Abstract
The modification of endodontic sealers with nanoparticles to confer antimicrobial activity allow greater effect, with interaction at a molecular level. The nanostructured silver vanadate decorated with silver nanoparticles (AgVO3) is a nanomaterial unprecedented in dentistry for this application. This study incorporated the AgVO3 into three endodontic sealers of different compositions and evaluate the cytotoxicity and release of compounds. The groups of commercially available AH Plus, Sealer 26, and Endomethasone N and groups of the same sealers with incorporated AgVO3 (at concentrations 2.5, 5, 10%) were prepared, and extracts of the specimens were obtained for 24 h. The cell viability (cytotoxicity) of human gingival fibroblasts (HGF) was assessed after 24 h, 7 and 14 days. Silver (Ag+) and vanadium (V4+/V5+) ion release was quantified after 24 h by ICP-MS. Data were analyzed by Kruskal–Wallis and Dunn’s post-hoc (α = 0.05). The cell viability was inversely proportional to treatment time. The Sealer 26 and Endomethasone N groups were cytotoxic for HGF cells, regardless of the incorporation of the AgVO3 (p > 0.05), and the incorporation reduced cell viability of AH Plus (p < 0.05). The release of ions was proportional to the concentration of AgVO3. AH Plus released more Ag+ ions, and Sealer 26 and Endomethasone N releases more V4+/V5+ ions. In conclusion, it was not possible to confirm the influence of AgVO3 on HGF cell viability to Sealer 26 and Endomethasone N, however, nanomaterial influenced cell-viability to AH Plus, so the commercial sealers can be cytotoxic in synergy with the nanomaterial. The release of Ag+ and V4+/V5+ was proportional to the AgVO3 incorporated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The complete elimination of bacteria in endodontic treatments only with chemo-mechanical preparation is practically impossible. The complexity of the root canal system and virulence factors of microorganisms may cause treatment failure and subsequent need for retreatment [1, 2]. The use of obturation materials with antimicrobial activity can be advantageous in the reduction of persistent bacteria [1].
Modified endodontic sealers with various antimicrobial agents still present limited information regarding their biocompatibility, long-term maintenance of their properties, and antibacterial effect [2,3,4]. Advances in this area include the incorporation of nanoparticles, since nanomaterials present higher chemical reactivity due to the small size, with more atoms on their surface relative to the nucleus [5,6,7,8].
The nanostructured silver vanadate decorated with silver nanoparticles (AgVO3) is an innovative proposal in dentistry for the modification of endodontic sealers, presenting advantages over the use of silver nanoparticles [9]. According to Holtz et al. [10], this hybrid nanomaterial combines the action of silver nanoparticles and vanadate nanowires, which dissociate into silver (Ag+) and vanadium (V4+ and V5+) ions that interact at the cellular level with bacterial cells. Silver nanoparticles have been applied in nanobiotechnology and are known for their antimicrobial potential, however, problems such as the cluster formation and color change limit its use [10,11,12,13].
Previous studies indicated an antimicrobial activity of endodontic sealers incorporated with AgVO3 [14, 15], being necessary to evaluate the cytotoxic effect on human cells and the amount of Ag+ and V4+/V5+ ions that are released for future application in clinical practice. The released ions and other sealer components interact with the bacterial membrane and are thus essential for the antimicrobial efficacy of sealers. In contrast, these substances can lead to inflammatory reactions in the periapical cells, affecting biocompatibility [16,17,18].
Given this perspective, the AgVO3 can contribute to the seeks an endodontic sealer with ideal physicochemical properties, biocompatibility, and antimicrobial effectiveness. However, its cytotoxic effect on human cells is still unknown. Therefore, the objective of this study was to incorporate the AgVO3 to three endodontic sealers of different compositions, at concentrations: 2.5%, 5% and 10%; and evaluate the cell viability of human gingival fibroblasts (HGF) in contact with extracts of the specimens for 24 h, 7 and 14 days. The concentration of Ag+ and V4+/V5+ ions released in distilled water in 24 h was also assessed. The null hypothesis tested was that the cell viability of HGF and the amount of ions released are not affected by the concentration of the nanomaterial incorporated into the endodontic sealers.
Materials and methods
Specimens preparation
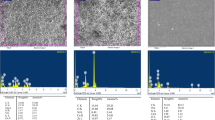
The AgVO3 was synthetized by dissolving 1.3569 g of silver nitrate (AgNO3, Merck, 99.8%; Merck KGaA, Darmstadt, Germany) and 0.9736 g of ammonium vanadate (NH4VO3; 99%; Merck KGaA, Darmstadt, Germany) each in 200 mL of distilled water, and then these solutions were mixed. The silver vanadate solution was vacuum filtered, washed with distilled water and absolute ethanol, and vacuum dried for 10 h [11, 19]. The morphology of the powder obtained was analyzed by scanning transmission electron microscopy (Magellan 400L, FEI Company, Hillsboro, OR, EUA), and demonstrated semi-spherical silver nanoparticles with an average size of 25 nm coated the nanowires of silver vanadate with dimensions nano and micrometric, and an average diameter of 150 nm (Fig. 1).
The endodontic sealers commercially available AH Plus, Sealer 26, and Endomethasone N were incorporated with AgVO3 in concentrations of 2.5, 5 and 10% (by mass), based on previous pilot studies. A commercial group of each sealer without the incorporation of AgVO3 were prepared. The powder or base paste of the sealers and the AgVO3 were weighed on a precision scale, and mixed with their respective liquid or catalyst paste in an unpolished glass plate, to facilitate the incorporation of the nanomaterial (Table 1). The mixed sealers were inserted into silicon matrices (Zetalabor© Zhermack SpA, Badia Polenise, RO, Italy) of Ø7.75 × 1.5 mm and incubated in a stove (DeLeo, B2DG) at 37 °C for 7 days for setting [9].
Cell viability
The specimens (n = 3) were sterilized with ethylene oxide, and to obtain the extracts, the specimens were placed in polypropylene tubes (BD Falcon, Juiz de Fora, Brazil) with 436 μL of DMEM (Dulbecco's Modified Eagle Medium–Gibco, Thermo Fisher Scientific, Waltham, MA, USA) (3 cm2/mL area-volume ratio, ISO 10993-5 Part 5: Cytotoxicity tests), and incubated at 37 °C in 5% CO2 and 95% oxygen (Series, Shell Lab, Cornelius, OR, USA) for 24 h.
The culture of human gingival fibroblasts (HGF) was performed in DMEM supplemented with 10% fetal bovine serum (FBS, Cultilab, Campinas, SP, Brazil) and 1% penicillin and streptomycin (Sigma, St. Louis, MO, USA) at 37 °C in 5% CO2 and 95% O2 (Series). 3 × 103 cells were seeded per well of a 96-well plate, incubated for 24 h. The cells were treated for 24 h, 7 and 14 days with 200 μL of the specimens extracts, DMEM (without FBS) for the negative control, and distilled water for the positive control group (n = 3). After treatment, the wells were washed with PBS solution, and 500 μL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (5 mg of MTT/1 mL of DMEM without phenol red) was added for 4 h at 37 °C. The MTT solution was removed and dimethyl sulfoxide solvent (DMSO, Synth, Diadema, SP, Brazil) was added for 20 min at room temperature. Absorbance reading was performed on a spectrophotometer (SYNERGY MX Monochromator-based reader, Biotek, Winooski, VT, USA) at 550 nm. The experiment was performed in triplicate, and cell viability was reported as percentage, considering the negative control as 100% cell viability [20].
Release of metal ions
The release of Ag+ and V4+/V5+ ions was evaluated by inductively coupled plasma mass spectrometry (ICP-MS) on NexIon 300X equipment (PerkinElmer, Waltham, MA, USA) [21]. The samples were suspended by a nylon strand in polypropylene tubes (BD Falcon) with 9 mL of deionized water, and incubated at 37 °C for 24 h. Samples were then removed from the tubes and the liquid was quantitatively analyzed by calibration curves of the equipment. All experiments were performed independently in triplicate (n = 3).
Statistical analysis
Data analysis was performed using the Kruskal–Wallis and Dunn’s post-hoc (p = 0.05), using SPSS software v 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Cell viability
All groups presented a reduction in cell viability of HGF in comparison to the negative control (p < 0.05), regardless of treatment time and AgVO3 concentration. The Sealer 26 and Endomethasone N groups presented a reduction of more than 95% in HGF cell viability regardless of treatment time. After 24 h of treatment, the AH Plus commercial group presented 55.17% of cell viability, which is greater than the cell viability of the AH Plus groups incorporated with AgVO3 (p < 0.05). However, after 7 and 14 days of treatment, all AH Plus groups presented a reduction of more than 95% in HGF cell viability. The Sealer 26 and Endomethasone N groups presented statistical similarity in comparison to the positive control after 24 h of treatment (p > 0.05) indicating cytotoxicity. The AH Plus groups presented higher cell viability than positive control in 24 h treatment (p < 0.05) (Table 2).
Release of metal ions
The release of Ag+ and V4+/V5+ was proportional to the concentration of AgVO3 incorporated in the sealers. The commercial groups and the AH Plus groups had the lowest release and the Endomethasone N groups had the highest release, especially the 10% group.
The AH Plus and Endomethasone N incorporated with 10% of AgVO3 presented the highest release of Ag+ and significant difference of 2.5% group (p < 0.05). The Sealer 26–10% group released significantly more Ag+ than the other groups of this sealer (p < 0.05), and the Sealer 26–2.5% group showed no statistical difference in comparison to the commercial group (p > 0.05) (Fig. 2).
Release Ag+ and V4+/V5+ ions (µg/L) in distilled water for 24 h, of endodontic sealers incorporated with different AgVO3 concentrations determined by ICP-MS (n = 3). Median [interquartile range]. *Represents statistical difference in comparison to AH Plus commercial group (p < 0.05). #Represents statistical difference compared to Sealer 26 commercial group (p < 0.05). £Represents a statistical difference compared to Endomethasone N commercial group (p < 0.05).-Represents statistical difference between the groups incorporated with AgVO3 (p < 0.05)
The AH Plus groups incorporated with AgVO3 presented no release of V4+/V5+ (p > 0.05) and showed a statistical difference of Sealer 26 and Endomethasone N groups with and without nanomaterial incorporation (p < 0.05). The highest V4+/V5+ release was significantly observed in the Sealer 26–10% and Endomethasone N—5% and 10% groups (p < 0.05) (Fig. 2).
Discussion
The modification of endodontic sealers to confer long-term effective antimicrobial activity requires the continued release of components and maintenance of properties and biocompatibility. In this study, the biocompatibility and ion release of endodontic sealers added with an AgVO3-based nanomaterial was investigated. The antimicrobial activity and physicochemical properties of these sealers have been reported elsewhere [14, 15, 22]. These conventional root canal sealers, with different chemical compositions, were selected for the initial study with the incorporation of AgVO3, because the objective was to verify the best type of composition to incorporate the nanomaterial. Besides, the AH Plus is considered the gold standard in endodontics; sealers based on zinc oxide and eugenol, such as Endomethasone, were the first types of sealer to appear in dentistry; and the Sealer 26 with calcium hydroxide and epoxy resin in the composition, it is a low cost sealer widely commercialized [23, 24].
The null hypothesis tested of this study was partially accepted, since the incorporation of AgVO3 to the AH Plus sealer influenced HGF viability. However, the different concentrations of AgVO3 incorporated in Sealer 26 and Endomethasone N sealers, did not affect cell viability compared to the commercial group, indicating that these endodontic sealers are cytotoxic for HGF cells, regardless of the modification. The cell viability was inversely proportional to the exposure time with the extracts, and the concentration of released silver and vanadium ions was proportional to the concentration of incorporated AgVO3.
The cytotoxicity evaluation with extracts was performed according to ISO 10993-5 [25], which considers that a material is potentially cytotoxic if cell viability is reduced to less than 70% and has a cytotoxic effect if cell viability is reduced to less than 30%. All experimental groups presented HGF viability reduction compared to the negative control, including the commercial groups.
The biocompatibility of endodontic materials directly influences treatment success. The contact of materials with periapical tissues should not interfere with the repair process [5], but some induce irritation or degeneration of the surrounding tissues [18, 26]. Biocompatibility is determined by the physicochemical characteristics of materials (composition, element release, solubility, setting time) [27], and certain sealer components, such as zinc oxide, eugenol, formaldehyde, resinous components, and calcium hydroxide, have cytotoxic effects on human and animal cells [28,29,30].
The AH Plus groups had the lowest reduction of HGF viability, compared to the negative control, in 24 h of contact of the extracts with the cells, presenting only cytotoxic potential. The toxicity of AH Plus is attributed to the release of formaldehyde during the setting and to resinous components, such as amines that accelerate the polymerization reaction, besides the presence of bisphenol-A, epoxy resin component [16,17,18, 29]. With a longer exposure of cells to the extracts, all experimental groups showed a cytotoxic effect, with no significant difference among the commercial group, 2.5, 5 and 10% concentrations.
In addition to the cytotoxicity of sealers components, nanoparticles can also have this effect depending on the chemistry of their surface, since they interact with cellular DNA, in addition to proteins and lipids, which may damage the integrity of the membrane [31]. The incorporation of AgVO3 increased the cytotoxic potential of AH Plus, compared to the commercial group in 24 h of treatment, an effect that can be attributed to silver. Once Artal et al. [32] evaluated the cytotoxicity of the AgVO3 to Daphinia similis, an aquatic organism, and it found that the silver IC50 (concentration capable of inhibiting 50% of cell viability of D. similis) was 1.1 µg/L, while the vanadium IC50 was 1400 µg/L, proving the high cytotoxicity of silver. This study was to estimate that the silver IC50 for HGF was approximately 6.67 µg/L (based on the concentration of the ions released and the cell viability values for the AH Plus 10% in 24 h of treatment—Table 2 and Fig. 2). The silver IC50 estimated in this study is higher than the silver nanoparticle IC50 for HGF reported in the literature, whose concentration is approximately 0.04 µg/L [33, 34], giving preference to the use of AgVO3.
Besides, the AH Plus commercial group caused a reduction in HGF cell viability in 44.83% in 24 h of treatment, while AH Plus 10% caused a reduction of 65.38% of HGF viability. Thus, the incorporation of 10% of AgVO3 was responsible for reducing just 20.55% of HGF viability, acting in synergy with the components of the AH Plus commercial sealer.
For Sealer 26 and Endomethasone N, it cannot be affirmed that AgVO3 influenced the cytotoxicity of these sealers since all the experimental groups had a cytotoxic effect. The observed cytotoxicity may be inherent to the components of these materials. The Sealer 26, is an epoxy resin-based sealer containing calcium hydroxide. This sealer came from a modification in the AH 26 (Dentsply), whose silver present in the original formulation was replaced by 20% of calcium hydroxide. Although it is an epoxy resin sealer as AH Plus, it is polymerization reaction is characterized by polycondensation between the bisphenol epoxy resin and hexamethylenetetramine [24, 35]. Its initial irritating effect is due to the substances present in the formulation, mainly calcium hydroxide which, in contact with the culture medium, dissociates into calcium and hydroxyl ions, resulting in a higher pH [36, 37].
Endomethasone sealer (Septodont, Saint-Maur-des-Fossés, France) is based on zinc oxide and eugenol (ZOE) and has hydroquinone acetate in its composition to inhibit tissue inflammatory responses. Initially, this sealer contained paraformaldehyde, which despite the potent antimicrobial action, it was highly cytotoxic [26], inducing COX-2 expression and causing inflammation [30]. Endomethasone N was then developed with a formaldehyde-free composition. However, this sealer showed a cytotoxic effect for HGF in the present study, which may be due to the sealer base formula. ZOE extracts have been related to chronic inflammation of the periodontal ligament and shown to promote the release of signaling molecules that lead to bone resorption [18]. In addition, they have been reported to be cytotoxic for dental pulp stem cells, gingival fibroblasts, and keratinocytes [28], and released eugenol has a cytotoxic effect and might be related to the development of periapical lesions [18, 29].
Despite their cytotoxic effects, the release of chemical components of endodontic sealers is essential for the antimicrobial effect. Positively charged metal ions interact electrostatically with the negatively charged bacterial cell membrane, causing cell death [38]. The AgVO3 presents this activity, since the silver ions and vanadium ions in the 5+ state of oxidation (V5+) interact with the thiol groups present in the enzymes of bacterial metabolism, forming stable complexes [11]. In addition, the contact of Ag+ ions with bacterial DNA prevents its replication. Moreover, the oxidation–reduction reaction between V4+ and V5+can cause oxidative stress to the bacterial cell [11].
In the present study, the concentration of released silver and vanadium ions was evaluated in the 24 h period, corresponding to the release time of the extracts for cell viability. It was found that the release was proportional to the concentration of AgVO3 incorporated. The AH Plus modified groups showed the lowest concentration of released ions. This may have been due to the way this sealer is mixed; the manufacturer recommendations ask for equal proportions of the two pastes, and the higher proportion of resinous components could prevent the release of ions from the nanomaterial [39].
Sealer 26 and Endomethasone N released higher concentrations of vanadium ions, especially for the groups incorporated with 10% AgVO3. The greater solubility of Endomethasone N, verified in a previous study [14], corroborates the findings of this study, and can be related to the higher cytotoxic effect. In this study, only the release of silver and vanadium was evaluated, however, the chemical elements of the original sealer might also influence the cytotoxicity, as mentioned previously.
Despite the low concentrations of Ag+ released by the AH Plus groups, these were higher than the concentrations of V4+ and V5+. Thus, the influence of AgVO3 on the cytotoxicity of modified AH Plus groups could be attributed to silver, although present in low concentrations. For Sealer 26 and Endomethasone N sealers, high concentrations of vanadium ions were released. However, the cytotoxicity cannot be attributed to vanadium, as there was no statistical difference between groups with and without AgVO3.
The results observed in this study support that, despite the effects observed on HGF viability, the original compositions of the commercial sealers are cytotoxic for HGF cells. In addition, the release of silver and vanadium ions assists in the antimicrobial activity of these sealers. Future investigations about genotoxic potential should be undertaken.
Conclusion
HGF cells viability was inversely proportional to the exposure time of the cells to the extracts. It was not possible to confirm the influence of AgVO3 on HGF cell viability to Sealer 26 and Endomethasone N, however, nanomaterial influenced cell viability to AH Plus, so the commercial sealers can be cytotoxic in synergy with the nanomaterial. Nevertheless, the release of Ag+ and V4+/V5+ was proportional to the AgVO3 incorporated, being essential for the maintenance of the antimicrobial effect.
References
Kapralos V, Koutroulis A, Orstavik D, Sunde PT, Rukke HV. Antibacterial activity of endodontic sealers against planktonic bacteria and bacteria in biofilms. J Endod. 2018;44:149–54.
Dornelles NB Jr, Collares FM, Genari B, Balbinot GS, Samuel SMW, Arthur RA, Visioli F, Guterres SS, Leitune VCB. Influence of the addition of microsphere load amoxicillin in the physical, chemical and biological properties of an experimental endodontic sealer. J Dent. 2018;68:28–33.
Ruiz-Linares M, Bailón-Sánchez ME, Baca P, Valderrama M, Ferrer-Luque CM. Physical properties of AH Plus with chlorhexidine and cetrimide. J Endod. 2013;39:1611–4.
Silva GO, Cavalcanti BN, Oliveira TR, Bin CV, Camargo SEA, Camargo CHR. Cytotoxicity and genotoxicity of natural resin-based experimental endodontic sealers. Clin Oral Investig. 2016;20:815–9.
Shrestha A, Kishen A. Antibacterial nanoparticles in endodontics: a review. J Endod. 2016;42:1417–26.
Carpio-Perochena A, Kishen A, Shrestha A, Bramante CM. Antibacterial properties associated with chitosan nanoparticle treatment on root dentin and 2 types of endodontic sealers. J Endod. 2015;41:1353–8.
Samiei M, Farjami A, Dizaj SM, Lotfipour F. Nanoparticles for antimicrobial purposes in Endodontics: a systematic review of in vitro studies. Mater Sci Eng C. 2016;58:1269–78.
Nair N, James B, Devadathan A, Johny M, Mathew J, Jacob J. Comparative evaluation of antibiofilm efficacy of chitosan nanoparticle- and zinc oxide nanoparticle-incorporated calcium hydroxide-based sealer: an in vitro study. Contemp Clin Dent. 2018;9:434–9.
Teixeira ABV, Silva CCH, Alves OL, Reis AC. Endodontic sealers modified with silver vanadate: antibacterial, compositional, and setting time evaluation. Biomed Res Int. 2019;2019:1–9.
Holtz RD, Souza Filho AG, Brocchi M, Martins D, Durán N, Alves OL. Development of nanostructured silver vanadates decorated with silver nanoparticles as a novel antibacterial agent. Nanotechnology. 2010;21:1–8.
Holtz RD, Lima BA, Souza Filho AG, Brocchi M, Alves OL. Nanostructured silver vanadate as a promising antibacterial additive to water-based paints. Nanomedicine. 2012;8:935–40.
Corrêa JM, Mori M, Sanches HL, Cruz AD, Poiate E Jr, Poiate IAVP. Silver nanoparticles in dental biomaterials—review article. Int J Biomater. 2015;2015:1–9.
Salas-Orozco M, Niño-Martínez N, Martínez-Castañón GA, Méndez FT, Jasso MEC, Ruiz F. Mechanisms of resistance to silver nanoparticles in endodontic bacteria: a literature review. J Nanomater. 2019;7630316:1–11.
Teixeira ABV, Vidal CL, Albiasetti T, Castro DT, Reis AC. Influence of adding nanoparticles of silver vanadate on antibacterial effect and physicochemical properties of endodontic sealers. Iran Endod J. 2019;14:7–13.
Teixeira ABV, Vidal CL, Castro DT, Oliveira-Santos C, Schiavon MA, Reis AC. Incorporating antimicrobial nanomaterial and its effect on the antimicrobial activity, flow and radiopacity of endodontic sealers. Eur Endod J. 2017;2:2–8.
Candeiro GTM, Moura-Netto C, D’Almeida-Couto RS, Azambuja-Júnior N, Marques MM, Cai S, Gavini G. Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int Endod J. 2016;49:858–64.
Eldeniz AU, Shehata M, Högg C, Reichl FX. DNA double-strand breaks caused by new and contemporary endodontic sealers. Int Endod J. 2016;49:1141–51.
Saraiva JA, Fonseca TS, Silva GF, Sasso-Cerri E, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Cerri PS. Reduced interleukin-6 immunoexpression and birefringent collagen formation indicate that MTA Plus and MTA Fillapex are biocompatible. Biomed Mater. 2018;13:1–14.
Castro DT, Valente MLC, Agnelli JAM, Silva CHL, Watanabe E, Siqueira RL, Alves OL, Holtz RD, Reis AC. In vitro study of the antibacterial properties and impact strength of dental acrylic resins modified with a nanomaterial. J Prosthet Dent. 2016;115:238–46.
Procópio ALF, da Silva RA, Maciel JG, Sugio CYC, Soares S, Urban VM, Neppelenbroek KH. Antimicrobial and cytotoxic effects of denture base acrylic resin impregnated with cleaning agents after long-term immersion. Toxicol in Vitro. 2018;52:8–13.
de Souza JR, da Silva L, da Rocha MS, Saint Pierre TD. Dynamic reaction Cell-ICP-MS as a powerful tool for quality control of a se-enriched dietary supplement. Food Anal Methods. 2017;10:3088–97.
Teixeira ABV, Vidal CL, Castro DT, Valente MLC, Oliveira-Santos C, Alves OL, Reis AC. Effect of the incorporation of a new antimicrobial nanomaterial on the physical-chemical properties of endodontic sealers. J Conserv Dent. 2017;20:392–7.
Cintra LTA, Benetti F, Queiroz IOA, Ferreira LL, Massunari L, Bueno CRE, Oliveira SHP, Gomes-Filho JE. Evaluation of the cytotoxicity and biocompatibility of new resin epoxy–based endodontic sealer containing calcium hydroxide. J Endod. 2017;43:2088–92.
Kuga MC, Faria G, Só MV, Keine KC, Santos AD, Duarte MAH, Kopper PMP. The impact of the addition of iodoform on the physicochemical properties of an epoxy-based endodontic sealer. J Appl Oral Sci. 2014;22:125–30.
ISO Report. ISO 10993–5: biological evaluation of medical devices - Part 5: tests for in vitro cytotoxicity. Geneva: International Standard Organization; 2009
Trichês KM, Simi Junior J, Calixto JB, Machado R, Rosa TP, Silva EJNL, Vansan LP. Connective tissue reaction of rats to a new zinc-oxide-eugenol endodontic sealer. Microsc Res Tech. 2013;76:1292–6.
Rodrigues C, Costa-Rodrigues J, Capelas JA, Fernandes MH. Long-term dose- and time-dependent effects of endodontic sealers in human in vitro osteoclastogenesis. J Endod. 2013;39:833–8.
Lee JH, Lee HH, Kim HW, Yu JW, Kim KN, Kim KM. Immunomodulatory/anti-inflammatory effect of ZOE-based dental materials. Dent Mater. 2017;33:e1–e12.
Silva EJNL, Accorsi-Mendonça T, Almeida JFA, Ferraz CCR, Gomes BPFA, Zaia AA. Evaluation of cytotoxicity and up-regulation of gelatinases in human fibroblast cells by four root canal sealers. Int Endod J. 2012;45:49–56.
Bae WJ, Chang SW, Lee SI, Kum KY, Bae KS, Kim EC. Human periodontal ligament cell response to a newly developed calcium phosphate–based root canal sealer. J Endod. 2010;36:1658–63.
Deng J, Yao M, Gao C. Cytotoxicity of gold nanoparticles with different structures and surface-anchored chiral polymers. Acta Biomater. 2017;53:610–8.
Artal MC, Holtz RD, Kummrow F, Alves OL, Umbuzeiro GA. The role of silver and vanadium release in the toxicity of silver vanadate nanowires toward Daphnia similis. Environ Toxicol Chem. 2013;32:908–12.
Yin IX, Yu OY, Zhao IS, Mei ML, Li QL, Tang J, Chu CH. Developing biocompatible silver nanoparticles using epigallocatechin gallate for dental use. Arch Oral Biol. 2019;102:106–12.
Niska K, Knap N, Kędzia A, Jaskiewicz M, Kamysz W, Inkielewicz-Stepniak I. Capping agent-dependent toxicity and antimicrobial activity of silver nanoparticles: an in vitro study. Concerns about potential application in dental practice. Int J Med Sci. 2016;13:772–82.
Borlina SC, Souza V, Holland R, Murata SS, Gomes-Filho JE, Dezan E Jr, Marion JJC, Anjos ND. Influence of apical foramen widening and sealer on the healing of chronic periapical lesions induced in dogs’ teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:932–40.
Chang SW, Lee SY, Kang SK, Kum KY, Kim EC. In vitro biocompatibility, inflammatory response, and osteogenic potential of 4 root canal sealers: sealapex, sankin apatite root sealer, MTA fillapex, and iroot sp root canal sealer. J Endod. 2014;40:1642–8.
Silva LAB, Azevedo LU, Consolaro A, Barnett F, Xu Y, Battaglino RA, Cañadas PS, Oliveira KMH, Silva RAB. Novel endodontic sealers induce cell cytotoxicity and apoptosis in a dose-dependent behavior and favorable response in mice subcutaneous tissue. Clin Oral Investig. 2017;21:2851–61.
Şuhani MF, Băciuţ G, Băciuţ M, Şuhani R, Bran S. Current perspectives regarding the application and incorporation of silver nanoparticles into dental biomaterials. Clujul Med. 2018;91:274–9.
Arias-Moliz MT, Ruiz-Linares M, Cassar G, Ferrer-Luque CM, Baca P, Ordinola-Zapata R, Camilleri J. The effect of benzalkonium chloride additions to AH Plus sealer: antimicrobial, physical and chemical properties. J Dent. 2015;43:846–54.
Acknowledgements
The authors thank the cell brank of integrated research center of School of Dentistry of Bauru/University of São Paulo, Brazil.
Funding
This work was supported by the Foundation for Research Support of the State of São Paulo [FAPESP—Grant Number 2017/04667-0]; Coordination for the improvement of higher education personnel [CAPES—Grant Number 001]; and National Council for Scientific and Technological Development [CNPq—Grant Number 140139/2017-6].
Author information
Authors and Affiliations
Contributions
The authors ABVT and ACDR contributed to the study conception and design. Material preparation, data collection and analysis were performed by ABVT, DTDC and MAS. The first draft of the manuscript was written by ABVT and ACDR. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teixeira, A.B.V., de Castro, D.T., Schiavon, M.A. et al. Cytotoxicity and release ions of endodontic sealers incorporated with a silver and vanadium base nanomaterial. Odontology 108, 661–668 (2020). https://doi.org/10.1007/s10266-020-00507-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-020-00507-x