Abstract
Chemical fertilizers and pesticides are an integral part of modern agriculture and are often associated with numerous environmental problems. Biological agents such as microorganisms can largely replace chemical fertilizers and pesticides. The proper use of selected microorganisms such as bacteria, fungi and viruses have several benefits for agriculture. These include a healthy soil microbiota, biological production of important compounds that promote plant health, and to be used as biocontrol agents (BCAs) that provide protection from plant pathogenic microorganisms. Scientists have found that several bacterial genera including Bacillus and Pseudomonas have antimicrobial activity against numerous pathogenic bacterial and fungal plant pathogens. Trichoderma, Aspergillus, and Penicillium are among the most common fungal genera used as BCAs against both bacterial and fungal plant pathogens. Several bacteriophages and mycoviruses are also found effective as BCAs against selective plant pathogens. Fusarium oxysporum is a commonly found microbial plant pathogen causing wilts and rots in plants. Overall, it can be concluded that the use of microbial BCAs is an effective practice against microbial plant pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agriculture has been one of the most important practices in the world for centuries. Fertilizers and pesticides are widely used to increase crop productivity, promote plant growth, and inhibit growth and damage caused by plant pathogenic microorganisms. These chemicals, referred to as agrochemicals, are an integral part of modern agriculture, providing farmers with high crop yields at low costs (Malik et al. 2017). However, these chemical compounds degrade soil quality, cause pollution, disrupt ecological balance by disturbing natural microflora and food chains, and cause problems for human health (De Silva et al. 2019). With simultaneous growth of human population and with it the growth of demand for food, producing high-quality crops with limited application of chemical fertilizers and chemical pesticides is a major challenge. Organic farming with minimal use of chemical fertilizers has gained popularity in recent decades, as organic crops are considered healthier and more environmentally friendly.
Various biological strategies are implemented to control plant diseases mediated by pathogenic microorganisms, including the introduction of natural predators and inhibitors of microbial pests. These microbial pests are eliminated either by direct inhibition strategies such as the production of enzymes, antibiotics, or toxins, or by indirect inhibition through competition for niches and nutrients. Microbial biocontrol agents (BCAs) have been in use for decades to control plant diseases and to repel pathogens. Commonly used BCAs include bacteria such as Agrobacterium sp., Bacillus sp., and Pseudomonas sp.; and fungi such as Aspergillus spp., Ampelomyces sp., Candida sp., Coniothyrium sp., Gliocladium sp., and Trichoderma sp. (Gawai 2018). The use of microorganisms and microbial products has several advantages over chemical fertilizers: they are target-specific, can be used in small quantities, are more rapidly degradable, and can be used for both disease control and plant growth promotion. However, besides the advantages, such microbial agents also have some major disadvantages, such as resistance adaptation of target pests with repeated use of the same biocontrol species, susceptibility to abiotic conditions, and lower economics of the process due to high cost of biofertilizers.
Biocontrol agents or BCAs are natural or modified organisms that eliminate undesirable disease-causing pests and organisms while promoting the growth of beneficial insects and microorganisms (Singh et al. 2020). In terms of disease control and increasing agricultural yields, BCAs such as microorganisms (bacteria, fungi, and viruses), plant feedstocks, and vermicompost are effective. These BCAs can effectively control plant diseases and improve pathogen resistance by interacting with pathogens through parasitism, competition, release of antimicrobial compounds, and destruction of spores, mycelium, cells, and endospores of the pathogens. Biological pesticides are substitutes for chemical fertilizers and pesticides, but have secondary effects on the environment and human health. Biofertilizers are microbial inoculants used in agriculture. They can be either active or latent strains of microorganisms as sole species or in combination with algal or fungal components that activate microbial activity and enhance nutrient uptake from the soil (Suyal et al. 2016).
The criteria of modern agriculture, which tend to use a minimum of chemical pesticides, can be met by the application of biopesticides, but are considered expensive compared to chemical agents. Several biopesticides are available in the market, of which about 75% are bacterial (Siegwart et al. 2015). In European countries, biopesticides have attracted much attention compared to Asian countries. In the Indian pesticide market, biopesticides accounted for only 4% in 2014 (Singh 2014), which increased to 9% of the overall pesticide consumption in India by 2020 with an overall increase of 40% from 2014 to 2019 (Nayak and Solanki 2021). About 970 biopesticides were registered till 2021 by the Central Insecticides Board and Registration Committee (CIBRC) in India (Nayak and Solanki 2021). One of the major problems with the use of biopesticides is the development of resistance in pests, which calls into question the sustainability of such strategies (Siegwart et al. 2015). Bacillus thuringiensis (Bt) is considered one of the most successful biopesticides to date, used both as a Bt spray and as genetically engineered plants with Bt insecticide genes. Resistance to Bt pesticides and plants has been found in a wide range of pest species (Jurat-Fuentes et al. 2021).
Biological pest control can be briefly divided into natural, conservation, classical, and supplemental biocontrol. Natural biocontrol refers to pest control by natural means without human intervention in all types of ecosystems around the world. In the conservation type biocontrol strategy, the natural antagonists of pests and microbial plant pathogens are stimulated by human intervention (van Lenteren et al. 2018). In classical biological control, natural inhibitor organisms of a pest are collected from its area of origin and introduced into areas where the pests have become invasive. This method is called classical because it is one of the first widely used biological control methods. In complementary biological control, specific BCAs are applied to specific crops to inhibit the target pests, but the inhibitory effect is not carried over to the next crop cycle (Cock et al. 2010; van Lenteren et al. 2018). Much research has been done on various aspects of biological control, but we need to continue to look forward and conduct new research to enable new biocontrol technologies and applications by improving the efficacy of biocontrol agents and their biocontrol potential.
Microorganisms as BCA
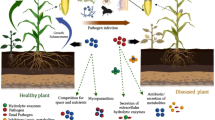
Microbial biocontrol agents help control plant pathogens through various methods, including inducing resistance or priming of plants to pathogenic microorganisms, competing for space and nutrients, or other methods that involve disrupting growth conditions necessary for pathogen growth. Various microorganisms are known to produce antibiotics, enzymes, and various other metabolites and compounds that help control plant pathogens. Microorganisms with such properties are used as antagonists (Köhl et al. 2019). Several microorganisms have both pathogen-inhibiting and plant growth-promoting properties. Microorganisms colonize plant surfaces and form plant–microbe interactions. These microorganisms may be bacteria, fungi, protozoa, actinomycetes, nematodes, or microarthropods that promote plant growth and crop yield. Phytopathogenic fungi and bacteria cause many diseases in agricultural crops and postharvest agricultural products, leading to global food security problems that are difficult to control. Chemical fungicides and bactericides are commonly used by farmers to control microbial diseases because they are usually cheaper and readily available in markets. A biological and environmentally friendly approach is to use microorganisms with antagonistic properties for the biological control of plant pathogenic microorganisms (Köhl et al. 2019). Various bacteria, fungi, and viruses are known to have antagonistic properties against a variety of fungal pathogens that cause disease in agricultural crops to biocontrol them. As part of an environmentally friendly approach, BCAs can be used effectively alone or in combination with chemical fertilizers to reduce plant diseases. Several microbial species have been found to have antagonistic activities and to be effective in biocontrol of phytopathogenic bacteria and associated plant disease control. Figure 1 shows the different microbial BCAs and their general modes of biocontrol. Commercially, there are many microbial products available in the market all over the world for disease control in agricultural crops. Some of these products are tabulated in Table 1.
Fungi as BCAs
Several species of fungi have properties that make them ideal BCAs of plant pathogens. Trichoderma has been found to be effective against a wide range of plant pathogens and is therefore considered one of the best biocontrol agents (Mukhopadhyay and Kumar 2020). Many Trichoderma strains and its secondary metabolites are found to suppress proliferation of microbial plant pathogens, stimulate plant growth, enhance root systems, as well as suppress several plant diseases (Zin and Badaluddin 2020). Some common secondary metabolites and their related Trichoderma strains are mentioned in Table 2. Penicillium, Gliocladium, Aspergillus and Saccharomyces are some of the other common fungal genera that have antagonistic properties like direct parasitism and secondary metabolite production (e.g., antibiotics, toxins) (Prajapati et al. 2020; Ram et al. 2018) against fungal plant pathogens such as Phytophthora, Fusarium, Alternaria, Pythium, Rhizoctonia, Botrytis, Pyricularia and Gaeumannomyces (Tariq et al. 2020).
Fungal species are used as BCAs because of their ability to produce antibiotics and enzymes to compete for space and nutrients, induce systemic resistance, or produce lytic enzymes that lead to direct mycoparasitism (Kumar and Ashraf 2017; Ghorbanpour et al. 2018). Besides these properties, fungal BCAs are also host specific, efficient, cause high levels of host destruction, easy maintenance and environmentally friendly in nature, which makes fungal BCAs ideal for plant disease control (Savita and Sharma 2019). The arbuscular mycorrhizal fungus (AMF) Funnelliformis mosseae and the rhizobium Sinorhizobium medicae can effectively inhibit the fungus Fusarium oxysporum, which is responsible for wilt and root rot in alfalfa (Wang et al. 2019). Antagonistic fungi belonging to the genera Trichoderma, Gliocladium, Pythium sp., non-pathogenic Fusarium sp., Rhizoctonia sp. and Laestisaria sp. and antagonistic bacterial genera Bacillus, Pseudomonas and Streptomyces are widely studied for biological control of fungal plant pathogens in soil (Jensen and Lumsden 1999).
Fungi, particularly Trichoderma sp, are widely known to biocontrol phytopathogenic bacteria by producing antimicrobial secondary metabolites (Table 2). Fungi of the genus Trichoderma, including T. pseudoharzianum (T113), T. asperelloides (T136), T. pseudoharzianum (T129), and T. pseudoharzianum (T160), have been found to produce secondary metabolites that have antimicrobial properties against phytopathogenic bacteria Ralstonia solanacearum and Xanthomonas compestris (Khan et al. 2020).
Bacteria as BCAs
Bacterial species commonly known as plant growth promoting bacteria (PGPB) are effectively used as biofertilizers and BCAs (Singh et al. 2019). PGPB is a group of bacterial species that enhance plant growth by improving nutrients for host plants, secretion of important enzymes and phytohormones, as well as induction and enhancement of pathogen resistance in host plant. Some common bacteria belonging to the PGPB group includes Agrobacterium radiobacter, Azospirillum lipoferum, Bacillus licheniformis, B. cereus, B. subtilis, B. amyloliquefaciens, Pseudomonas fluorescens, P. solanacearum, P. syringae, Rhizobium spp., etc. (Khabbaz et al. 2019). The introduction of bacteria into soil and plants for the promotion of plant growth and development is widely known. Bacteria of certain species perform nitrogen fixation that provides usable nitrogen to the plant body, degrades toxic compounds, and helps control plant pathogenic microorganisms (Tariq et al. 2020). PGPB like Rhizobacteria, stimulate plant growth by colonizing the root system of plants as well as the soil surrounding the root system (known as the rhizosphere) up to a certain radius that can be of 4 mm or above which is determined by numerous factors like pH, N- and other compounds, soil water content, root exudates, and several other organic and inorganic compounds and nutrients (Kuzyakov and Razavi, 2019). In many cases, rhizobacteria have been found to colonize as endophytes by entering the plant system through the roots. Several rhizobial strains have antagonistic properties and growth inhibitory activity against phytopathogenic fungi in soil such as Fusarium, Rhizoctonia, Sclerotium and Macrophomina (Das et al. 2017). The antagonistic activity of rhizobia is primarily by the production of antibiotics, hydrocyanic acid (HCN), mycolytic enzymes, and siderophores under iron-limiting conditions. Kanouni et al. (2018) reported the growth inhibitory properties of various Rhizobia isolates against pathogenic Alternaria sp., Penicillium sp., Cladosporium sp. and Humicola sp. There are several other PGPB that occur naturally as endophytic colonizers. PGPB mediate biocontrol of pathogenic microorganisms through mechanisms such as competition for niches and substrates, secretion of allelochemicals such as siderophores, and activation of the host plant immune system to induce systemic resistance to pathogens and environmental stress (Compant et al. 2005; Reddy 2014).
Numerous bacterial species are known for their antifungal properties. Saechow et al. (2018) reported the mycelial inhibitory potential of Bacillus amyloliquefaciens against some fungal pathogens of rice, including Curvularia lunata, Fusarium semitectum, and Helminthosporium oryzae. Several PGPB species like Bacillus simplex, B. subtilis, B. amyloliquefaciens, B. licheniformis, B. velezensis etc., produce antifungal compounds that inhibit fungal pathogens and compete with them for space and food (Choub et al. 2022). There are reports of antagonistic activities of Streptomyces isolates from soil with high antagonistic activity against Curvularia sp., Helminthosporium sp., A. niger, and Fusarium sp. (Evangelista-Martínez 2014). The bacterial genus Bacillus produces several secondary metabolites such as bacteriocins, antimicrobial peptides and lipopeptides, polyketides, and siderophores with antagonistic properties (Fira et al. 2018). Bacillus amyloliquefaciens FZB42, a commercially available bacterial strain, produces bacillomycin D, which contributes to the antifungal properties of the bacterial strain against the fungus Fusarium graminearum that infects wheat and barley (Gu et al. 2017). Fengycin BS155, a cyclic lipopeptide produced by Bacillus subtilis BS155, was described by Zhang and Sun (2018) as the major antifungal compound against the phytopathogen Magnaporthe grisea, the agent that cause rice blast. Fengycin BS155 was found to alter the morphology of the M. grisea hyphal plasma membrane and cell walls, causing organelle dysfunction, disrupting mitochondrial membrane potential, oxidative stress, and chromatin condensation, leading to hyphal cell death. Several bacteria found in the plant microbiome have antifungal properties mediated by the production of specific compounds. Chen et al. (2018c) reported that Pseudomonas piscium ZJU60 isolated from the wheat microbiome exhibited antifungal activity against Fusarium graminearum by inhibiting growth, reducing virulence, and suppressing mycotoxin production by releasing the bacterial compound phenazine-1-carboxamide (PCN). This report suggests that ZJU60 can be used for biocontrol of the plant disease Fusarium head blight (FHB) caused by F. graminearum. Durairaj et al. (2017) reported the bacterial species Pseudomonas aeruginosa and Bacillus stratosphericus with antagonistic activity against several bacterial plant pathogens such as Pseudomonas syringae, Burkholderia glumae, Xanthomonas oxyzae pv. Oryzae, Pectobacterium carotovorum and Ralstonia solanacearum. The bacterial species P. aeruginosa and B. stratosphericus produce siderophores and secondary metabolites such as chitinase, protease, and amylase, which contribute to antagonistic activity. The authors also reported that local application of P. aeruginosa and B. stratosphericus on tomato plants stimulated the expression of the gene PR -1a, which was able to stimulate the salicylic acid defence mechanism against infecting pathogens.
Plant growth promoting bacteria (PGPB) such as many strains of the genera Bacillus (strains of B. subtilis, B. amyloliquefaciens, B. velezensis), Pseudomonas (strains of P. aeruginosa, P. fluorescens), Streptomyces sp. and various other PGPB proteobacteria and Firmicutes have bio-controlling properties against bacterial plant pathogens. PGPB can control phytopathogenic plant diseases through several mechanisms, including repression and physical suppression of the phytopathogen, secretion of siderophores, synthesis of antibiotics, synthesis of growth inhibitory compounds and enzymes, and stimulation of systemic resistance in the plant (Glick and Bashan 1997). A few recent reports of bacteria as BCA have been depicted in Table 3.
Viruses as BCAs
Several DNA and RNA viruses are used in biocontrol of fungal plant pathogens. Mycoviruses cause hypovirulence in fungi, where the pathogenicity of phytopathogenic fungi is reduced due to mycovirus infection (Abid et al. 2018). Although the presence of mycoviral dsRNAs has been reported in Fusarium solani, F. oxysporum, F. poae, and F. graminearum, their incorporation into hypovirulent host has been detected in only a few isolates, including F. graminearum strain DK21. Transmissible viruses carried by hypovirulent strains of plant pathogenic fungi have attracted the attention of researchers because they can be used as BCAs and serve for understanding the fungal pathogenesis mechanisms. Some cases of mycovirus-associated hypovirulence include mitoviruses infecting Ophiostoma novo-ulmi and Sclerotinia homoeocarpa, as well as mycoviruses infecting Diaporthe ambigua, Fusarium graminearum, and Botrytis cinerea (Sharma et al. 2018). Viruses are responsible for natural suppression of the fungal pathogens such as, Cryphonectria parasitica, Gaeumannomyces graminis, Sclerotinia sp. and Ophiostoma novo-ulmi, which cause chestnut blight, powdery mildew, Sclerotinia rot and Dutch elm disease, respectively. Thus, a broad spectrum of microbial pathogens, including viruses, bacteria, and fungi, possess antagonistic activities against an equally broad spectrum of fungal pathogens (Elad and Freeman 2002).
Bacteriophages are parasites for bacteria and depend on host bacteria to complete their life cycle. Virulent lytic phages are of greatest interest for biocontrol of phytopathogenic bacteria in agriculture because they are capable of lysing host cells and are highly host specific with a narrow target range. To use phages for biocontrol, it is important to select those phage that do not undergo a lysogenic life cycle and do not induce transduction of bacterial genes as in such case resistance may be induced in host bacteria due to integration of bits of previous host bacterial genes into phage genetic material (Barua and Nath 2018). Biocontrol by phages helps in addressing the problem of bacterial antibiotic resistance. Voronina et al. (2019) reported a podovirus PP16 that infects strains of Pectobacterium carotovorum, the causal agent of blackleg and soft rot of potato, and inhibits bacterial infection both in vitro and in planta. Because phages are highly host specific, the use of a cocktail of phage particles is often considered to increase the reach of biocontrol. Carstens et al. (2019) reported the preparation of a phage cocktail of 6 species to reduce soft rot of potato caused by a number of Pectobacterium atrosepticum strains. They reported 61% and 64% reduction in disease occurrence and severity, respectively, indicating the potential of phage control of P. atrosepticum strains.
Mechanism of biological control
Severity of plant diseases may be influenced by numerous factors, such as, plant sensitivity, virulence of the pathogens, and the abiotic environment. Several researches have been conducted to understand the mechanisms of action of various BCAs. The basic biocontrol mechanism of bacteria and fungi against microbial phytopathogens involves various strategies of antagonism. Viruses such as bacteriophages, on the other hand, follow the lytic and lysogenic life cycles to infect the host, which in some cases lead to the production of microbial toxins with antimicrobial properties.
Microbial antagonism
Antagonistic properties of various fungi, bacteria, PGPB, and rhizobacteria are commonly used as a biocontrol strategy, whereby these microbial species secrete substances that are harmful to the target organisms. Such antagonistic microbial species employ various antagonistic strategies, including hyper-parasitism, production of inhibitory compounds such as enzymes, toxins, or antibiotics, and competition for space and food (Prajapati et al. 2020; De Silva et al. 2019). The use of antagonistic properties of various endophytic microbial species as a biocontrol strategy has attracted considerable attention. Endophytic fungal species belonging to different genera such as nonpathogenic mutant strains of Colletotrichum magna, C. gloeosporioides, Cladosporium oxysporum, Fusarium verticillioides, F. graminearum, Pestalotiopsis cocculi, and several Trichoderma species such as T. gamsii, T. polysporum etc., have been shown to be effective in controlling plant diseases (De Silva et al. 2019). Chen et al. (2018b) reported the antagonistic property of Galactomyces candidum JYC1146 against the fungus Botrytis cinerea that causes gray mold. Yang et al. (2019) reported the antagonistic properties of Streptomyces corchorusii stain AUH -1 through the production of secondary metabolites against a variety of soilborne plant pathogens, including Fusarium oxysporum f. sp. niveum, Phytophthora parasitica var. nicotianae, Rhizoctonia solani, P. capsica, Botryosphaeria dothidea, F. oxysporum f. sp. vasinfectum, Verticillium dahliae, and F. oxysporum f. sp. cucumerinum. Chen et al. (2020a) reported the antagonistic nature of the novel endophytic LAB Lactobacillus plantarum CM-3 isolated from strawberry against the fungus Botrytis cinerea, which causes gray mold rot in strawberries.
The mode of action of antagonistic microorganisms against pathogenic targets varies depending on the biocontrol agent, pathogen species, host species, and abiotic conditions. One known mode of action is antibiosis, in which microbial BCAs secrete toxins or antibiotic compounds that inhibit pathogen growth. Antibiotics are secondary metabolites whose efficacy depends on their biochemical composition, which inhibits the metabolism and growth of pathogens (Ram et al. 2018). Various microbial species, including both bacteria and fungi, are known for producing several antibiotics, of which the most common fungal genera includes Trichoderma and Gliocladium, and several bacterial species belonging to the genera Bacillus and Pseudomonas (Prajapati et al. 2020). Bacillus amyloliquefaciens strain DH-4 has shown antagonistic activity by producing some heat tolerant compounds such as macrolactin, bacillaene, iturins, fengycin, and surfactin, that damage the ultrastructure of Penicillium digitatum pathogenic cells that cause green mold rot of citrus (Chen et al. 2018a). The rhizobacterium Lysobacter capsici AZ78 releases antimicrobial secondary metabolites of the families 2,5-diketopiperazines, cyclic lipodepsipeptides, macrolactones and macrolides that are active against various plant pathogens and Gram-positive bacteria, as well as AZ78 was also found to produce dihydromaltophilin or Heat Stable Antifungal Factor effective against Plasmopara viticola (Brescia et al. 2021). Several plant growth-promoting rhizobacteria (PGPR) such as Pseudomonas fluorescens (David et al. 2018), several Bacillus sp. (Choub et al. 2022), Azospirillum e.g., Azospirillum baldaniorum Sp245 (Pandey et al. 2022), Rhizobium, and Serratia are involved in plant protection by producing antibiotics through a cascade of signals that regulate antibiotic synthesis, such as sensor kinases, N-acylhomoserine lactones, and sigma factors (Kenawy et al. 2019). Several broad-spectrum antibiotics belonging to polyketides, heterocyclic nitrogen compounds, and lipopeptides are produced by PGPR that promote induced systemic resistance in plant systems in addition to antipathogenic activity (Kenawy et al. 2019).
Hyper parasitism is a widely used method of controlling plant pathogens in which a microbial hyperparasite invades or kills the cells and spores of pathogenic bacteria and fungi. Several bacterial and fungal species produce specific enzymes that target specific microbial pathogens. Chen et al. (2020b) reported the production of enzymes such as protease, phosphatase, lysozyme, and siderophores, and 12 other secondary metabolites by Lysobacter enzymogenes LE16, which has antagonistic properties against various plant pathogens such as Colletotrichum gloeosporioides, Penicillium italicum, Alternaria alternate, Rhizoctonia solani, Didymella bryoniae, Sclerotinia sclerotiorum, Phytophthora nicotianae, and Phytophthora capsica. Hyperparasites can repel plant pathogens by several mechanisms, including antibiosis induced resistance in plants, and competitive exclusion of pathogens from the host plant. Trichoderma, a rhizospheric fungus and a commonly used BCA against pathogenic fungi, is capable of secreting antibiotics and lytic enzymes such as chitinases and glucanases that aid in cell wall degradation (Viterbo et al. 2002). Penicillium brevicompactum, Clonostachys rosea, and Simpicillium aogashimaense were able to significantly reduce the germination rate of various Puccinia sp. rust urediospores through antibiotic and antifungal effects (Wilson et al. 2020). The hyperparasitic Cladosporium cladosporioides isolated from the rust fungus Puccinia striiformis f. sp. tritici (Pst) was able to reduce the production and germination rate of Pst urediospores (Zhang et al. 2022a). The fungal virus Cryphonectria hypovirus 1 (CHV1) reduced the virulence of the chestnut blight pathogen Cryphonectria parasitica, mainly by reducing the metabolism of the fungus (Stauber et al. 2022).
Competition
Competition between microbial communities for space and food occurs when two or more microbial species are present in the same space and require the same nutrients. This characteristic is cleverly exploited to inhibit plant pathogens using biocontrol agents. Sarrocco et al. (2020) reported endophytic colonization of wheat roots by Trichoderma gamsii T6085 reduced colonization of Fusarium graminearum, the causal agent of late blight in wheat, and Fusarium oxysporum isolate (7121), a natural competitor of wheat plant residues. T6085 was also responsible for increased expression of plant defense mechanism PAL1 genes. Plant microbes are thought to rapidly colonize and consume all available substrates and surfaces to make them inaccessible to plant pathogens. In doing so, they compete primarily for macronutrients produced by plants. Iron is one such macronutrient that attracts multiple plant pathogens, leading to competition between rhizospheric and pathogenic microorganisms. Francesco and Baraldi (2021) reported that strains L1 and L8 of Aureobasidium pullulans compete with the fungal pathogen Monilinia laxa for iron by producing siderophores that reduce pathogen growth and germination at low iron concentrations. Plant root exudates serve as a food source for microorganisms in the rhizosphere. The rhizosphere consists of a limited supply of iron, resulting in a competitive situation. Many microorganisms secrete siderophores to scavenge iron. Siderophores are secondary metabolites with high affinity for iron and have the ability to promote or inhibit competing microbial species, depending on the presence of transporters or channels for siderophore uptake in competitors (Niu et al. 2020).
Induced resistance
Induced resistance in plants may consist of activation of resistance in plants using external agents by nonspecifically activating plant defense genes without causing any changes in the plant genome. Endophytic plant microorganisms are an option associated with strengthening host plant induced defenses. Endophytes are responsible for inducing resistance in plants by several mechanisms including increasing synthesis of phytoalexins (Asghari et al. 2020) and pathogenesis-related (PR) proteins (Jha, 2022), depositing lignin and glucans that thickens plant cell walls (Benhamou et al. 2000), as well as thickening cuticles which prevents pathogens from development and plant cell wall penetration (Fontana et al. 2021). Therefore, it is critical to understand the role of endophytic microorganisms to enhance agricultural productivity using natural biocontrol strategies that are often compromised by the use of chemical bactericides and fungicides. The endophytic Burkholderia gladioli BG -E39 exhibited fungicidal activity that caused mycelial cell death of several fungal saffron pathogens including Fusarium oxysporum by producing chitinase and β-1,3-glucanase that degraded the fungal cell wall. The endophyte prevented tuber blight by antibiosis, increased endogenous jasmonic acid content (JA), and increased expression of the JA signaling pathway for systemic resistance in the host plant (Ahmad et al. 2022).
Factors affecting the efficacy of biocontrol
Numerous biotic and abiotic factors influence the activity and behavior of BCAs, including pH, temperature, moisture, texture and components of the soil, native field microflora, and pesticide use. Experiments and trials with BCAs are first conducted under laboratory conditions on a small scale to avoid natural antagonists of the agricultural field environment. When these BCAs are tried on a large scale in natural field environments, they often do not show acceptable biocontrol activities. Controlled greenhouse conditions that meet environmental criteria such as pH, temperature, and other biotic and abiotic factors may be suitable for the application of antagonistic BCAs. When planning the use of BCAs against microbial plant pathogens, it is important to consider the various environmental factors that affect the proper functioning of BCAs. Growth and reproduction of several BCAs depend on substrates excreted by host plants through roots and shoots. These strongly influence microbial composition and the expression of genes responsible for the production of antibiotics and other microbial metabolites.
Mycoparasitic Trichoderma strains are strongly influenced by pH properties and water activity (Aw) (Kredics et al. 2004; Daryaeiet et al. 2016), which varies from strain to strain. pH 6 has been reported as the optimum for growth inhibition of Fusarium sp. and sporulation of Trichoderma sp. from onion soils in Sri Lanka (Abeyratne and Deshappriya 2018).
Antibiotic production by bacterial BCAs can be influenced by various abiotic factors such as oxygen, temperature, soil moisture, pH, carbon and nitrogen sources, and microelements, while biotic factors include the type of plant host and pathogen, local microflora, and cell density of the producing strain (Raaijmakers et al. 2002).
Combination of BCAs to enhance efficacy of biocontrol
The efficacy of BCAs can be synergistically enhanced by combining them with several other BCAs or with several agronomic, physical, or chemical agents so that all partners in the combination reap the benefits. Combining BCAs with other control agents provides a broader control spectrum and allows the use of control methods that are typically less effective as stand-alone agents. The combination of BCAs with chemical pesticides against target organisms has attracted much attention. This reduces chemical pesticide use, which is replaced by BCAs in the control of plant diseases. For example, a BCA can be combined with a chemical fungicide to improve the efficacy of the BCA and minimize the dosage of the fungicide. For such combinations, BCAs must be resistant to such chemicals, which can occur naturally or be produced by genetic manipulation. In a report by Zhang et al. (2021), cowpea wilt disease caused by Fusarium oxysporum was very effectively controlled by the combined application of the BCA Trichoderma asperellum strain SC012 and a fungicide hymexazol. The Trichoderma strain proved resistant to hymexazol and showed hyperparasitism to F. oxysporum. The fungicides Nativo (tebuconazole 50% + trifloxystobin 25%) and propiconazole were found to be compatible with T. harzianum strain IRRI-1 in vitro at concentrations of 10 ppm, 15 ppm, 20 ppm, and 25 ppm for control of various plant pathogens in an integrated system (Singh et al. 2021a). Dubey et al. (2020) reported in a study that Fusarium wilt of tomato could be controlled by the combined application of Pusa biopellet (T. harzianum or T. viride) in soil along with dipping seedling roots in Pusa 5SD (T. harzianum/T. viride) + Vitavax Power.
Several agronomic practices promote BCAs. These include informative practices for crop selection, soil types, crop rotations, soil amendments, and use of appropriate plowing, planting, and seeding regimes. Combining BCAs with appropriate agronomic practices can be an effective biological control strategy that further enhances the effectiveness of BCAs.
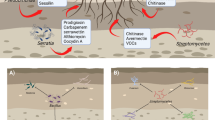
Figure 2 depicts the general factors that have both positive and negative effects on the efficacy of BCAs.
Effects of agrochemicals on BCAs
Agrochemicals include various chemical compounds used in agriculture in the form of pesticides, fertilizers, and chemical growth promoters. It is important to understand the effects of agrochemicals on natural and human-introduced microbial biocontrol agents. Several complex agrochemicals are used in the form of fertilizers and pesticides that reach non-target soil microorganisms (Patibanda and Ranganathswamy 2018). Some agrochemicals have drastic effects on non-target soil microorganisms and disrupt vital cellular functions leading to microbial cell death (Kalia and Gosal 2011). Such toxic agrochemicals have direct effects on the soil microbiota and lead to a decrease in soil fertility (Meena et al. 2020). Fungal pathogens are known to be one of the major causes of diseases in agricultural crops. Since chemical fungicides are cheap and readily available, they are used all over the world. Many of these fungicides are known to destroy non-target microorganisms (Yang et al. 2011).
Agrochemicals usually have a negative image in people's minds. However, several researchers have found that agrochemicals can have both harmful and nontoxic (sometimes beneficial) effects on soil microbial communities. Baćmaga et al. (2018) reported that chlorothalonil, a widely used agrochemical pesticide, increased the number of heterotrophic bacteria and actinobacteria at an acceptable dosage, while it had inhibitory effects on fungi. Chlorothalonil was also reported to improve soil biochemical properties. Fournier et al. (2020) reported that both chemical fungicides (Fosetyl-Al and propamocarb hydrochloride) and biopesticides (Clonostachys rosea) showed non-significant but different changes in soil microbial community composition.
Although BCAs have been shown to be effective in controlling diseases in plants as a replacement to various chemical pesticides, disease management is incomplete and efficacy is largely dependent on environmental abiotic conditions. In this regard, an integrated system combining BCAs with agrochemicals could be a perfect solution. It is expected that the combination of BCAs with fungicides in an integrated pest management of fungal plant pathogens will reduce the dosage of chemical fungicides as well as the selectivity of BCAs and adaptation of resistance (Ons et al. 2020).
Recent advances in biological control
In general, BCAs do not achieve satisfactory results under natural conditions compared to chemical pesticides and laboratory conditions. Advances in molecular methods have improved the efficacy and properties of BCAs in recent decades, and this is to the credit of numerous researchers in the field. Genetic modification of BCAs is intended to achieve a variety of goals. However, among the most common are improving efficacy in controlling plant pathogens, increasing resistance to chemical pesticides, and improving the stability of BCA strains to a variety of biotic and abiotic factors. The goals are to be achieved with minimal risk to the natural ecosystem.
The most common entomopathogenic bacterial genera to which genetic engineering has been applied include Bacillus, Lysinibacillus, Pseudomonas, Serratia, Photorhabdus, and Xenorhabdus (Azizoglu et al. 2020). Jing et al. (2020) reported the genetic modification of the root-colonizing bacterium Pseudomonas protegens Pf-5 as a biocontrol agent. Inactivation of retS genes generated a retS mutant strain that increased the production of the antibiotic 2,4-diacetylphloroglucinol and effectively inhibited the growth of Rhizoctonia solani. They also reported the introduction of nif genes into the retS mutant, which showed significant nitrogenase activity.
Genetic modification in the host plant
Genes can be modified, silenced, or introduced into host plants using vectors and other means as a biocontrol strategy against microbial pathogens. The process is complicated but not impossible, as there are reports of it. However, more research is needed in this area to reduce the use of chemical pesticides. Gene silencing strategy was used to suppress Asian soybean rust (ASR) in soybean leaves caused by Phakopsora pachyrhizi. The host-induced gene silencing strategy using modified bean pod mottle virus (BPMV) vectors in soybean was able to suppress the expression of 45%–80% of target genes, thereby reducing ASR symptoms in soybean leaves after inoculation with P. pachyrhizi. Direct spraying of dsRNAs for genes encoding an acetyl-CoA acyltransferase, a 40S ribosomal protein S16, and a glycine cleavage system H protein onto detached soybean leaves before inoculation with P. pachyrhizi also reduced P. pachyrhizi accumulation by up to 75% (Hu et al. 2020).
Expression of bacteriocins in crops through genetic modification is an effective strategy for controlling bacterial diseases in agricultural crops. Rooney et al. (2020) demonstrated efficient expression of PL1 in Nicotiana benthamiana and Arabidopsis, resulting in quantitative and qualitative disease resistance to PL1-susceptible strains of Pseudomonas syringae (Ps). They also reported that the link between the LPS biosynthetic machinery and mutations associated with PL1 insensitivity/tolerance and that increased PL1-tolerant Ps mutants are still unable to induce disease symptoms in transgenic plants. They conclude that transgenic expression of a bacteriocin in plants can lead to robust disease resistance to the bacterial phytopathogen Ps.
A gene editing system to improve resistance in crops for biocontrol of microbial plant pathogens has been recently introduced and widely accepted by the name of clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 (Karmakar et al. 2022; Verma et al. 2022). This system consists of several steps that include selection of the gene of interest, preparation of ribonucleoprotein (RNP), incorporation of the foreign RNP into plant cells, identification of the transformation occurred using Next Generation Sequencing (NGS), and evaluation of the expression of the gene of interest, followed by breeding new varieties of the modified target plant (Liu et al. 2021). This technology has been found to be highly precise, low off-target edits and can edit multiple sites in the genome (Bansal et al. 2022). The use of CRISPR technology has been dramatically increasing to induce disease resistance in crop plants by introducing genes responsible for production of metabolites with antimicrobial and antibacterial properties against microbial plant pathogens in crop plants (Sharma et al. 2022).
Induction of fungicide resistance in the host plant
BCAs are not considered 100% effective when used as the sole means of controlling fungal plant pathogens. This requires that the BCAs have significant resistance to the chemical fungicides so that both the chemical fungicide and the biocontrolling microorganisms can work efficiently. Genetic engineering techniques can be used to improve the fungicide resistance of such BCAs. Hirpara and Gajera (2018) reported the development of Trichoderma interfusants by protoplast fusion of mycoparasitic T. virens NBAII Tvs12 and fungicide-tolerant T. koningii MTCC 796, tolerance to the fungicides mancozeb, thiram, tebuconazole, and carbendazim, and increased antagonistic activity against Sclerotium rolfsii sacc.
Genetic modification of biocontrol agents
Some mycoviruses are known to induce hypovirulence in phytopathogenic fungi. Hypovirulence is the reduction in the ability of pathogens to cause disease. Transgenic hypovirulent strains of Cryphonectria parasitica generated by integrating hypovirus cDNA into nuclear DNA result in hypovirulence-mediated biocontrol of pathogenic C. parasitica as viral cDNA is transferred to fungal progeny (Nuss 2005).
The efficiency of BCAs producing bacteriocins can be improved by genetic modification. Knowledge of the genetic organization and biosynthetic pathways of various bacteriocins has enabled the analysis and modification of bacteriocins and their production hosts to improve the efficacy of bacteriocins as antimicrobial agents. Bacteriocins with unique properties can be produced by mutation or fusion of genes from different bacterial species encoding bacteriocins. Genetic modification has several advantages over using bacteriocins and bacteriocin producers in their natural form, such as broadening the damage spectrum of bacteriocins using gene fusion techniques, as they often have a narrow killing range and may not be effective against all strains of a targeted pathogen (Gillor et al. 2005).
Conclusion
Microorganisms such as bacteria, fungi and viruses can be effectively used as a biocontrol agent to protect plants from other pathogenic microorganisms. Microbial biocontrol agents provide biocontrol by several mechanisms including direct antagonism, production of secondary metabolites, competition, as well as induction of resistance in the host plant. Though researches have been going on extensively in this field all around the globe, more research is required on the combined usage of microorganisms among themselves and chemical compounds for better and longer effects. New technologies such as CRISPR have been widely accepted as an efficient method for the induction of resistance in host plants as well as production of antimicrobial compounds by the host plants using genetic modification for plant disease control. Genetic modification of BCAs is also a futuristic approach for better biocontrol. These technologies can be more researched upon and can be further utilized for creating mutant plant species and genetically modified microbial BCAs with a wide range of target pathogens.
There are several practical aspects yet to be covered to replace chemical pesticides with microbial biocontrol agents, costs and availability being one of the major problems in this regard. Educating farmers to use microorganisms for biocontrol instead of chemical pesticides is another aspect to be considered.
References
Abeyratne GD, Deshappriya N (2018) The effect of pH on the biological control activities of a Trichoderma sp. against Fusarium sp. isolated from the commercial onion fields in Sri Lanka. Trop Plant Res 5(2):121–128
Abid M, Khan M, Mushtaq S, Afzaal S, Haider MS (2018) A comprehensive review on mycoviruses as biological control agent. World J Biol Biotechnol 3(2):187–192
Ahmad T, Bashir A, Farooq S, Riyaz S (2022) Burkholderia gladioli E39CS3, an endophyte of Crocus sativus Linn., induces host resistance against corm-rot caused by Fusarium oxysporum. J Appl Microbiol 132(1):495–508
Anand A, Chinchilla D, Tan C, Mène-Saffrané L, L’Haridon F, Weisskopf L (2020) Contribution of hydrogen cyanide to the antagonistic activity of Pseudomonas strains against Phytophthora infestans. Microorganisms 8(8):1144
Asghari S, Harighi B, Ashengroph M, Clement C, Aziz A, Esmaeel Q, Barka EA (2020) Induction of systemic resistance to Agrobacterium tumefaciens by endophytic bacteria in grapevine. Plant Pathol 69(5):827–837
Azizoglu U, Jouzani GS, Yilmaz N, Baz E, Ozkok D (2020) Genetically modified entomopathogenic bacteria, recent developments, benefits and impacts: a review. Sci Total Environ 734:139169
Baćmaga M, Wyszkowska J, Jan K (2018) The influence of chlorothalonil on the activity of soil microorganisms and enzymes. Ecotoxicology 27(9):1188–1202
Bansal S, Balamurugan A, Achary V, Kumar A, Reddy MK, Campos JO (2022) Editing plant genome with CRISPR/Cas: a sustainable strategy for disease management. Next-generation plant breeding approaches for stress resilience in cereal crops. Springer, Singapore, pp 369–396
Barra-Bucarei L, France Iglesias A, Gerding González M, Silva Aguayo G, Carrasco-Fernández J, Castro JF, Ortiz Campos J (2019) Antifungal activity of Beauveria bassiana endophyte against Botrytis cinerea in two solanaceae crops. Microorganisms 8(1):65
Barua P, Nath PD (2018) Bacteriophages: a potential next generation biocontrol tool for plant disease management. Int J Curr Microbiol App Sci 7(9):1103–1112
Benhamou N, Gagné S, Le Quéré D, Dehbi L (2000) Bacterial-mediated induced resistance in cucumber: beneficial effect of the endophytic bacterium Serratia plymuthica on the protection against infection by Pythium ultimum. Phytopathology 90(1):45–56
Brescia F, Vlassi A, Bejarano A, Seidl B, Marchetti-Deschmann M, Schuhmacher R, Puopolo G (2021) Characterisation of the antibiotic profile of Lysobacter capsici AZ78, an effective biological control agent of plant pathogenic microorganisms. Microorganisms 9(6):1320
Carstens AB, Djurhuus AM, Kot W, Hansen L (2019) A novel six-phage cocktail reduces Pectobacterium atrosepticum soft rot infection in potato tubers under simulated storage conditions. FEMS Microbiol Lett 366(9):fnz101
Chen K, Tian Z, Luo Y, Cheng Y, Cho L (2018a) Antagonistic activity and the mechanism of Bacillus amyloliquefaciens DH-4 against citrus green mold. Phytopathology 108(11):1253–1262
Chen PH, Chen RY, Chou JY (2018b) Screening and evaluation of yeast antagonists for biological control of Botrytis cinerea on strawberry fruits. Mycobiology 46(1):33–46
Chen Y, Wang J, Yang N, Wen Z, Sun X, Chai Y, Ma Z (2018c) Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat Commun 9(1):1–4
Chen C, Cao Z, Li J, Tao C, Feng Y, Yuan H (2020a) A novel endophytic strain of Lactobacillus plantarum CM-3 with antagonistic activity against Botrytis cinerea on strawberry fruit. Biol Control 148:104306
Chen DM, Yang HJ, Huang JG, Yuan H (2020b) Lysobacter enzymogenes LE16 autolysates have potential as biocontrol agents—Lysobacter sp. autolysates as biofungicide. J Appl Microbiol 129(6):1684–1692
Chenniappan C, Narayanasamy M, Daniel G, Ramaraj G, Ponnusamy P, Sekar J, Ramalingam PV (2019) Biocontrol efficiency of native plant growth promoting rhizobacteria against rhizome rot disease of turmeric. Biol Control 129:55–64
Choub V, Won SJ, Ajuna HB, Moon JH, Choi SI, Lim HI, Ahn S (2022) Antifungal activity of volatile organic compounds from Bacillus velezensis CE 100 against Colletotrichum gloeosporioides. Horticulturae 8(6):557
Cock MJ, van Lenteren JC, Brodeur J, Barratt BI, Bigler F, Bolckmans K, Cônsoli FL, Haas F, Mason PG, Parra J (2010) Do new access and benefit sharing procedures under the convention on biological diversity threaten the future of biological control? Biocontrol 55(2):199–218
Compant S, Duffy B, Nowak J, Clément C, Baraka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9):4951–4959
Daryaei A, Jones EE, Glare TR, Richard EF (2016) pH and water activity in culture media affect biological control activity of Trichoderma atroviride against Rhizoctonia solani. Biol Control 92:24–30
Das K, Prasanna R, Anil A (2017) Rhizobia: a potential biocontrol agent for soilborne fungal pathogens. Folia Microbiol 62(5):425–435
David BV, Chandrasehar G, Selvam P (2018) Pseudomonas fluorescens: a plant-growth-promoting rhizobacterium (PGPR) with potential role in biocontrol of pests of crops. Crop Improvement through microbial biotechnology chapter 10. New and future developments in microbial biotechnology and bioengineering. Springer, Berlin, pp 221–243
De Silva NI, Brooks S, Lumyong S, Hyde KD (2019) Use of endophytes as biocontrol agents. Fungal Biol Rev 33(2):133–148
Di Francesco A, Baraldi E (2021) How siderophore production can influence the biocontrol activity of Aureobasidium pullulans against Monilinia laxa on peaches. Biol Control 152:104456
Dubey SC, Tripathi A, Tak R, Devi I (2020) Evaluation of bio-formulations of fungal and bacterial biological control agents in combination with fungicide in different mode of application for integrated management of tomato wilt. Indian Phytopathol 73(3):425–432
Durairaj K, Velmurugan P, Park JH, Chang WS, Park YJ, Senthilkumar P, Choi KM, Lee JH, Oh B (2017) Potential for plant biocontrol activity of isolated Pseudomonas aeruginosa and Bacillus stratosphericus strains against bacterial pathogens acting through both induced plant resistance and direct antagonism. FEMS Microbiol Lett 364(23):fnx225
Elad Y, Freeman S (2002) Biological control of fungal plant pathogens. Agricultural applications. Springer, Berlin, pp 93–109
Evangelista-Martínez Z (2014) Isolation and characterization of soil Streptomyces species as potential biological control agents against fungal plant pathogens. World J Microbiol Biotechnol 30(5):1639–1647
Fira D, Dimkić I, Berić T, Lozo J, Slavisa S (2018) Biological control of plant pathogens by Bacillus species. J Biotechnol 285:44–55
Fontana DC, de Paula S, Torres AG, de Souza VH, Pascholati SF, Schmidt D, Durval DN (2021) Endophytic fungi: Biological control and induced resistance to phytopathogens and abiotic stresses. Pathogens 10(5):570
Fournier B, Dos Santos SP, Gustavsen JA, Imfeld G, Lamy F, Mitchell EA, Mota M, Noll D, Planchamp C, Heger TJ (2020) Impact of a synthetic fungicide (fosetyl-Al and propamocarb-hydrochloride) and a biopesticide (Clonostachys rosea) on soil bacterial, fungal, and protist communities. Sci Total Environ 738:139635
Gawai D (2018) Role of fungi as biocontrol agents for the control of plant diseases in sustainable agriculture. Fungi and their role in sustainable development: current perspectives. Springer, Berlin, pp 283–291
Ghorbanpour M, Omidvari M, Abbaszadeh-Dahaji P, Omidvar R, Khalil K (2018) Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol Control 117:147–157
Glick BR, Bashan Y (1997) Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol Adv 15(2):353–378
Gillor O, Nigro LM, Margaret R (2005) Genetically engineered bacteriocins and their potential as the next generation of antimicrobials. Curr Pharm Des 11(8):1067–1075
Gu Q, Yang Y, Yuan Q, Shi G, Wu L, Lou Z, Huo R, Wu H, Borriss R, Gao X (2017) Bacillomycin D produced by Bacillus amyloliquefaciens is involved in the antagonistic interaction with the plant-pathogenic fungus Fusarium graminearum. Appl Environ Microbiol 83(19):e01075-e1117
Hirpara DG, Gajera HP (2018) Molecular heterozygosity and genetic exploitations of Trichoderma inter-fusants enhancing tolerance to fungicides and mycoparasitism against Sclerotium rolfsii Sacc. Infect Genet Evol 66:26–36
Hu D, Chen ZY, Zhang C, Mala G (2020) Reduction of Phakopsora pachyrhizi infection on soybean through host-and spray-induced gene silencing. Mol Plant Pathol 21(6):794–807
Jensen FD, Lumsden RD (1999) Biological control of soilborne pathogens. Integrated pest and disease management in greenhouse crops. Springer, Berlin, pp 319–337
Jha Y (2022) Enhanced cell viability with induction of pathogenesis related proteins against Aspergillus niger in maize by endo-rhizospheric bacteria. Jordan J Biol Sci 15(1):139–147
Jing X, Cui Q, Li X, Yin J, Ravichandran V, Pan D, Fu J, Tu Q, Wang H, Bian X, Zhang Y (2020) Engineering Pseudomonas protegens Pf-5 to improve its antifungal activity and nitrogen fixation. Microb Biotechnol 13(1):118–133
Jurat-Fuentes JL, Heckel DG, Ferre J (2021) Mechanisms of resistance to insecticidal proteins from Bacillus thuringiensis. Annu Rev Entomol 66:121–140
Kalia A, Gosal SK (2011) Effect of pesticide application on soil microorganisms. Arch Agron Soil Sci 57(6):569–596
Kanouni L, Larous L, Samia M-A (2018) Inhibitory effect of rhizobia isolated from several leguminous against phytopathogenic fungi. Annual Research & Review in Biology. https://doi.org/10.9734/ARRB/2018/38161
Karmakar A, Taufiqa S, Baig MJ, Molla KA (2022) Increasing disease resistance in host plants through genome editing. Proc Indian Natl Sci Acad. https://doi.org/10.1007/s43538-022-00100-6
Kenawy A, Dailin DJ, Abo-Zaid GA, Malek RA, Ambehabati KK, Zakaria KH, Sayyed RZ, Enshasy H (2019) Biosynthesis of antibiotics by PGPR and their roles in biocontrol of plant diseases. Plant growth promoting rhizobacteria for sustainable stress management. Springer, Singapore, pp 1–35
Khabbaz SE, Ladhalakshmi D, Babu M, Kandan A, Ramamoorthy V, Saravanakumar D, Al-Mughrabi T, Kandasamy S (2019) Plant growth promoting bacteria (PGPB)—a versatile tool for plant health management. Canad J Pestic Pest Manag 1(1):1–25
Khan RA, Najeeb S, Mao Z, Ling J, Yang Y, Li Y, Xie B (2020) Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and Root-knot nematode. Microorganisms 8(3):401
Köhl J, Kolnaar R, Willem R (2019) Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front Plant Sci 10:845
Kredics L, Manczinger L, Antal Z, Pénzes Z, Szekeres A, Kevei F, Nagy E (2004) In vitro water activity and pH dependence of mycelial growth and extracellular enzyme activities of Trichoderma strains with biocontrol potential. J Appl Microbiol 96(3):491–498
Kumar M, Ashraf S (2017) Role of Trichoderma spp. as a biocontrol agent of fungal plant pathogens. Probiotics and plant health. Springer, Berlin, pp 497–506
Kuzyakov Y, Razavi BS (2019) Rhizosphere size and shape: temporal dynamics and spatial stationarity. Soil Biol Biochem 135:343–360
Liu Q, Yang F, Zhang J, Liu H, Rahman S, Islam S, Ma W, She M (2021) Application of CRISPR/Cas9 in crop quality improvement. Int J Mol Sci 22(8):4206
Malik Z, Ahmad M, Abassi GH, Dawood M, Hussain A, Jamil M (2017) Agrochemicals and soil microbes: interaction for soil health. Xenobiotics in the soil environment. Springer, Berlin, pp 139–152
Meena RS, Kumar S, Datta R, Lal R, Vijayakumar V, Brtnicky M, Sharma MP, Yadav GS, Jhariya MK, Jangir CK, Pathan SI (2020) Impact of agrochemicals on soil microbiota and management: a review. Land 9(2):34
Mukhopadhyay R, Kumar D (2020) Trichoderma: a beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egypt J Biol Pest Control 30(1):1–8
Nayak P, Solanki H (2021) Pesticides and Indian agriculture—a review. Int J Res Granthaalayah 10(5):250–263
Niu B, Wang W, Yuan Z, Sederoff RR, Sederoff H, Chiang VL, Borriss R (2020) Microbial interactions within multiple-strain biological control agents impact soil-borne plant disease. Front Microbiol 11:2452
Nuss D (2005) Hypovirulence: mycoviruses at the fungal–plant interface. Nat Rev Microbiol 3(8):632–642
Ons L, Bylemans D, Thevissen K, Bruno C (2020) Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 8(12):1930
Pandey P, Dubey AP, Mishra S, Singh VS, Singh C, Tripathy A (2022) β-lactam resistance in Azospirillum baldaniorum Sp245 is mediated by lytic transglycosylase and β-lactamase and regulated by a cascade of RpoE7→ RpoH3 sigma factors. J Bacteriol 204(4):e00010-22
Patibanda AK, Ranganathswamy M (2018) Effect of agrichemicals on biocontrol agents of plant disease control. Microorganisms for green revolution. Springer, Berlin, pp 1–21
Prajapati S, Kumar N, Kumar S, Lakharan L (2020) Biological control a sustainable approach for plant diseases management: a review. J Pharmacogn Phytochem 9(2):1514–1523
Raaijmakers JM, Vlami M, Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 81(1):537–547
Rahimi Tamandegani P, Marik T, Zafari D, Balázs D, Vágvölgyi C, Szekeres A, Kredics L (2020) Changes in peptaibol production of Trichoderma species during in vitro antagonistic interactions with fungal plant pathogens. Biomolecules 10(5):730
Ram RM, Keswani C, Bisen K, Tripathi R, Singh SP, Singh HB (2018) Biocontrol technology: eco-friendly approaches for sustainable agriculture. Omics technologies and Bio-engineering. Academic Press, London, pp 177–190
Reddy PP (2014) Mechanisms of biocontrol. Plant growth promoting rhizobacteria for horticultural crop protection. Springer, New Delhi, pp 55–68
Rooney WM, Grinter RW, Correia A, Parkhill J, Walker DC, M. J. (2020) Engineering bacteriocin-mediated resistance against the plant pathogen Pseudomonas syringae. Plant Biotechnol J 18(5):1296–1306
Saechow S, Thammasittirong A, Kittakoop P, Prachya S, Ranong T (2018) Antagonistic activity against dirty panicle rice fungal pathogens and plant growth-promoting activity of Bacillus amyloliquefaciens BAS23. J Microbiol Biotechnol 28(9):1527–1535
Sallam N, Ali EF, Seleim MA, Khalil BH (2021) Endophytic fungi associated with soybean plants and their antagonistic activity against Rhizoctonia solani. Egypt J Biol Pest Control 31(1):1–9
Sarrocco S, Esteban P, Vicente I, Bernardi R, Plainchamp T, Domenichini S, Puntoni G, Baroncelli R, Vannacci G, D. M. (2020) Straw competition and wheat root endophytism of Trichoderma gamsii T6085 as useful traits in the biocontrol of Fusarium head blight. Phytopathology 111:1129–1136
Savita A, Sharma A (2019) Fungi as biological control agents. Biofertilizers for sustainable agriculture and environment. Springer, Cham, pp 395–411
Sharma M, Guleria S, Singh K, Chauhan A, Saurabh K (2018) Mycovirus associated hypovirulence, a potential method for biological control of Fusarium species. Virus Dis 29:134–140
Sharma A, Abrahamian P, Carvalho R, Choudhary M, Paret ML, Vallad GE, Jones J (2022) Future of bacterial disease management in crop production. Annu Rev Phytopathol 60:259–282
Siegwart M, Graillot B, Blachere Lopez C, Besse S, Bardin M, Nicot PC, Lopez-Ferber M (2015) Resistance to bio-insecticides or how to enhance their sustainability: a review. Front Plant Sci 6:381
Singh HB (2014) Management of plant pathogens with microorganisms. Proc Indian Natl Sci Acad 80(2):443–454
Singh M, Singh D, Gupta A, Pandey KD, Singh PK, Kumar A (2019) Plant growth promoting rhizobacteria: application in biofertilizers and biocontrol of phytopathogens. PGPR amelioration in sustainable agriculture. Woodhead Publishing, Sawston, pp 41–66
Singh S, Kumar V, Dhanjal DS, Singh J (2020) Biological control agents: diversity, ecological significances, and biotechnological applications. Natural bioactive products in sustainable agriculture. Springer, Berlin, pp 31–44
Singh M, Singh R, Mishra P, Sengar RS, Shahi UP (2021a) In-vitro compatibility of Trichoderma harzianum with systemic fungicides. Int J Chem Stud 9(1):2884–2888
Singh P, Singh RK, Guo D-J, Sharma A, Singh RN, Li D-P, Malviya MK, Song X-P, Lakshmanan P, Yang L-T (2021b) Whole genome analysis of sugarcane root-associated endophyte Pseudomonas aeruginosa B18—a plant growth-promoting bacterium with antagonistic potential against Sporisorium scitamineum. Front Microbiol 12:628376
Stauber L, Croll D, Simone P (2022) Temporal changes in pathogen diversity in a perennial plant–pathogen–hyperparasite system. Mol Ecol 31(7):2073–2088
Suyal DC, Soni R, Sai S, goel R (2016) Microbial inoculants as biofertilizer. Microbial inoculants in sustainable agricultural productivity. Springer, Berlin, pp 311–318
Tariq M, Khan A, Asif M, Khan F, Ansari T, Shariq M, Siddiqui MA (2020) Biological control: a sustainable and practical approach for plant disease management. Acta Agric Scand Sect B 70(6):507–524
Tchameni SN, Cotârleț M, Ghinea IO, Bedine MA, Sameza ML, Borda D, Bahrim G, Dinică R (2020) Involvement of lytic enzymes and secondary metabolites produced by Trichoderma spp. in the biological control of Pythium myriotylum. Int Microbiol 23(2):179–188
van Lenteren JC, Bolckmans K, Köhl J, Ravensberg WJ, Urbaneja A (2018) Biological control using invertebrates and microorganisms: plenty of new opportunities. Biocontrol 63(1):39–59
Verma AK, Chettri D, Verma AK (2022) Potential of CRISPR/Cas9-based genome editing in the fields of industrial biotechnology: strategies, challenges, and applications. Industrial microbiology and biotechnology. Springer, Berlin, pp 667–690
Viterbo A, Ramot O, Chernin L, Chet I (2002) Significance of lytic enzymes from Trichoderma spp. in the biocontrol of fungal plant pathogens. Antonie Van Leeuwenhoek 81(1):549–556
Voronina MV, Bugaeva EN, Vasiliev DM, Kabanova AP, Barannik AP, Shneider MM, Kulikov EE, Korzhenkov AA, Toschakov SV, Ignatov AN, Miroshnikov KA (2019) Characterization of Pectobacterium carotovorum subsp. carotovorum bacteriophage PP16 prospective for biocontrol of potato soft rot. Microbiology 88(4):451–460
Wang X, Ding T, Li Y, D. T. (2019) Effects of an arbuscular mycorrhizal fungus and a rhizobium species on Medicago sativa wilt and Fusarium oxysporum root rot. Acta Pratacul Sin 28(8):139–149
Wang M, Geng L, Sun X, Shu C, Song F, Zhang J (2020) Screening of Bacillus thuringiensis strains to identify new potential biocontrol agents against Sclerotinia sclerotiorum and Plutella xylostella in Brassica campestris L. Biol Control 145:104262
Wilson A, Cuddy WS, Park RF, Harm GF, Priest MJ, Bailey J, Moffitt M (2020) Investigating hyperparasites as potential biological control agents of rust pathogens on cereal crops. Australas Plant Pathol 49(3):231–238
Yang C, Hamel C, Vujanovic V, gan Y (2011) Fungicide: modes of action and possible impact on nontarget microorganisms. Int Sch Res Not. https://doi.org/10.5402/2011/130289
Yang Y, Zhang SW, Li KT (2019) Antagonistic activity and mechanism of an isolated Streptomyces corchorusii stain AUH-1 against phytopathogenic fungi. World J Microbiol Biotechnol 35(9):1–9
Yassin MT, Mostafa AA, Al-Askar A (2022) In vitro antagonistic activity of Trichoderma spp. against fungal pathogens causing black point disease of wheat. J Taibah Univ Sci 16(1):57–65
Yin X, Li T, Jiang X, Tang X, Zhang J, Yuan L, Wei Y (2022) Suppression of grape white rot caused by Coniella vitis using the potential biocontrol agent Bacillus Velezensis GSBZ09. Pathogens 11(2):248
Zhang L, Sun C (2018) Fengycins, cyclic lipopeptides from marine Bacillus subtilis strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl Environ Microbiol 84(18):e00445-e518
Zhang C, Wang W, Xue M, Liu Z, Zhang Q, Hou J, Xing M, Wang R, Lang T (2021) The Combination of a biocontrol agent Trichoderma asperellum SC012 and hymexazol reduces the effective fungicide dose to control fusarium wilt in cowpea. J Fungi 7(9):685
Zhang H, He M, Fan X, Dai L, Zhang S, Hu Z, Wang N (2022a) Isolation, identification and hyperparasitism of a novel Cladosporium cladosporioides isolate hyperparasitic to Puccinia striiformis f. sp. tritici, the wheat stripe rust pathogen. Biology 11(6):892
Zhang J, Zhu W, Goodwin PH, Lin Q, Xia M, Xu W, Sun R, Liang J, Wu C, Li H (2022b) Response of Fusarium pseudograminearum to biocontrol agent Bacillus velezensis YB-185 by phenotypic and transcriptome analysis. Journal of Fungi 8(8):763
Zin NA, Badaluddin N (2020) Biological functions of Trichoderma spp. for agriculture applications. Ann Agric Sci 65(2):168–178
Acknowledgements
The authors would like to thank the Department of Microbiology, Sikkim University, for providing the computational infrastructure and central library facilities for procuring references and plagiarism analysis (Ouriginal: Plagiarism Detection Software).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the framework design of the manuscript. The manuscript was written by MB and SS, and the manuscript was reviewed by DC and AKV. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boro, M., Sannyasi, S., Chettri, D. et al. Microorganisms in biological control strategies to manage microbial plant pathogens: a review. Arch Microbiol 204, 666 (2022). https://doi.org/10.1007/s00203-022-03279-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03279-w