H2O2 addition. Similar to TEGDMA a significant decrease of the EC50 values was found when the cells were exposed to UDMA in combination with H2O2.
The strongest toxicity potentiation of TEGDMA in combination with H2O2 occurred between 0.06 and 0.1 mmol/l H2O2 (the EC50 value of TEGDMA decreased about 81% from 2.1 to 0.4 mmol/l). These results indicate that the toxicity potentiation of TEGDMA is more sensitive to higher H2O2 concentrations, as compared to UDMA, whereas the EC50 value decreased only about 27% (from 0.27 to 0.11 mmol/l) between 0.06 and 0.1 mmol/l H2O2.
An explanation for the toxicity potentiation of TEGDMA and/or UDMA with H2O2 might be not the precursor itself, but a time-dependent formation of toxic metabolites in combination with H2O2 leads to oxidative stress and thereby enhances glutathione consumption. Indeed, in this study a significant (P < 0.05) decrease in glutathione content was found, when HGF received a non-toxic dose of TEGDMA or UDMA, each in combination with a non-toxic dose of H2O2. Also a decline of the intracellular glutathione level in HGF after TEGDMA addition was already described (Stanislawski et al. 2003; Engelmann et al. 2004; Lefeuvre et al. 2005). TEGDMA and UDMA can possibly more effectively enter the cells (higher lipid solubility) resulting in the formation of oxidative stress in combination with H2O2 at earlier times, as compared to HEMA. A decrease of compounds with reactive SH groups (e.g., glutathione) and a reduction of functions of relevant mechanisms for detoxifications may result. These results are also in agreement with the findings, described by Volk et al. (in press), that a decrease of the intracellular GSH content was found when the cells were exposed to TEGDMA in combination with H2O2, as compared to the experiments without H2O2, or the findings, described by Engelmann et al. (Engelmann et al. 2005), that TEGDMA and campherchinone released into an aqueous environment from resinous materials might interact, thus generating significant cytotoxic effects. These data show that the investigated substances may cause cell damage due to various mechanisms, glutathione decrease, and/or ROS increase.
2-Hydroxyethylmethacrylate or BisGMA in combination with non-toxic H2O2 concentrations showed no toxicity potentiation in HGF or HPF in all experiments. This indicates that the influence of H2O2 on HEMA or BisGMA is only of minor relevance.
In the previous experiments it could be demonstrated that TEGDMA or UDMA is metabolized to methacrylic acid (Seiss et al. 2004). In further experiments it could be found that methacrylic acid is metabolized to 2,3-epoxymethacrylic acid in human liver microsomes by cytochrome P450 systems (Seiss et al. 2007). Epoxides are regarded as very toxic (cancerogenic, mutageneous) compounds (Ali et al. 2005). It has been recently demonstrated that 2,3-epoxymethacrylic acid can cause teratogeneous effects on mouse embryonic stem cells (Schwengberg et al. 2005). Furthermore this toxic dental epoxy-metabolite can be additionally formed by H2O2 catalytic activated hydrogen carbonate whereas increasing H2O2 concentrations can increase the formation of the intermediate 2,3-epoxymethacrylic acid (Yao and Richardson 2000).
The addition of H2O2 resulted in a toxicity potentiation of TEGDMA and UDMA in HGF and HPF. However it is noted that in the human physiological situation the interactions between a peroxide (applied short term from bleaching) and leached resins (that are released long term at low concentrations) seem unlikely to be of much consequence in the gingival tissues because most of the resin components are simply washed away and are highly diluted. However, the pulp represents the more likely locus, where degree of conversion of monomers/comonomers is lowest, resin release is highest, dentin is thinnest and where peroxides are known to gain access readily. Therefore the use of dental restorative materials containing only HEMA and/or BisGMA may contribute to preventive measures as compared to the use of dental materials containing TEGDMA and/or UDMA when H2O2 is applied simultaneously, e.g., during bleaching procedures.
Conclusion
The addition of H2O2 (0.06 or 0.1 mmol/l, respectively) resulted in a toxicity potentiation of TEGDMA and UDMA, but not of HEMA and BisGMA, on HGF or HPF.
References
Al-Hiyasat AS, Darmani H, Milhem MM (2005) Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin Oral Investig 9:21–25
Ali I, Aboul-Enein H, Ghanem A (2005) Enantioselective toxicity and carcinogenesis. Curr Pharm Anal 1:109–125
Bouillaguet S, Wataha J, Hanks C, Ciucchi B, Holz J (1996) In vitro cytotoxicity and dentin permeability of HEMA. J Endod 22:244–248
Cavalcanti B, Rode S, Marques M (2005) Cytotoxicity of substances leached or dissolved from pulp capping materials. Int Endond J 38:505–509
Cuttle L, Zhang X, Endre Z, Winterford C, Gobe G (2001) Bcl-X-L translocation in renal tubular epithelial cells in vitro protects distal cells from oxidative stress. Kidney Int 59:1779–1788
Datar R, Rueggeberg F, Caughman G, Wataha J, Lewis J, Schuster G (2004) Effects of subtoxic concentrations of benzoyl peroxide on cell lipid metabolism. J Biomed Mater Res A 71:685–692
Engelmann J, Janke V, Volk J, Leyhausen G, Von Neuhoff N, Schlegelberger B, Geurtsen W (2004) Effects of BisGMA on glutathione metabolism and apoptosis in human gingival fibroblasts in vitro. Biomaterials 25:4573–4580
Engelmann J, Volk J, Leyhausen G, Geurtsen W (2005) ROS formation and glutathione levels in human oral fibroblasts exposed to TEGDMA and camphorquinone. J Biomed Mater Res B Appl Biomater 75:272–276
Ferracane JL (1994) Elution of leachable components from composites. J Oral Rehabil 21:441–452
Ferracane JL, Condon JR (1990) Rate of elution of leachable components from composite. Dent Mater 6:282–287
Forst HT (1985) Probleme des multiplen Testens und Schätzens in der Arzneimittelforschung. Arzneim Forsch/Drug Res 35:3–5
Fugaro J, Nordahl I, Fugaro O, Matis B, Mjor I (2004) Pulp reaction to vital bleaching. Oper Dent 29:363–368
Gerzina TM, Hume WR (1996) Diffusion of monomers from bonding resin-resin composite combinations through dentine in vitro. J Dent 24:125–128
Geurtsen W, Leyhausen G (2001) Chemical–biological interactions of the resin monomer triethyleneglycol-dimethacrylate (TEGDMA). J Dent Res 80:2046–2050
Haywood VB, Heymann HO (1989) Nightguard vital bleaching. Quintessence Int 20:173–177
Hegedus C, Bistey T, Flora-Nagy E, Keszthelyi G, Jenei A (1999) An atomic force microscopy study on the effect of bleaching agents on enamel surface. J Dent 27:509–515
Heil J, Reifferscheid G, Waldmann P, Leyhausen G, Geurtsen W (1996) Genotoxicity of dental materials. Mutat Res 368:181–194
Jones T, Henderson J, Johnson R (2005) Effects of doxorubicin on human pulp cells in vitro. Cell Biol Toxicol 21:207–214
Kedjarune U, Charoenworaluk N, Koontongkaew S (1999) Release of methyl methacrylate from heat-cured and autopolymerized resins: cytotoxicity testing related to residual monomer. Aust Dent J 44:25–30
Kehe K, Reichl FX, Durner J, Walther U, Hickel R, Forth W (2001) Cytotoxicity of dental composite components and mercury compounds in pulmonary cells. Biomaterials 22:317–322
Kleinsasser NH, Wallner BC, Harreus UA, Kleinjung T, Folwaczny M, Hickel R, Kehe K, Reichl FX (2004) Genotoxicity and cytotoxicity of dental materials in human lymphocytes as assessed by the single cell microgel electrophoresis (comet) assay. J Dent 32:229–234
Lai YL, Yang ML, Lee SY (2003) Microhardness and color changes of human dentin with repeated intracoronal bleaching. Oper Dent 28:786–792
Lee S, Yoon Y, Jang YY, Song JH, Han ES, Lee CS (2001) Effect of iron and ascorbate on cyclosporine-induced oxidative damage of kidney mitochondria and microsomes. Pharmacol Res 43:161–171
Lefeuvre M, Amjaad W, Goldberg M, Stanislawski L (2005) TEGDMA induces mitochondrial damage and oxidative stress in human gingival fibroblasts. Biomaterials 26:5130–5137
Lonnroth EC, Shahnavaz H (1997) Use of polymer materials in dental clinics, case study. Swed Dent J 21:149–159
Mathias CGT, Caldwell TM, Maibach HI (1979) Contact-dermatitis and gastro-intestinal symptoms from hydroxyethylmethacrylate. Br J Dermatol 100:447–449
Mazzaoui SA, Burrow MF, Tyas MJ (2002) Long-term quantification of the release of monomers from dental resin composites and a resin-modified glass ionomer cement. J Biomed Mater Res 63:299–305
Nakabayashi N, Takarada K (1992) Effect of Hema on bonding to dentin. Dent Mater 8:125–130
Ratanasathien S, Wataha JC, Hanks CT, Dennsion JB (1995) Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. J Dent Res 74:1602–1606
Reichl FX, Durner J, Hickel R, Kunzelmann KH, Jewett A, Wang MY, Spahl W, Kreppel H, Moes GW, Kehe K, Walther U, Forth W, Hume WR (2001) Distribution and excretion of TEGDMA in guinea pigs and mice. J Dent Res 80:1412–1415
Reichl F, Durner J, Kehe K, Folwaczny M, Kleinsasser N, Schwarz M, El-Mahdy K, Hickel R (2003) Synergistic effects of H2O2 with components of dental restorative materials on gluconeogenesis in rat kidney tubules. Biomaterials 24:1909–1916
Rueggeberg FA, Caughman WF (1993) The influence of light exposure on polymerization of dual-cure resin cements. Oper Dent 18:48–55
Salahudeen AK, Huang H, Patel P, Jenkins JK (2000) Mechanism and prevention of cold storage-induced human renal tubular cell injury. Transplantation 70:1424–1431
Sasaki N, Okuda K, Kato T, Kakishima H, Okuma H, Abe K, Tachino H, Tuchida K, Kubono K (2005) Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. J Mater Sci Mater Med 16:297–300
Schweikl H, Schmalz G (1997) Glutaraldehyde-containing dentin bonding agents are mutagens in mammalian cells in vitro. J Biomed Mater Res 36:284–288
Schwengberg S, Bohlen H, Kleinsasser N, Kehe K, Seiss M, Walther UI, Hickel R, Reichl FX (2005) In vitro embryotoxicity assessment with dental restorative materials. J Dent 33:49–55
Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Curren MJ, Seniff D, Boyd MR (1988) Evaluation of a soluble tetrazolium formazan assay for cell-growth and drug sensitivity in culture using human and other tumor-cell lines. Cancer Res 48:4827–4833
Seiss M, Kehe K, Haffner C, El-Mahdy K, Hickel R, Nitz S, Walther UI, Manhart J, Reichl FX (2004) Analytic of (toxic) intermediates from metabolized dental restorative materials. Naunyn Schmiedebergs Arch Pharmacol 369:R107
Seiss M, Nitz S, Kleinsasser N, Buters J, Behrendt H, Hickel R, Reichl F (2007) Identification of 2,3-epoxymethacrylic acid as an intermediate in the metabolism of dental materials in human liver microsomes. Dent Mater 23:9–16
Sofou A, Tsoupi I, Emmanouil J, Karayannis M (2005) HPLC determination of residual monomers released from heat-cured acrylic resins. Anal Bioanal Chem 381:1336–1346
Spahl W, Budzikiewicz H, Geurtsen W (1998) Determination of leachable components from four commercial dental composites by gas and liquid chromatography mass spectrometry. J Dent 26:137–145
Stanislawski L, Lefeuvre M, Bourd K, Soheili-Majd E, Goldberg M, Perianin A (2003) TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species. J Biomed Mater Res A 66A:476–482
Terasaka H, Kadoma Y, Sakagami H, Fujisawa S (2005) Cytotoxicity and apoptosis-inducing activity of bisphenol A and hydroquinone in HL-60 cells. Anticancer Res 25:2241–2247
Volk J, Leyhausen G, Dogan S, Geurtsen W (in press) Additive effects of TEGDMA and hydrogen peroxide on the cellular glutathione content of human gingival fibroblasts. Dent Mater
Walther UI, Siagian II, Walther SC, Reichl FX, Hickel R (2004) Antioxidative vitamins decrease cytotoxicity of HEMA and TEGDMA in cultured cell lines. Arch Oral Biol 49:125–131
Yao HR, Richardson DE (2000) Epoxidation of alkenes with bicarbonate-activated hydrogen peroxide. J Am Chem Soc 122:3220–3221
Acknowledgments
This work has been supported by the Deutsche Forschungsgemeinschaft (DFG), Germany; number RE 633/2-1/4. The authors gratefully acknowledge the excellent technical assistance of Sabine Domes and Stefan Schulz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reichl, FX., Seiss, M., Marquardt, W. et al. Toxicity potentiation by H2O2 with components of dental restorative materials on human oral cells. Arch Toxicol 82, 21–28 (2008). https://doi.org/10.1007/s00204-007-0226-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-007-0226-1