Abstract
Globally, phytopathogenic fungi infections cause crop diseases, resulting in crop yield and quality loss. Extensive use of chemical fungicides leads to resistance and high costs for growers as well as environmental pollution; thus, researchers are exploring a more sustainable approach using biological control tactics. This review highlights the critical processes involved in biological control by bacteria, fungi, viruses, and archaea, i.e., the synthesis of various metabolites, enzymes, and signaling molecules, as well as competitive tactics or soil suppressiveness that can effectively control the phytopathogenic fungi. The global increase in registrations for biological products reflects the rising demand and requirement for more organic agriculture and achieving some sustainable development targets. Understanding the complicated interplay between microorganisms in this environment can aid in managing soil diversity and inhibiting phytopathogenic fungi without chemical residues. Therefore, microorganisms are recommended as a sustainable alternative biological control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant diseases yearly cost the world economy more than 220 billion USD, according to The Food and Agriculture Organization of the United Nations (FAO) reports; moreover, phytopathogenic fungi cause an effective loss in crops worldwide (Jantasorn et al. 2016). They are responsible for approximately 30% of all agricultural plant diseases, including rice, wheat, maize, barley, soybean, cotton, bananas, coffee, etc. (Figueroa et al. 2021). The significant economic effects of fungal plant diseases result from declining agricultural output and quality (Bonaterra et al. 2022; Bhat et al. (2023). Their exportability is at stake, and the expense of disease surveillance and possible fungicide spraying can increase (Palm 2001; Fisher et al. 2012; Brauer et al. 2019; Jain et al. 2019).
Agriculture has increased its use of pesticides, e.g., herbicides, insecticides, and fungicides, to increase production (Grützmacher et al. 2008), but the extensive usage of these pesticides has polluted the ecosystems. Nowadays, the focus has shifted to microorganisms with the potential to control pathogenic fungi. Therefore, microbial agents may be viewed as an alternate technique for disease control because it is ecologically benign and simultaneously reduces the potentially harmful effects of chemical fungicides (Moreira et al. 2002; Khunnamwong et al. 2020). Along with the lifecycles of plants and diseases, one may see dynamic interactions with other creatures. These interactions are known to impact plant health in various ways. It is feasible to manage fungal plant diseases through the various actions of biocontrol-active microorganisms. Biological management of plant diseases is still moving at a snail’s pace, mostly because of its inconsistent efficacy under various climatic circumstances (Heydari and Pessarakli 2010).
Numerous microbes generate and release one or more antimicrobial compounds. In low quantities, these substances are microbial poisons that can harm or kill bacteria and others (El-Tarabily et al. 1996; El-Saadony et al. 2022; Gómez-Godínez et al. 2023). Numerous biocontrol-active bacteria create compounds that can inhibit the development and activity of pathogens. Lytic enzymes are metabolites that degrade polymeric materials, such as DNA, chitin, proteins, cellulose, and hemicellulose (El-Tarabily et al. 1997; Anderson et al. 2004). This characteristic enables these microbes to have a range of biocontrol uses (Badalyan 2001). It is feasible to mention hyperparasitism, predation, antibiosis, cross-protection, competition concepts for location and/or resources, and induced resistance among the several recognized microbial biocontrol effects. According to Heyedari and Pessarakli (2010), the presence and actions of other microorganisms that a pathogen encounters are always detrimental. Mejía et al. (2008) stated that biological controller microorganisms must be effective colonizers. They have a combined growth rate and antibiosis action, indicating the significance of integrating field data with in vitro experiments to get good results when selecting biological pesticides (Elnahal et al. 2022; Bonaterra et al. 2022).
Moreover, in many instances, a single microbe cannot suppress the appearance of the disease; a community of microorganisms can play a crucial role in regulating soilborne diseases. In search of a more sustainable option in agriculture, this method has exploded in popularity worldwide. In light of this, it is necessary to study some under-developed parts of biocontrol to build more effective biological control tactics in the future (De Vrieze et al. 2020; Elnahal et al. 2022). The biocontrol of plant diseases has a bright and hopeful future. With the increasing demand for biocontrol products among farmers, it is conceivable to employ biological control as an effective way to manage plant diseases, boost crop yields, and safeguard the environment and biological resources as we move toward a more sustainable agricultural system (Heydari and Pessarakli 2010; Daranas et al. 2019; Legein et al. 2020; Bonaterra et al. 2022).
Endophytic microorganisms as a source of potential antifungal compounds
Endophytic bacteria are plant-beneficial bacteria that exist within plants and can enhance plant development under normal and demanding environmental circumstances. The capacity of certain microorganisms, mostly bacteria and fungi, to create secondary metabolites relevant to the food, pharmaceutical, and agricultural sectors has been studied (Gao et al. 2017). Diverse sources exist for isolating and characterizing microorganisms of biotechnological relevance; however, endophytic microbes provide a new reservoir of novel metabolites (Rana et al. 2020). Endophytes are a category of microorganisms that may infiltrate plant tissues without having detrimental effects (Duong et al. 2021). According to Ali et al. (2020), endophytes are rhizosphere microorganisms that connect effectively with their host plants. Endophytic microbes can be used to isolate and characterize natural compounds.
Bacteria and fungi isolated from plants have the potential to create antibiotics, antifungals, anthelmintics, and anticancer compounds (Daranas et al. 2019; Montes-Osuna et al. 2021). In addition, they can increase the defenses of plants and exhibit antagonistic features, such as the creation of antibiotics, siderophores, HCN, and several enzymes (Duong et al. 2021; Sabra et al. 2022; Saad et al. 2022; Ashry et al. 2022). Multiple plants were chosen as sources for isolating bacteria with antibiotic properties. Most research focuses on the production of antimicrobials with therapeutic significance or the separation of endophytes from plants with medicinal qualities (Musa et al. 2020). In recent years, however, there has been a growing interest in discovering and characterizing microorganisms with valuable antibacterial capabilities for agriculture. The utilization of endophytes as biocontrol agents is advantageous since the evolutionary process has already chosen microorganisms that compete ecologically for the same niche, such as fungi and phytopathogenic bacteria (Rojas-Solís et al. 2018; Bungtongdee et al. 2019).
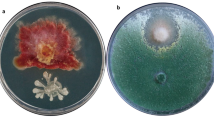
Certain secondary metabolites generated by bacterial and fungal strains, such as dimethyl disulfide (DMDS), which can promote systemic resistance. In a greenhouse experiment, Huang et al. (2012) revealed that Bacillus cereus C1L elicited a defensive immunity against Botrytis cinerea and Cochliobolus heterostrophus by developing systemic resistance in maize and tobacco plants. Some investigations try to discover bacterial strains capable of inhibiting the growth of phytopathogenic fungi. By isolating 282 bacteria from the leaves, stems, and roots of chilli pepper plants (Capsicum annum L.), four isolates can prevent the growth of phytopathogenic fungus Alternaria panax, B. cinerea, Colletrotichum acutum, Fusarium oxysporum, and Phytophthora capsici could be identified. Bacillus tequilensis (CNU082075), Burkholderia cepacia (CNU082111), and Pseudomonas aeruginosa were the designations given to these bacterial strains (CNU082137 and CNU082142). Another 18 strains inhibited at least one of the examined phytopathogenic fungi (Fig. 1), demonstrating the significance of chilli pepper isolates in isolating microorganisms of agricultural significance (Pei et al. 2019). Bacillus subtilis SC1.4, isolated from sugar cane leaves, inhibits the development of many phytopathogenic fungi, including Alternaria, Cochliobolus, Curvularia, Fusarium, Neodeightonia, Phomopsis, and Saccharicola. The secondary metabolites in chloroform and methanol extracts of bacterial strains confirmed their antifungal activity (Fig. 1).
Surfactin and other volatile organic compounds (VOCs) may inhibit fungal development and, as a result, exert antifungal action (El-Tarabily 2003; Hazarika et al. 2019). The use of endophytic microbiota as a biocontrol agent can still present as an additional characteristic in promoting plant growth, such as the bacterium Arthrobacter agilis UMCV2 that generates metabolite dimethyl hexadecylamine (DMHDA), which is characterized as an antagonist against phytopathogenic fungi B. cinerea and the oomycete Phytophthora cinnamomic. Selim et al. (2017) demonstrated the antifungal ability of three bacterial strains, namely P. aeroginosa H40 (isolated from the roots of Pisum sativum L.), Stenotrophomonas maltophilia H8 (isolated from the roots of Brassica oleracea L.), and B. subtilis H18 (isolated from the stem of Cicer arietinum L.) (Selim et al. 2016). The three examined strains inhibited the development of the disease in cotton cultivars caused by the fungus Rhizoctonia solani, based on the inoculum of the bacterial strains in the soil. Thus, it may be emphasized that the protective ability mediated by endophytes might be conferred by the presence of the microbe in the soil and not necessarily by entry into the plant tissues (Fig. 2). Additionally, crop yield increased, resulting in higher dry and fresh weight and shoot length (Legein et al. 2020; Bonaterra et al. 2022; El-Mageed et al. 2022; Elsayed et al. 2023).
Although several endophytic fungal strains are known for suppressing phytopathogenic fungi, most research focus on isolating fungi for their agricultural significance (Hassan et al. 2021; El-Mageed et al. 2022; de Andrade et al. 2023). Ginkgo biloba is a plant with therapeutic applications; nonetheless, it was used to isolate endophytic fungi, which were then evaluated for their capacity to prevent the growth of phytopathogenic fungi such as Fusarium graminearum, Sclerotia sclerotiorum, and Phytophthora capsici. Twelve of the 80 isolated endophytic fungal strains could inhibit at least one of the analyzed fungi, with Chaetomium globosum CDW7 being the most effective because producing 1,2-benzenedicarboxaldehyde-3,4,5-trihydroxy-6-methyl (flavin), which can protect the development of phytopathogenic fungi (in vivo, Fig. 1) and has healing properties for symptoms caused by pathogenic fungi (Xiao et al. 2013; El-Saadony et al. 2021; Alblooshi et al. 2022).
Lutfia et al. (2020) noted that medicinal plants are often distinguished by their capacity to suppress human infections. However, these microorganisms can also be employed for phytopathogenic fungus characterization. Seven endophytic fungi of Etlingera elatior were isolated, described, and evaluated for their capacity to inhibit F. oxysporum, Ganoderma boninense, and Rigidoporus lignus in their investigation (Elnahal et al. 2022; de Andrade et al. 2023). Given the potential of benefit from the endophytic plant interaction, using endophytic microorganisms in the control of phytopathogens is contingent on several factors, including host specificity, moving in plant tissues, inducing systemic resistance, adaptation to environmental conditions, and adaptation to the physiological state of the plant (Fig. 1). However, using these specific microorganisms can benefit economically and environmentally since bacteria and fungi can produce cheaper costs and less environmental harm than chemical fungicides. In addition to controlling phytopathogens, developing biotechnological techniques that manipulate endophytic microbes may promote plant growth (El-Saadony et al. 2022; Elnahal et al. 2022) (Fig. 2).
Bacteria as antifungal biocontrol agents
Many environmental bacteria inhabiting soil and rhizosphere are sources of antifungal secondary metabolites (Desoky et al. 2022; Ahmed et al. 2022; Abdelkhalik et al. 2023). These chemicals are useful in human and animal therapy and plant protection against phytopathogens (Hassan et al. 2021; de Andrade et al. 2023; Elsayed et al. 2023). The secondary metabolism is not engaged in survival but provides producers with adaptive benefits (Katz and Baltz 2016). Most bacteria that generate bioactive natural chemicals are actinomycetes, known as actinobacteria in recent years. Among them, soil-dwelling filamentous Streptomyces are most frequently associated with producing antibiotic chemicals (Saeed et al. 2017; Kamil et al. 2018; Al Hamad et al. 2021; Al Raish et al. 2021; Alwahshi et al. 2022). In addition to antibacterial chemicals, actinomycetes produce natural molecules with insecticide and anticancer characteristics (Table 1). Competition, protection, and signaling-mediated host interaction can account for the developing antimicrobial chemicals found mostly in soil bacteria (El-Tarabily and Sivasithamparam 2006; Hutchings et al. 2019).
Phytopathogenic fungi such as Fusarium, Colletotrichum, Ceratocystis, and Rhizoctonia, among others, can cause illnesses in different crops such as vascular wilt (Ma et al. 2013), anthracnose (Yan et al. 2018), black rot (Stahr and Quesada-Ocampo, 2020), and sheath blight (Rao et al. 2019), respectively. Biological control is an indirect plant growth-promoting strategy that occurs when an organism kills or halts the growth of pathogens through competition for space or resources, induction of plant systemic resistance, or antibiosis mediated by the creation of secondary metabolites (Olanrewaju et al. 2017). Beneficial microorganisms that colonize plants and promote plant development are plant growth–promoting bacteria (PGPB). The use of PGPB as biofertilizers in agriculture has increased in recent years, with Pseudomonas, Bacillus, Enterobacter, Klebsiella, Azobacter, Azospirillum, and Rhizobium, among others, being the predominant organisms (Vejan et al., 2016).
Bacterial secondary metabolites
Diffusible antifungal substances
Secondary metabolites with effective antifungal action include phenazine (phenazine-1-carboxylic acid), pyrrolnitrin, surfactin, iturin, fengcin, and hydrogen cyanide (Fig. 3). The plant growth-promoting rhizobacteria (PGPR), Pseudomonas spp. strain ST–TJ4, was able to effectively suppress 6 phytopathogenic fungi in agriculture and forestry, including Botryosphaeria berengeriana, Colletotrichum tropicale, Fusarium oxysporum, Fusarium graminearum, Phytophthora cinnamomic, and Rhizoctonia solani. The production of phenazines, pyrrolnitrins, and hydrogen cyanide was associated with aggressive action (Kong et al. 2020).
Pseudomonas spp., were also discovered to produce ecomycins, pseudomonic acid, pyoluteorin, oomycin A, cepaciamides, butyrolactones, aerugines, azomycins, rhamnolipids, cepafungins (Goswami et al. 2016). Bacillus spp., are significant producers of antifungal metabolites, including fengycin, iturin, and surfactin, which are bioactive against, among others, Monilinia fructicola, S. sclerotiorum, Phoma medicaginis, F. oxysporum, Penicillium expansum, and Aspergillus flavus (Penha et al. 2020). Bacillus velezensis HC6 has antagonistic activity against Aspergillus and Fusarium in maize by suppressing mycelial growth and lowering toxin generation via lipopeptides release (Liu et al. 2020).
Bacillus metabolites are often generated from the ribosome or non-ribosomal peptide synthetases (NRPS) and polyketide synthetases (PKS). Examples include bacilysin, bacillaene, chlorotetain, sublancin, subtilosin, and subtilin, in addition to fengycin, iturin, and surfactin (Olanrewaju et al. 2017) (Table 2). The species of this genus are promising for the biocontrol of fungi that have already been marketed to control various diseases in a vast array of crop cultures. Bacillus pumilus GB34 is a component of Bayer Crop Science’s (United States) Yield Shield product with antifungal action against R. solani and Fusarium spp., in soybean crops. The Bio-Yield (3Bar Biologics, USA) is another example of a Bacillus-based inoculant that targets Rhizoctonia, Pythium, and Fusarium in bedding plants (El-Tarabily 2006; El-Tarabily et al. 2009; Lopes et al. 2018).
Fungi resistance to the active fungicides used on crops is evolving daily, and the utilization of alternate natural resources, such as beneficial bacteria, is increasing (Lamichhane et al. 2016). B. cinerea is a fungus that infects most greenhouse crops, and its global resistance to conventional fungicides is growing. Three strains of Pseudomonas (P. protegens AP54, P. chlororaphis 14B11, and P. fluorescens 89F1) were able to reduce B. cinerea infection in petunia under greenhouse conditions, so providing a viable solution to the fungicide-resistance issue (South et al. 2020).
Volatile organic compounds
The VOCs generated by microorganisms may travel great distances, facilitating indirect interaction between organisms even at low concentrations, limiting the development of phytopathogenic fungi, and establishing systemic resistance in plants (Fig. 3; Table 2). VOCs have smaller molecules than non-volatile chemicals, allowing for easy dispersion in soil and air (Chen et al. 2020). Bacillus, Arthrobacter, Pseudomonas, Serratia, and Stenotrophomonas are examples of bacteria that generate VOCs that affect plant development (Vejan et al., 2016).
Many molecules, including acetaldehyde, butanoic acid, camphene, camphor, methanol, geosmin, propanoic acid, 5-hydroxy-methyl-furfural, and caryophyllene can be emitted as VOCs (Kanchiswamy et al. 2015). Several studies demonstrated that bacterial volatile organic compounds inhibit phytopathogenic fungi. B. velezensis strain ZSY-1 generates the volatile organic compounds 2,5-dimethylpyrazine, benzothiazole, 4-chloro-3-methyl-phenol, and 2,4- bis(1,1-dimethylethyl)-phenol, which manages tomato fungal infections by inhibiting Alternaria solani and B. cinerea (Gao et al. 2017) (Fig. 3; Table 2).
Pyrrolnitrin is a volatile organic compound generated by Burkholderia spp. with efficacy against significant. B. cinerea, R. solani, S. sclerotiorum, and Verticillium dahlia are examples of phytopathogens (Kanchiswamy et al. 2015). Pseudomonas spp. ST–TJ4 (1-undecene) can prevent the growth of phytopathogenic fungi, such as C. tropicale, F. oxysporum, P. cinnamomic, and R. solani, by emitting volatile organic compounds. VOCs inhibited more fungal growth than the diffusible chemicals in the culture media (Kong et al. 2020). Burkholderia cenocepacia ETR-B22 produces 32 distinct volatile organic compounds, including dimethyl trisulfide, indole, methyl anthranilate, methyl salicylate, and benzyl propionate, with potent antagonistic action against 12 fungal phytopathogens (Chen et al. 2020). Staphylococcus sciuri MarR44 decreased anthracnose disease at post-harvest on strawberries by 72.17%, caused by Colletotrichum nymphaeae detected VOCs, mesityl oxide (81.4%) being the most prevalent (Alijani et al. 2019).
Hydrolytic enzymes
Multiple PGPBs can generate protease, lipase, chitinase, and cellulase. Enzymes generated by bacteria can degrade phytopathogenic fungus cell walls, preventing their growth. Due to the presence of chitin and b-glucan in fungal cell walls, the bacterial enzymes chitinases and b-glucanases can impede fungal growth (Ali et al., 2020). Pseudomonas spp. and Sinorhizobium fredii are examples of hydrolytic enzyme makers that can inhibit Fusarium spp., and R. solani (Vejan et al., 2016) (Fig. 3; Table 2).
The mechanism of killing phytopathogenic fungi
Competition
Struggle for nutrients or space and competition for binding sites on roots hinder the phytopathogen’s ability to reach and colonize the plant. This rivalry is often tied to biocontrol processes, such as releasing secondary metabolites (Olanrewaju et al. 2017). Primarily in soil, VOCs have a role in competitive interactions between bacteria and fungi. A collective volatile-mediated antagonism of soil bacteria against invading fungi is an integral approach for bacteria to fight invading fungi for nutritional locations (Li et al. 2020) (Fig. 3).
Some bacteria create a siderophore, a tiny molecular weight molecule whose role is to bind the iron in the environment and transfer it to the cell interior, providing a competitive advantage to the organism (Hutchings et al. 2019). The siderophore pyoverdine produced by P. aeruginosa is essential for combating Aspergillus fumigatus (Sass et al. 2018).
Strains REC2, REC3, and PGPB of A. brasilense release catechol-type siderophores that are efficient against the phytopathogen Colletotrichum acutatum, hence protecting strawberry crops against anthracnose disease (Tortora et al. 2011). Studies conducted in vitro with the siderophore producer Acinetobacter calcoaceticus HIRFA32 from wheat rhizosphere indicated a significant reduction of F. oxysporum mycelium growth (Maindad et al. 2014).
Induced systemic resistance (ISR)
Several PGPBs can be protective agents by generating ISR, activating the plants’ resistance to certain diseases. Signaling chemicals, such as salicylic acid, released by PGPB, which may protect the plant from phytopathogens, coordinate the process (Olanrewaju et al. 2017). Rhizobacteria produced systemic resistance to F. oxysporum in chickpeas, safeguarding the plant (Kumari and Khanna 2020). By releasing the protein elicitor PeBL2, Brevibacillus laterosporus A60 can generate systemic resistance to B. cinerea in tobacco (Jatoi et al. 2019). Beneficial bacteria and rhizobacteria can protect plants from phytopathogenic fungi through several processes. Using microorganisms or their metabolites in agriculture is a more ecologically friendly alternative to chemical fungicides that must be progressively implemented to maintain sustained crop yield (Fig. 3; Table 2).
Fungi as biological control agents
Because they occupy a similar ecological niche, some fungi can regulate phytopathogenic fungi by competing for resources, creating antagonistic chemicals, and developing plant resistance (Schardl and Phillips 1997; Hallmann et al. 1998; Peixoto Neto et al. 2002). Thus, Santos et al. (2019) revealed that an endophytic fungus (Diaporthe) isolated from Sapindus saponaria L. leaves may inhibit phytopathogenic fungi in vitro. Several phytopathogens, including Fusarium solani, Glomerella spp., and Monilophthora perniciosa, had their growth reduced by touch, showing that competition for space was part of the inhibitory process (Table 1).
Mycoparasitism, in which fungi colonize the surface of the phytopathogen colony, is another method utilized by fungicides to inhibit the proliferation of phytopathogenic fungi. Wall-degrading enzymes are essential antagonists in this process (De Vrieze et al. 2020; Montes-Osuna et al. 2021; Díaz-Díaz et al. 2022). Since most phytopathogenic fungi have cell walls consisting of complex polymers, glucanases, chitinases, and proteases are primarily extra cellular enzymes responsible for the destruction of cell walls (Silva et al. 2019; Khunnamwong et al. 2020; Peters et al. 2020). Trichoderma species are recognized for their ability to generate lytic enzymes, which play crucial functions in the cell wall (da Silva Ribeiro et al. 2018), making this genus a significant biological control agent. Khatri et al. (2017) examined the effectiveness of 12 soil-isolated species of Trichoderma against fungal plant diseases, including A. niger, F. oxysporum, and Sclerotium rolfsii.
Trichoderma viride was shown to be the most effective antagonist against fungal plant diseases among the examined species, generating three important cell wall degrading enzymes (chitinase, protease, and glucanase) and playing a significant part in the adversary process. In a separate investigation, Díaz-Gutiérrez et al. (2021) evaluated the growth inhibition capacity of rhizospheric Trichoderma asperellum against F. oxysporum on rooted cuttings of Stevia rebaudiana L. T. asperellum prevented the development of F. oxysporum and five other pathogenic fungi, demonstrating the capacity to hydrolyze chitin and cellulose by releasing chitinase and cellulase. Antimicrobial secondary metabolites are an additional mode of action utilized by fungi against phytopathogenic organisms (Köhl et al. 2019). In this instance, endophytic fungi have attracted interest due to their mutualistic interaction with plants, which produces bioactive chemicals (de Almeida et al. 2018).
Using a crude hexane extract of the fungal mycelium from the endophytic fungus Diatrype palmicola, isolated from the medicinal plant Polyscias fruticosa, Tanapichatsakul et al. (2020) observed significant antifungal activity, with a 64.71% inhibition rate, against the common tomato infected by Athelia rolfsii. The bioactive chemical generated by D. palmicola has been discovered as 8-methoxynaphthalen-1-ol, suggesting that this substance, which inhibits the growth of A. rolfsii, may be a viable alternative for managing this phytopathogen (Table 2). The endophytic fungus can also form combinations of carbon-based molecules known as volatile organic compounds (VOCs) have shown promise as antifungal plant defenses (Kaddes et al. 2019).
Yeh et al. (2021) subjected volatile organic compounds from the endophytic fungus Nodulisporium spp., isolated from the medicinal plant Peperomia dindygulensis, to inhibitory assays. The isolated fungus was cultivated in various media to yield active compounds tested against Penicillium digitatum, the fungus responsible for citrus green mould. 2-ethyl-2-hexenal and 2,4-dimethyl-1,3-cyclopentanedione were the substances produced by the fungus cultivated in bagasse that reduced the prevalence of green mold, indicating that Nodulisporium spp. might be employed as a bioagent to control P. digitatum.
Like bacteria, fungi may reduce plant diseases via two kinds of induced resistance: systemic acquired resistance (SAR) and induced systemic resistance (ISR). They produce signaling chemicals such as jasmonic acid (JA), salicylic acid (SA), ethylene (ET), phytoalexins, protein-related chitinases, and glucanases (Hermosa et al. 2012; Nawrocka and Małolepsza 2013; Desoky et al. 2020; Olowe et al. 2020). El-Maraghy et al. (2020) investigated the capability of plant growth-promoting fungi (PGPF) strains to produce ISR against wilt disease caused by R. solani R43 in wheat (Triticum aestivum L.). According to their findings, the PGPF A. flavus, A. niger, Penicillium citrinum, Penicillium chrysogenum, and Trichoderma koningiopsis were identified from the rhizosphere of Triticum aestivum (ISR) infected with R. solani R43 and resistant to wilt disease.
This was accomplished by activating the pathogenesis-related gene (PR1, 2), the plant defensive chitinase (Chit-1), and − 1,3-glucanase (Glu-2) genes and increasing the R. solani-specific plant-specific defensive proteins. Consequently, biological management against phytopathogenic organisms employing fungus has been demonstrated to be a potential solution for managing some plant diseases. These microorganisms employ diverse modes of action against the pathogen, making them helpful in developing novel and more effective pesticides, improving cropping systems, reducing the use of chemically synthesized pesticides, and diminishing the latter’s environmental impact (Mckenna et al. 2001; De Vrieze et al. 2020; Montes-Osuna et al. 2021; Díaz-Díaz et al. 2022).
Virus as biological control agents
Hyper-parasitism by obligatory parasites of a plant pathogen would be regarded as the most direct mechanism since no other organism’s activities would be necessary to provide a suppressive impact (Harman et al. 2004). Four critical categories of hyperparasites, including hypoviruses, facultative parasites, obligate bacterial pathogens, and predators, have been identified. Mycoviruses are fungal viruses, and most have dsRNAs as their genome. There are at least 12 families of mycoviruses; some resemble plant and animal viruses, while others comprise their own families (Marzano and Domier 2016; Nerva et al. 2016). Partitiviridae, Totiviridae, Chrysoviridae, and Endornaviridae are the primary families into which double-stranded RNA (dsRNA) mycoviruses fall. However, it has been suggested that newly found mycoviruses should be placed into a new genus and family (Ghabrial et al. 2015). In general, dsRNA mycoviruses consistently infect the host fungus, are propagated vertically during host cell division, and cause asymptomatic infections (Osaki et al. 2006).
In the worst-case scenario, viruses can infect plant-associated fungi with an incidence rate ranging from a few percentage points to 90%. Even though most viral infections are asymptomatic, phenotypic changes can be damaging or advantageous to the host fungus. This review will cover harmful interactions between viruses and hosts, including hypovirulence and debilitation in various phytopathogenic fungi (Marzano and Domier 2016; Nerva et al. 2016). Hypovirulence is a prime example of a negative interaction in which several viruses diminish the virulence of fungi (Ghabrial et al. 2015). Additionally, it is frequently accompanied by additional phenotypic abnormalities, such as diminished pigmentation, sporulation, and growth problems (Hillman et al. 2018). Due to their parasitic nature, viruses cannot create secondary metabolites that can serve as antifungal substances. Therefore, if fungal viruses infect harmful plant fungi, they might serve as biocontrol agents (Yu et al. 2013).
Numerous studies have found fungal viruses that infect agriculturally significant phytopathogenic fungi; hence, a subset of these viruses might serve as biological control agents for fungal crop diseases (Xie and Jiang 2014; García-Pedrajas et al. 2019). However, hypovirulence-associated viruses have a variety of phenotypes that affect fungal properties and reproductive performance, such as reduced growth and fertility, together with modified pigment and metabolite synthesis, can render infected fungus fewer competitive than untreated fungi or less able to facilitate viral transmission (Nuss 2005).
In a small number of plant endophytic and pathogenic fungi, beneficial interactions are identified when heat tolerance and virulence are improved (Marzano and Domier 2016; Nerva et al. 2016). Despite viral infection, certain fungal plant diseases are biologically controlled by viruses with harmful RNA. For instance, in the chestnut blight (Cryphonectria parasitica), take-all disease (Gaeumannomyces graminis), Sclerotinia moulds (Sclerotinia spp.), and Dutch elm disease (Ophiostoma novoulmi) pathosystems, viruses are responsible for natural disease suppression under field conditions, and research is currently being conducted to utilize this hypovirulence phenomenon (Duffy and Weller 1996; Zhou and Boland 1998; Brasier 2001).
The viral infection of C. parasitica is a prominent example of hypo-parasitism when it causes hypovirulence, or a reduction in the pathogenicity of the pathogen, hence reducing chestnut blight in several locations (Milgroom and Cortesi 2004). Based on the infection system between plant disease fungi and viruses, there are possible in viro-control agents to determine if they infect plant pathogen fungi, such as the hypo viruses, to manage the chestnut blight in Europe (Rigling and Prospero 2018).
Notably, the most well-described mycoviruses responsible for affecting the phytobiome (as the American Phytopathological Society defines plant pathology as “plants and their environments”, and the related groups of organisms”) are the C. parasitica hypovirulence-inducing viruses. Several critical historical texts Anagnostakis (1982); Heiniger and Rigling (1994); Nuss (2005) describe chestnut blight and hypovirulence viruses. Chestnut blight fungus was first described in North America in 1906 by Merkel apud Schoelz and Stewart and in Europe in 1946 by Biraghi with devastating effects (Schoelz and Stewart 2018). Hypovirulent strains were first identified in Italy when Biraghi discovered chestnut trees (Castanea sativa) healing from cankers (Biraghi 1953). Subsequently, Day (1977) identified dsRNAs in hypo-virulent strains, and these dsRNAs were ultimately shown to be viral.
Anagnostakis (1982) outlined how more hypovirulent strains were subsequently discovered on American chestnut and how, over time, several viruses associated with C. parasitica hypovirulence of diverse viral lineages were discovered (Nuss 2005). The Cryphonectria hypovirulence viruses 1–4 (CHV1–4) are infectious clones of CHV1 (Chen and Nuss 1999). From 1982 to 2005, the biocontrol of chestnut disease in North America was less effective than anticipated, although it looked more influential in Europe (Heiniger and Rigling 1994). This may be because the biocontrol does not proliferate effectively in the less dense American chestnut trees and because the mating kinds (regulated by six vic loci) of chestnut disease are incompatible with the mating types of biological control in North America are more diverse and complex than in Europe. There may be susceptibility variations between the host trees (American and European chestnut) (Anagnostakis 1982).
In addition to Cryphonectria hypoviruses, other hypo viruses for other phytopathogenic fungi, such as Rhizoctonia, dollar spot, Dutch elm, Victoria blight on oats, and white root rot pathogens, have been identified. The majority of hypovirulence-associated viruses found so far belong to the families Totiviridae, Chrysoviridae, and Reoviridae or are unclassified viruses; viruses of phytopathogenic fungi also include Endornaviridae, Partitiviridae, and Hypoviridae viruses (Nuss 2005; Ghabrial and Suzuki 2009).
Xie and Jiang (2014) discussed the bottlenecks in using these viruses as biocontrol tools. They suggested they are comparable to those for Cryphonectria hypovirulence viruses (CHV), such as establishing knowledge about the related virus-causing hypovirulence and discovering effective, sustainable delivery strategies to resolve vegetative incongruence and other dispersion obstacles. Also, S. sclerotiorum is a crucial plant-pathogenic fungus for which the viruses with the highest hypovirulence were found (Xie and Jiang 2014). Notably, it was the first report of a Gemini-like SsDNA virus, SsHADV1, and a mono-negavirus, S. sclerotiorum negative-stranded RNA virus 1 (SsNSRV-1), linked with hypovirulence (Yu et al. 2010; Liu et al. 2014).
Sclerotinia sclerotiorum hypovirus 2 (SsHV2) (Marzano et al. 2015) and Sclerotinia sclerotiorum partitivirus 1 (SsPV1) (Xiao et al. 2014) cause hypovirulence in S. sclerotiorum. The latter is notable because most partiti-viruses, whether plants or fungi cause asymptomatic infections in their hosts. There are a large number of hypovirulence-causing viruses from a variety of viral families. Host fungi exhibiting hypo-virulent phenotypes are primarily limited to Ascomycota, and virtually little is known about viral or host mechanisms causing the phenotypic alterations generated by these viruses (Hillman et al. 2018).
The interactions between species in natural systems are many and intricate. One of the field’s most significant challenges is sorting out the numerous little connections constituting a complicated tapestry of interconnected interactions. Schoelz and Stewart (2018) stated that, similar to bacteria, viruses may be bio-prospected and utilized for bio-controlling projects or other valuable objectives. They thought it was conceivable owing to the hypovirulence factor’s potential but detecting these interactions is not yet simple.
The research on using viruses to control the effects of various biotic and abiotic stressors has just begun (Schoelz and Stewart 2018). Sustainable agriculture is a movement supported in most Latin American nations, intending to profit from the increased usage of registered viral pesticides, novel viral products undergoing registration, and other products in the applied research pipeline (Haas and Défago 2005). The baculovirus family (Baculoviridae) was regarded to have produced the most commercial viral biopesticides among natural insect viruses (Haase et al. 2015) (Fig. 4).
General factors affected the biocontrol agent mechanism. An effective biocontrol agent contains both biocontrol and adaptability aspects. Biocontrol factor interactions can be categorized as either direct or indirect. These are interactions between the biocontrol agent (blue) and the pathogen (red). Indirect connections are interactions between the biocontrol agent and the host plant that result in the host plant’s greater resistance to pathogen infection. Adaptation factors are variables necessary to adapt to the particular phyllosphere circumstances, such as elevated levels of UV stress, inadequate water availability, nutrients, and host immune system responses
In the 1930s, the very first well-documented introduction of a baculovirus to the environment led to the efficient eradication of a pest when an NPV specific for the spruce sawfly Diprion hercyniae was accidentally introduced along with a parasitoid imported from Europe to the United States and Canada to control the spruce sawfly (Bird and Elgee 1957). They are deemed safe for vertebrates; no incidences of baculovirus pathogenicity in vertebrates have been identified (Black et al. 1997).
In addition, its host-specificity is often restricted to a particular insect species. Several effective baculovirus-based pest management programs have been implemented in Latin American nations, as (Haase et al. 2015) described in detail (Fig. 4).
Baculovirus-based pesticides are a potential example of virus-based biocontrol agents; once they are compatible with integrated pest management systems, their use will substantially minimize the dangers associated with synthetic chemical insecticides. Given the high host-specificity of baculoviruses and their restricted use without causing any harm to vertebrates, it is believed that the naturally isolated fungal RNA virus represents a potential biocontrol tool for improved agricultural management, causing little harm to non-target creatures, such as beneficial fungi, vertebrates, and plants.
Archaea: a possible source of antimicrobial compounds
Archaea is a significant domain that could serve as an alternative method to increase agricultural production due to their unique characteristics, particularly their prominent role in nutrient (nitrogen, sulfur, and carbon) recycling in agricultural productions when the plant requires these nutrients in large quantities (Odelade and Babalola 2019). Chelius and Triplett (2001) have documented the presence of archaea in the rhizospheres of plant roots. In addition, archaea act as hosts for a unique class of potentially beneficial antibiotics. In general, bacteriocins are ribosomally generated, tiny (10 kDa), bactericidal, heat-stable, antibiotic-like compounds, amphiphilic peptides, or proteins produced by bacteria that may kill the same strain or closely related strains of bacteria (Cascales et al. 2007).
Moreover, bacteriocins show antifungal efficacy against three spoilage fungi (P. citrinum, A. niger, and A. flavus), as described (Adebayo and Aderiye 2010). The archaea generate their own separate family of bacteriocin-like antimicrobial peptides called archaeocins; they have been found and described in the Sulfolobus and Haloarchaea classes of archaea, but it is estimated that one hundred more archaeocins exist (Shand and Leyva 2008). The word “archaeocin” was employed to distinguish peptide- and protein-based antibiotics produced by archaea from those produced by bacteria (Kumar and Tiwari 2017). The first archaeocin found was halocin S8 from Haloarchaea, a short hydrophobic peptide of 36 amino acids. The cells create these chemicals when they enter the stationary phase. When bacteria deplete a local environment’s resources, the producer strain lyses the target cells by secreting archaeocins, lowering competition (Heng et al. 2007; El-Tarabily et al. 2009, 2010).
Because these chemicals differ structurally from antibiotics generated by bacteria, their mechanism of action may be unique. In the molecular biology of archaea, it is possible to produce new selectable markers for application (Shand and Leyva 2008). Archaeocins generated by Haloarchaea, a type of euryarchaea that are severe halophiles, are called “halocins,“ whereas the crenarchaeal genus Sulfolobus, which is an aerobic hyperthermophile, generates “sulfolobicins” (Quehenberger et al. 2017). Halocins (Hal) have a broad spectrum of action against Haloarchaea and Halobacteriaceae (Mazguene et al. 2018) (Table 2).
Rani et al. (2021) provided a comprehensive assessment of antimicrobial compounds generated by various microbes, including archaea; in their review, no authors presented any evidence, however scant, of archeocins as possible bioactive agents against the development of fungi. Rani et al. (2021) evaluated the reported halocins HalH1, HalH4, HalH6, HalS8, HalC8, HalR1, and sulfolobicins, which all exhibited anti-archaeal action. It is uncertain if there is no bioactivity against plant disease fungi because archeocin compounds do not inhibit their growth or if there is no evidence because no one has tested archeocins against this fungal group (Table 2). Archaea-produced bioactive antifungal chemicals (against plant pathogen species of fungus) against plant pathogen fungi have not been demonstrated to exist. Despite this, Roscetto et al. (2018) discovered antifungal activity from the first cryptic antimicrobial peptide generated by an archaeon against clinical isolates of Candida spp., worldwide, Candida species are the most common opportunistic fungal infections. Candida spp., as commensal fungi colonizes the skin, oral cavities, and gastrointestinal and genitourinary systems of most healthy individuals (Calderone and Fonzi 2001).
The antifungal activity of an antimicrobial peptide (VLL-28) generated from an archaeal transcription factor was demonstrated against 10 clinical isolates of Candida spp., Candida forms biofilm through cell adhesion on the host cell tissues or abiotic surfaces. VLL-28 was found to inhibit biofilm formation by interfering with cell viability at sub-MIC doses, hence drastically lowering biofilm viability for C. tropicalis, C. albicans, C. parapsilosis, and C. glabrata, except C. krusei isolates, for which no inhibition of cell adhesion was observed. Through the fluoresceinated derivative of this peptide, Roscetto et al. (2018) discovered that VLL-28 attaches to the surface of planktonic Candida spp., cells with no evidence of internalization. In this instance, it was determined that VLL-28 operates as a typical cationic antimicrobial peptide (CAMP) by causing damage to the cell membrane and cell wall, hence reducing the possibility of strain resistance.
The most significant contribution of this research is that VLL-28 is the first example of an archaeal antimicrobial peptide active against Candida spp., a member of the kingdom of fungi. Rossetti et al. (2018) thought that the antifungal function of VLL-28 indicates that archaeal microbes may be a source of new antifungal drugs. In this way, a great deal of hope could be placed in the potential application of peptides produced by archaeons demonstrating bioactivity against human opportunistic pathogenic fungi such as Candida spp. or even other pathogens, such as phytopathogens that cause so much damage to agriculture; however, the field of study is vast, and there is still much to learn (Table 2). Regarding the archaeal source of antifungal chemicals, the findings of (Roscetto et al. 2018) represent merely the tip of the iceberg.
Advantages and disadvantages of using microorganisms as biocontrol
The secondary metabolites from PGBR microbes are naturally occurring and gaining importance in plant disease management. Considering its frequency, dominance, and potential as an agrochemical, it could be marketed commercially (Pang et al. 2022). Less harmful and eco-friendly; Targets the specific organism and decomposes quickly; Supplies micronutrients and balances the soil nutrient cycle; Regulates plant metabolism against diseases; efficient colonizer of roots and supports colonization of mycorrhiza. However, disadvantages related to the lack of commercially accessible and effective BCA hinder the widespread application of biological control. Future BCA research should focus on finding new and better BCAs, and learning more about how they work. This will help us develop new and more effective ways to use BCAs to treat diseases and improve human health.
Future prospective and economics
Recent years have witnessed significant advancements in BCA knowledge for developing commercial therapies for controlling bacterial and fungal diseases. However, the lack of commercially accessible and effective BCA hinders the widespread application of biological control. Future developments should involve the discovery of innovative BCA and necessitate efficient and effective screening approaches appropriate for evaluating many candidates. In addition, comprehensive research of model BCA utilizing comparative genome analysis, genome, transcriptome, and proteome analysis will provide a helpful foundation for a comprehensive examination of the biological processes of BCA and the development of ways to enhance its positive effect. In addition, this multi-omics technique will analyze bacterial application’s effect on plants’ indigenous microbiome. This study would permit studying the environmental impact of BCA, ensuring its biosafety, and understanding how to regulate the microbiota to enhance the efficacy of biocontrol.
Conclusion
The biocontrol of phytopathogenic fungi can be carried out directly by bacteria, fungus, viruses, and archaea; by the many metabolites, enzymes, and signaling molecules these microorganisms can create; by competitive tactics; or even by soil microbiomes. Extensive usage of different fungicides results in resistance; thus, researchers are pursuing a more sustainable alternative using biological control. The rise in biological product registrations globally demonstrated its widespread use. Understanding the intricate interplay between microorganisms can also aid in managing soil diversity and inhibiting phytopathogenic fungi without chemical residues.
Abbreviations
- FAO:
-
Food and Agriculture Organization of the United Nations
- DMDS:
-
dimethyl disulfide
- VOCs:
-
volatile organic compounds
- DMHDA:
-
dimethyl hexadecylamine
- PGPB:
-
plant growth–promoting bacteria
- PGPR:
-
plant growth-promoting rhizobacteria
- NRPS:
-
non-ribosomal peptide synthetases
- PKS:
-
polyketide synthetases
- SAR:
-
systemic acquired resistance
- ISR:
-
induced systemic resistance
- JA:
-
jasmonic acid
- SA:
-
salicylic acid
- ET:
-
ethylene
- PR1, 2:
-
pathogenesis-related gene
- Chit-1:
-
the plant defensive chitinase
- Glu-2:
-
1,3-glucanase
- dsRNA:
-
Double-stranded RNA
- CHV:
-
Cryphonectria hypovirulence viruses
- SsNSRV-1:
-
S. sclerotiorum negative-stranded RNA virus 1
- SsHV2:
-
S. sclerotiorum hypovirus 2
- SsPV1:
-
S. sclerotiorum partitivirus 1
References
Abdelkhalik A, El-Mageed A, Taia A, Mohamed IA, Semida WM, Al-Elwany OA, AbuQamar SF, El-Tarabily KA, Gyushi MA (2023) Soil application of effective microorganisms and nitrogen alleviates salt stress in hot pepper (Capsicum annum L.) plants. Front Plant Sci 13:1079260. https://doi.org/10.3389/fpls.2022.1079260
Adebayo C, Aderiye B (2010) Antifungal activity of bacteriocins of lactic acid bacteria from some Nigerian fermented foods. Res J Microbiol 5(11):1070–1082. https://doi.org/10.3923/jm.2010.1070.1082
Ahluwalia V, Kumar J, Rana VS, Sati OP, Walia S (2015) Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat Prod Res 29(10):914–920. https://doi.org/10.1080/14786419.2014.958739
Ahmed IH, Ali EF, Gad AA, Bardisi A, El-Tahan AM, Abd Esadek OA, El-Saadony MT, Gendy AS (2022) Impact of plant growth regulators spray on fruit quantity and quality of pepper (Capsicum annuum L.) cultivars grown under plastic tunnels. Saudi J Biol Sci 29(4):2291–2298. https://doi.org/10.1016/j.sjbs.2021.11.062
Alblooshi AA, Purayil GP, Saeed EE, Ramadan GA, Tariq S, Altaee AS, El-Tarabily KA, AbuQamar SF (2022) Biocontrol potential of endophytic actinobacteria against Fusarium solani, the causal agent of sudden decline syndrome on date palm in the UAE. J Fungi 8(1):8. https://doi.org/10.3390/jof8010008
Al Hamad BM, AlRaish SM, Ramadan GA, Alameri SSA, Al Senaani SS, AbuQamar SF, El-Tarabily KA (2021) Effectiveness of augmentative biological control of Streptomyces griseorubens UAE2 depends on 1-Aminocyclopropane-1-carboxylic acid deaminase activity against Neoscytalidium Dimidiatum. J Fungi 7(11):885. https://doi.org/10.3390/jof7110885
Alijani Z, Amini J, Ashengroph M, Bahramnejad B (2019) Antifungal activity of volatile compounds produced by Staphylococcus sciuri strain MarR44 and its potential for the biocontrol of Colletotrichum nymphaeae, causal agent strawberry anthracnose. Int J Food Microbiol 307:108276. https://doi.org/10.1016/j.ijfoodmicro.2019.108276
Ali S, Hameed S, Shahid M, Iqbal M, Lazarovits G, Imran A (2020) Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol Res 232:126389. https://doi.org/10.1016/j.micres.2019.126389
Al Raish SM, Saeed EE, Alyafei DM, El-Tarabily KA, AbuQamar SF (2021) Evaluation of Streptomycete Actinobacterial isolates as biocontrol agents against royal poinciana stem canker Disease caused by the fungal pathogen Neoscytalidium Dimidiatum. Biol Control 164:104783. https://doi.org/10.1016/j.biocontrol.2021.104783
Alwahshi KJ, Purayil GP, Saeed EE, Abufarajallah HA, Aldhaheri SJ, AbuQamar SF, El-Tarabily KA (2022) The 1-aminocyclopropane-1-carboxylic acid deaminase-producing Streptomyces violaceoruber UAE1 can provide protection from sudden decline syndrome on date palm. Front Plant Sci 13:904166. https://doi.org/10.3389/fpls.2022.904166
Anagnostakis SL (1982) Biological control of chestnut blight. Science 215:466–471
Anderson LM, Stockwell VO, Loper JE (2004) An extracellular protease of Pseudomonas fluorescens inactivates antibiotics of Pantoea agglomerans. Phytopathol 94:1228–1234. https://doi.org/10.1094/PHYTO.2004.94.11.1228
Ashry NM, Alaidaroos BA, Mohamed SA, Badr OA, El-Saadony MT, Esmael A (2022) Utilization of drought-tolerant bacterial strains isolated from harsh soils as a plant growth-promoting rhizobacteria (PGPR). Saudi J Biol Sci 29(3):1760–1769. https://doi.org/10.1016/j.sjbs.2021.10.054
Badalyan SM (2001) Higher basidiomycetes as prospective objects for mycopharmacological research. Int J Med Mushrooms 3(2–3):108. https://doi.org/10.1615/IntJMedMushr.v3.i2-3.270
Beris D, Theologidis I, Skandalis N, Vassilakos N (2018) Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against tomato spotted wilt virus and potato virus Y. Sci Rep 8(1):10320. https://doi.org/10.1038/s41598-018-28677-3
Bertuzzi T, Leni G, Bulla G, Giorni P (2022) Reduction of mycotoxigenic fungi growth and their mycotoxin production by Bacillus subtilis QST 713. Toxins 14(11):797. https://doi.org/10.3390/toxins14110797
Bhat MA, Mishra AK, Jan S, Bhat MA, Kamal MA, Rahman S, Shah AA, Jan AT (2023) Plant growth promoting rhizobacteria in plant health: a perspective study of the underground interaction. Plants 12(3):629. https://doi.org/10.3390/plants12030629
Biraghi A (1953) Possible active resistance to Endothia Parasitica in Castanea crenata. Congress International Union Forest Research Organization, pp 149–157
Bird F, Elgee D (1957) A virus Disease and introduced parasites as factors controlling the European spruce sawfly, Diprion hercyniae (htg.), in Central New Brunswick1. Can Entomol 89:371–378
Black BC, Brennan LA, Dierks PM, Gard IE (1997) Commercialization of baculoviral insecticides. The baculoviruses. Springer US, Boston, MA, pp 341–387
Bonaterra A, Badosa E, Daranas N, Francés J, Roselló G, Montesinos E (2022) Bacteria as biological control agents of plant diseases. Microorganisms10(9): 1759. https://doi.org/10.3390/microorganisms10091759
Brasier CM (2001) Rapid evolution of introduced plant pathogens via interspecific hybridization: hybridization is leading to rapid evolution of Dutch elm Disease and other fungal plant pathogens. Biosci 51(2):123–133. https://doi.org/10.1641/0006-3568(2001)051[0123:REOIPP]2.0.CO;2
Brauer VS, Rezende CP, Pessoni AM, De Paula RG, Rangappa KS, Nayaka SC, Gupta VK, Almeida F (2019) Antifungal agents in agriculture: friends and foes of public health. Biomolecules 9(10):521. https://doi.org/10.3390/biom9100521
Bungtongdee N, Sopalun K, Laosripaiboon W, Iamtham S (2019) The chemical composition, antifungal, antioxidant and antimutagenicity properties of bioactive compounds from fungal endophytes associated with Thai orchids. J Phytopathol 167:56–64. https://doi.org/10.1111/jph.12773
Calderone RA, Fonzi WA (2001) Virulence factors of Candida albicans. Trends Microbiol 9:327–335. https://doi.org/10.1016/s0966-842x(01)02094-7
Cascales E, Buchanan SK, Duché D, Kleanthous C, Lloubes R, Postle K, Riley M, Slatin S, Cavard D (2007) Colicin biology. Microbiol Mol Biol Rev 71:158–229. https://doi.org/10.1128/mmbr.00036-06
Chelius M, Triplett E (2001) The diversity of archaea and bacteria in association with the roots of Zea mays L. Microb Ecol 41(3):252–263. https://doi.org/10.1007/s002480000087
Chen B, Nuss DL (1999) Infectious cDNA clone of hypovirus CHV1-Euro7: a comparative virology approach to investigate virus-mediated hypovirulence of the chestnut blight fungus Cryphonectria parasitica. J Virol 73(2):985–992. https://doi.org/10.1128/JVI.73.2.985-992.1999
Chen JH, Xiang W, Cao KX, Lu X, Yao SC, Hung D, Huang RS, Li LB (2020) Characterization of volatile organic compounds emitted from endophytic Burkholderia cenocepacia ETR-B22 by SPME-GC-MS and their inhibitory activity against various plant fungal pathogens. Molecules 25(17):3765. https://doi.org/10.3390/molecules25173765
Conrad AM (2022) Management of Sclerotinia sclerotiorum in soybean using the biofungicides Bacillus amyloliquefaciens and Coniothyrium minitans. Purdue University, Thesis
Conrad AM, Telenko DE (2023) Efficacy of biocontrol agents Coniothyrium minitans and Bacillus amyloliquefaciens for managing Sclerotinia sclerotiorum in Indiana soybean. PhytoFrontiers 3:1–29. https://doi.org/10.1094/PHYTOFR-07-22-0080-R
Daranas N, Roselló G, Cabrefiga J, Donati I, Francés J, Badosa E, Spinelli F, Montesinos E, Bonaterra A (2019) Biological control of bacterial plant Diseases with Lactobacillus plantarum strains selected for their broad-spectrum activity. Ann Appl Biol 174(1):92–105. https://doi.org/10.1111/aab.12476
da Silva Ribeiro A, Polonio JC, Costa AT, Dos Santos CM, Rhoden SA, Azevedo JL, Pamphile JA (2018) Bioprospection of culturable endophytic fungi associated with the ornamental plant Pachystachys lutea. Curr Opin Microbiol 75:588–596. https://doi.org/10.1007/s00284-017-1421-9
Day P (1977) Double-stranded RNA in Endothia parasitica PR Day, JA Dodds, JE Elliston, R. A. Jaynes, and S. L. Anagnostakis Authors two and three, Department of Plant Pathology; and authors one, four, and five, Department of Genetics; all at The Connecticut Agricultural Experiment Station, New Haven, CT 06504
Díaz-Díaz M, Bernal-Cabrera A, Trapero A, Medina-Marrero R, Sifontes-Rodríguez S, Cupull-Santana RD, Cupull-Santana RD, García-Bernal M, Agustí-Brisach C (2022) Characterization of actinobacterial strains as potential biocontrol agents against Macrophomina Phaseolina and rhizoctonia solani, the main soilborne pathogens of Phaseolus vulgaris in Cuba. Plants 11(5):645. https://doi.org/10.3390/plants11050645
Díaz-Gutiérrez C, Arroyave C, Llugany M, Poschenrieder C, Martos S, Peláez C (2021) Trichoderma Asperellum as a preventive and curative agent to control Fusarium wilt in Stevia rebaudiana. Biol Control 155:104537. https://doi.org/10.1016/j.biocontrol.2021.104537
de Almeida TT, Ribeiro MAS, Polonio JC, Garcia FP, Nakamura CV, Meurer EC, Sarragiotto MH, Baldoqui DC, Azevedo JL, Pamphile JA (2018) Curvulin and spirostaphylotrichins R and U from extracts produced by two endophytic Bipolaris sp. associated to aquatic macrophytes with antileishmanial activity. Nat Prod Res 32:2783–2790. https://doi.org/10.1080/14786419.2017.1380011
de Andrade LA, Santos CHB, Frezarin ET, Sales LR, Rigobelo EC (2023) Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 11(4):1088. https://doi.org/10.3390/microorganisms11041088
Desoky ESM, Rady MM, Nader MM, Mostafa NG, Elrys AS, Mathai A, AbuQamar SF, El-Tarabily KA, El-Saadony MT (2022) Integrated application of bacterial carbonate precipitation and silicon nanoparticles enhances productivity, physiological attributes, and antioxidant defenses of wheat (Triticum aestivum L.) under semi-arid conditions. Front Plant Sci 13:947949. https://doi.org/10.3389/fpls.2022.947949
Desoky ESM, Saad AM, El-Saadony MT, Merwad ARM, Rady MM (2020) Plant growth-promoting rhizobacteria: potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal Agric Biotechnol 30:101878.https://doi.org/10.1016/j.bcab.2020.101878
De Vrieze M, Varadarajan AR, Schneeberger K, Bailly A, Rohr RP, Ahrens CH, Weisskopf L (2020) Linking comparative genomics of nine potato-associated Pseudomonas isolates with their differing biocontrol potential against late blight. Front Microbiol 11:857. https://doi.org/10.3389/fmicb.2020.00857
Duffy B, Weller D (1996) Biological control of take-all of wheat in the pacific northwest of the Usa using hypovirulent Gaeumannomyces Graminis var. Tritici and fluorescent pseudomonads. J Phytopathol 144:585–590. https://doi.org/10.1111/j.1439-0434.1996.tb00303.x
Duong B, Nguyen HX, Phan HV, Colella S, Trinh PQ, Hoang GT, Nguyen TT, Marraccini P, Lebrun M, Duponnois R (2021) Identification and characterization of Vietnamese coffee bacterial endophytes displaying in vitro antifungal and nematicidal activities. Microbiol Res 242:126613. https://doi.org/10.1016/j.micres.2020.126613
Eberl L, Vandamme P (2016) Members of the genus Burkholderia: good and bad guys. F1000Res 5:1–10. https://doi.org/10.12688/f1000research.8221.1
El-Mageed A, Taia A, Gyushi MA, Hemida KA, El-Saadony MT, El-Mageed A, AbuQamar SF, El-Tarabily KA, Abdelkhalik A (2022) Coapplication of effective microorganisms and nanomagnesium boosts the agronomic, physio-biochemical, osmolytes, and antioxidants defenses against salt stress in Ipomoea batatas. Front Plant Sci 13:883274. https://doi.org/10.3389/fpls.2022.883274
El-Maraghy SS, Tohamy TA, Hussein KA (2020) Role of plant-growth promoting fungi (PGPF) in defensive genes expression of Triticum aestivum against wilt Disease. Rhizosphere 15:100223. https://doi.org/10.1016/j.rhisph.2020.100223
Elnahal AS, El-Saadony MT, Saad AM, Desoky ESM, El-Tahan AM, Rady MM, AbuQamar SF, El-Tarabily KA (2022) The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: a review. Eur J Plant Pathol 162(4):759–792. https://doi.org/10.1007/s10658-021-02393-7
El-Saadony MT, Saad AM, Najjar AA, Alzahrani SO, Alkhatib FM, Shafi ME, Fouda SEE, El-Tahan AM, Hassan MA (2021) The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot Diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J Biol Sci 28(8):4461–4471. https://doi.org/10.1016/j.sjbs.2021.04.043
El-Saadony MT, Saad AM, Soliman SM, Salem HM, Ahmed AI, Mahmood M, Babalghith AO, Elrys AS, El-Tarabily KA, AbuQamar SF (2022) Plant growth-promoting microorganisms as biocontrol agents of plant Diseases: mechanisms, challenges and future perspectives. Front Plant Sci 13:923880. https://doi.org/10.3389/fpls.2022.923880
Elsayed SS, Sehsah MD, Oueslati MA, Ibrahim OM, Hamden S, Seddek NH, AbdElgawad H, El-Saadony MT, El-Tahan AM (2023) The effect of using fresh farmyard manure (animal manure) on the severity of Fusarium verticilioides in soil, root, stem, and kernels as well as lodging and borer incidence of maize plants. Front Plant Sci 13:998440. https://doi.org/10.3389/fpls.2022.998440
El-Tarabily KA (2003) An endophytic chitinase-producing isolate of Actinoplanes missouriensis, with potential for biological control of root rot of lupin caused by Plectosporium tabacinum. Aust J Bot 51:257–266. https://doi.org/10.1071/BT02107
El-Tarabily KA, Hardy GE, St J, Sivasithamparam K (2010) Performance of three endophytic actinomycetes in relation to plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber under commercial field production conditions in the United Arab Emirates. Eur J Plant Pathol 128:527–539. https://doi.org/10.1007/s10658-010-9689-7
El-Tarabily KA, Hardy GE, St J, Sivasithamparam K, Hussein AM, Kurtböke DI (1997) The potential for the biological control of cavity spot Disease of carrots caused by Pythium coloratum by Streptomycete and non-streptomycete actinomycetes in Western Australia. New Phytol 137:495–507. https://doi.org/10.1046/j.1469-8137.1997.00856.x
El-Tarabily KA, Nassar AH, Hardy GE, St J, Sivasithamparam K (2009) Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J Appl Microbiol 106:13–26. https://doi.org/10.1111/j.1365-2672.2008.03926.x
El-Tarabily KA, Sivasithamparam K (2006) Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol Biochem 38:1505–1520. https://doi.org/10.1016/j.soilbio.2005.12.017
El-Tarabily KA, Sykes ML, Kurtböke DI, Hardy GE, St J, Barbosa AM, Dekker RFH (1996) Synergistic effects of a cellulase-producing Micromonospora carbonacea and an antibiotic-producing Streptomyces violascens on the suppression of Phytophthora cinnamomi root-rot of Banksia Grandis. Canad J Bot 74:618–624. https://doi.org/10.1139/b96-078
Figueroa M, Ortiz D, Henningsen EC (2021) Tactics of host manipulation by intracellular effectors from plant pathogenic fungi. Curr Opin Plant Biol 62:102054. https://doi.org/10.1016/j.pbi.2021.102054
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194. https://doi.org/10.1038/nature10947
Gao Z, Zhang B, Liu H, Han J, Zhang Y (2017) Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis Cinerea. Biol Control 105:27–39. https://doi.org/10.1016/j.biocontrol.2016.11.007
García-Pedrajas M, Cañizares M, Sarmiento-Villamil JL, Jacquat AG, Dambolena JS (2019) Mycoviruses in biological control: from basic research to field implementation. Phytopathology 109:1828–1839. https://doi.org/10.1094/PHYTO-05-19-0166-RVW
Ghabrial SA, Castón JR, Jiang D, Nibert ML, Suzuki N (2015) 50-plus years of fungal viruses. Virology 479:356–368. https://doi.org/10.1016/j.virol.2015.02.034
Ghabrial SA, Suzuki N (2009) Viruses of plant pathogenic fungi. Annu Rev Phytopathol 47:353–384. https://doi.org/10.1146/annurev-phyto-080508-081932
Gómez-Godínez LJ, Aguirre-Noyola JL, Martínez-Romero E, Arteaga-Garibay RI, Ireta-Moreno J, Ruvalcaba-Gómez JM (2023) A look at plant-growth-promoting bacteria. Plants 12(8):1668. https://doi.org/10.3390/plants12081668
Goswami D, Thakker JN, Dhandhukia PC (2016) Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric 2(1):1127500. https://doi.org/10.1080/23311932.2015.1127500
Grützmacher DD, Grützmacher AD, Agostinetto D, Loeck AE, Roman R, Peixoto SC, Zanella R (2008) Monitoramento De agrotóxicos em dois mananciais hídricos no Sul do Brasil. Rev Bras Eng Agríc Ambient 12:632–637. https://doi.org/10.1590/S1415-43662008000600010
Haas D, Défago G (2005) Biological control of soilborne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3(4):307–319. https://doi.org/10.1038/nrmicro1129
Haase S, Sciocco-Cap A, Romanowski V (2015) Baculovirus insecticides in Latin America: historical overview, current status and future perspectives. Viruses 7(5):2230–2267. https://doi.org/10.3390/v7052230
Hallmann J, Quadt-Hallmann A, Rodrıguez-Kabana R, Kloepper J (1998) Interactions between Meloidogyne incognita and endophytic bacteria in cotton and cucumber. Soil Biol Biochem 30(7):925–937. https://doi.org/10.1016/S0038-0717(97)00183-1
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species—opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2(1):43–56. https://doi.org/10.1038/nrmicro797
Hassan MA, El-Saadony MT, Mostafa NG, El-Tahan AM, Mesiha PK, El-Saadony FM, Hassan AM, El-Shehawi AM, Ashry NM (2021) The use of previous crops as sustainable and eco-friendly management to fight Fusarium oxysporum in sesame plants. Saudi J Biol Sci 28(10):5849–5859. https://doi.org/10.1016/j.sjbs.2021.06.041
Hazarika DJ, Goswami G, Gautom T, Parveen A, Das P, Barooah M, Boro RC (2019) Lipopeptide mediated biocontrol activity of endophytic Bacillus subtilis against fungal phytopathogens. BMC Microbiol 2(1):71. https://doi.org/10.1186/s12866-019-1440-8
Heiniger U, Rigling D (1994) Biological control of chestnut blight in Europe. Annu Rev Phytopathol 32:581–599. https://doi.org/10.1146/annurev.py.32.090194.003053
Heng NC, Wescombe PA, Burton JP, Jack RW, Tagg JR (2007) The diversity of bacteriocins in Gram-positive bacteria. Bacteriocins: ecology and evolution. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 45–92
Hermosa R, Viterbo A, Chet I, Monte E (2012) Plant-beneficial effects of Trichoderma and of its genes. Microbiol 158(Pt 1):17–25. https://doi.org/10.1099/mic.0.052274-0
Heydari A, Pessarakli M (2010) A review on biological control of fungal plant pathogens using microbial antagonists. J Biol Sci 10(4):273–290. https://doi.org/10.3923/jbs.2010.273.290
Hillman BI, Annisa A, Suzuki N (2018) Viruses of plant-interacting fungi. Adv Virus Res 100:99–116. https://doi.org/10.1146/annurev-phyto-080508-081932
Huang CJ, Tsay JF, Chang SY, Yang HP, Wu WS, Chen CY (2012) Dimethyl disulfide is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag Sci 68:1306–1310. https://doi.org/10.1002/ps.3301
Hussain T, Khan AA, Mohamed HI (2022) Potential efficacy of biofilm-forming biosurfactant Bacillus firmus HussainT-Lab. 66 against Rhizoctonia solani and mass spectrometry analysis of its metabolites. Int J Pept Res Ther 38(2):90–101. https://doi.org/10.5423/PPJ.OA.11.2021.0173
Hutchings MI, Truman AW, Wilkinson B (2019) Antibiotics: past, present and future. Curr Opin Microbiol 51:72–80. https://doi.org/10.1016/j.mib.2019.10.008
Jain A, Sarsaiya S, Wu Q, Lu Y, Shi J (2019) A review of plant leaf fungal Diseases and its environment speciation. Bioengineered 10:409–424. https://doi.org/10.1080/21655979.2019.1649520
Jantasorn A, Moungsrimuangdee B, Dethoup T (2016) In vitro antifungal activity evaluation of five plant extracts against five plant pathogenic fungi causing rice and economic crop Diseases. J Biopestic 9(1):1–7. https://doi.org/10.57182/jbiopestic.9.1.01-07
Jatoi GH, Lihua G, Xiufen Y, Gadhi MA, Keerio AU, Abdulle YA, Qiu D (2019) A novel protein elicitor PeBL2, from Brevibacillus laterosporus A60, induces systemic resistance against Botrytis Cinerea in Tobacco plant. Plant Pathol J 35(3):208–218. https://doi.org/10.5423/PPJ.OA.11.2018.0276
Kaddes A, Fauconnier ML, Sassi K, Nasraoui B, Jijakli MH (2019) Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 24(6):1065. https://doi.org/10.3390/molecules24061065
Kamil FH, Saeed EE, El-Tarabily KA, AbuQamar SF (2018) Biological control of mango dieback Disease caused by Lasiodiplodia theobromae using Streptomycete and non-streptomycete actinobacteria in the United Arab Emirates. Front Microbiol 9:829. https://doi.org/10.3389/fmicb.2018.00829
Kanchiswamy CN, Malnoy M, Maffei ME (2015) Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci 6:151. https://doi.org/10.3389/fpls.2015.00151
Katz L, Baltz RH (2016) Natural product discovery: past, present, and future. J Ind Microbiol Biotechnol 43(2–3):155–176. https://doi.org/10.1007/s10295-015-1723-5
Khan RAA, Najeeb S, Hussain S, Xie B, Li Y (2020) Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 8(6):817. https://doi.org/10.3390/microorganisms8060817
Khatri DK, Tiwari DN, Bariya HS (2017) Chitinolytic efficacy and secretion of cell wall degrading enzymes from Trichoderma spp. in response to phyto-pathological fungi. J Appl Biol Biotechnol 5(6):1–8. https://doi.org/10.7324/JABB.2017.50601
Köhl J, Booij K, Kolnaar R, Ravensberg WJ (2019) Ecological arguments to reconsider data requirements regarding the environmental fate of microbial biocontrol agents in the registration procedure in the European Union. Biocontrol 64:469–487. https://doi.org/10.1007/s10526-019-09964-y
Khunnamwong P, Lertwattanasakul N, Jindamorakot S, Suwannarach N, Matsui K, Limtong S (2020) Evaluation of antagonistic activity and mechanisms of endophytic yeasts against pathogenic fungi causing economic crop Diseases. Folia Microbiol 65:573–590. https://doi.org/10.1007/s12223-019-00764-6
Kong WL, Li PS, Wu XQ, Wu TY, Sun XR (2020) Forest tree associated bacterial diffusible and volatile organic compounds against various phytopathogenic fungi. Microorganisms 8(4):590. https://doi.org/10.3390/microorganisms8040590
Kumari S, Khanna V (2020) Induction of systemic resistance in chickpea (Cicer arietinum L.) against Fusarium oxysporum f. sp. ciceris by antagonistic rhizobacteria in assistance with native mesorhizobium. Curr Microbiol 77:85–98. https://doi.org/10.1007/s00284-019-01805-6
Kumar V, Tiwari SK (2017) Halocin HA1: an archaeocin produced by the haloarchaeon Haloferax larsenii HA1. Process Biochem 61:202–208. https://doi.org/10.1016/j.procbio.2017.06.010
Lamichhane J, Dachbrodt-Saaydeh S, Kudsk P, Messéan A (2016) Conventional pesticides in agriculture: benefits versus risks. Plant Dis 100:10–24. https://doi.org/10.1094/PDIS-05-15-0574-FE
Legein M, Smets W, Vandenheuvel D, Eilers T, Muyshondt B, Prinsen E, Prinsen E, Samson R, Lebeer S (2020) Modes of action of microbial biocontrol in the phyllosphere. Front Microbiol 11:1619. https://doi.org/10.3389/fmicb.2020.01619
Liu L, Xie J, Cheng J, Fu Y, Li G, Yi X, Jiang D (2014) Fungal negative-stranded RNA virus that is related to bornaviruses and nyaviruses. Proc Natl Acad Sci 111:12205–12210. https://doi.org/10.1073/pnas.1401786111
Liu Y, Teng K, Wang T, Dong E, Zhang M, Tao Y, Zhong J (2020) Antimicrobial Bacillus velezensis HC6: production of three kinds of lipopeptides and biocontrol potential in maize. J Appl Microbiol 128(1):242–254. https://doi.org/10.1111/jam.14459
Li X, Garbeva P, Liu X klein Gunnewiek. PJA, Clocchiatti A, Hundscheid MPJ, Wang X, Boer W (2020) 1, 1025–1035
Lopes R, Tsui S, Gonçalves PJ, de Queiroz MV (2018) A look into a multifunctional toolbox: endophytic Bacillus species provide broad and underexploited benefits for plants. World J Microbiol Biotechnol 34:1–10. https://doi.org/10.1007/s11274-018-2479-7
Lund ME, Mourtzinis S, Conley SP, Ané JM (2018) Soybean cyst nematode control with Pasteuria nishizawae under different management practices. Agron J 110:2534–2540. https://doi.org/10.2134/agronj2018.05.0314
Lutfia A, Munir E, Yurnaliza Y (2020) Molecular identification of endophytic fungi from torch ginger (Etlingera elatior) antagonist to phytopathogenic fungi. Biodivers J Biol Diver 21(6):2681–2689. https://doi.org/10.13057/biodiv/d210641
Maindad D, Kasture V, Chaudhari H, Dhavale D, Chopade B, Sachdev D (2014) Characterization and fungal inhibition activity of siderophore from wheat rhizosphere associated Acinetobacter calcoaceticus strain HIRFA32. Indian J Med Microbiol 54(3):315–322. https://doi.org/10.1007/s12088-014-0446-z
Ma LJ, Geiser DM, Proctor RH, Rooney AP, O’Donnell K, Trail F, Gardiner DM, Manners JM, Kazan K (2013) Fusarium pathogenomics. Annu Rev Microbiol 67:399–416. https://doi.org/10.1146/annurev-micro-092412-155650
Marzano SYL, Domier LL (2016) Novel mycoviruses discovered from metatranscriptomics survey of soybean phyllosphere phytobiomes. Virus Res 213:332–342. https://doi.org/10.1016/j.virusres.2015.11.002
Marzano SYL, Hobbs HA, Nelson BD, Hartman GL, Eastburn DM, McCoppin NK, Domier LL (2015) Transfection of Sclerotinia sclerotiorum with in vitro transcripts of a naturally occurring interspecific recombinant of Sclerotinia Sclerotiorum hypovirus 2 significantly reduces virulence of the fungus. J Virol 89:5060–5071. https://doi.org/10.1128/JVI.03199-14
Mazguene S, Rossi M, Gogliettino M, Palmieri G, Cocca E, Mirino S, Imadalou-Idres N, Benallaoua S (2018) Isolation and characterization from solar salterns of North Algeria of a haloarchaeon producing a new halocin. Extremophiles 22(2):259–270. https://doi.org/10.1007/s00792-017-0994-3
Mckenna F, El-Tarabily KA, Hardy GE, St J, Dell B (2001) Novel in vivo use of a polyvalent Streptomyces phage to disinfest Streptomyces scabies infected seed potatoes. Plant Pathol 50:666–675. https://doi.org/10.1046/j.1365-3059.2001.00648.x
Mejía LC, Rojas EI, Maynard Z, Van Bael S, Arnold AE, Hebbar P, Samuels GJ, Robbins N, Herre EA (2008) Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol Control 46(1):4–14. https://doi.org/10.1016/j.biocontrol.2008.01.012
Milgroom MG, Cortesi P (2004) Biological control of chestnut blight with hypovirulence: a critical analysis. Annu Rev Phytopathol 42:311–338. https://doi.org/10.1146/annurev.phyto.42.040803.140325
Milijašević-Marčić S, Stepanović M, Todorović B, Duduk B, Stepanović J, Rekanović E, Potočnik I (2017) Biological control of green mould on Agaricus Bisporus by a native Bacillus subtilis strain from mushroom compost. Eur J Plant Pathol 148:509–519. https://doi.org/10.1007/s10658-016-1107-3
Montes-Osuna N, Gómez-Lama Cabanás C, Valverde-Corredor A, Berendsen RL, Prieto P, Mercado-Blanco J (2021) Assessing the involvement of selected phenotypes of Pseudomonas simiae PICF7 in olive root colonization and biological control of Verticillium Dahliae. Plants 10(2):412. https://doi.org/10.3390/plants10020412
Moreira JC, Jacob SC, Peres F, Lima JS, Meyer A, Oliveira-Silva JJ, Sarcinelli PN, Batista DF, Egler M, Faria MVC (2002) Integrated evaluation of the health impact of pesticide use in a community at Nova Friburgo, RJ. Ciência & saúde Coletiva 7(2):299–311. https://doi.org/10.1590/S1413-81232002000200010
Musa Z, Ma J, Egamberdieva D, Abdelshafy Mohamad OA, Abaydulla G, Liu Y, Li WJ, Li L (2020) Diversity and antimicrobial potential of cultivable endophytic actinobacteria associated with the medicinal plant Thymus roseus. Front Microbiol 11:191. https://doi.org/10.3389/fmicb.2020.00191
Nawrocka J, Małolepsza U (2013) Diversity in plant systemic resistance induced by Trichoderma. Biol Control 67(2):149–156. https://doi.org/10.1016/j.biocontrol.2013.07.005
Nerva L, Ciuffo M, Vallino M, Margaria P, Varese G, Gnavi G, Turina M (2016) Multiple approaches for the detection and characterization of viral and plasmid symbionts from a collection of marine fungi. Virus Res 219:22–38. https://doi.org/10.1016/j.virusres.2015.10.028
Ni L, Punja ZK (2019) Management of fungal diseases on cucumber (Cucumis sativus L.) and tomato (Solanum lycopersicum L.) crops in greenhouses using Bacillus subtilis (Chapter). Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol: Volume 2, 1–28
Nuss DL (2005) Hypovirulence: mycoviruses at the fungal–plant interface. Nat Rev Microbiol 3:632–642. https://doi.org/10.1038/nrmicro1206
Odelade KA, Babalola OO (2019) Bacteria, fungi and archaea domains in rhizospheric soil and their effects in enhancing agricultural productivity. Int J Environ Res Public Health 16(20):3873. https://doi.org/10.3390/ijerph16203873
Olanrewaju OS, Glick BR, Babalola OO (2017) Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotechnol 33(11):197. https://doi.org/10.1007/s11274-017-2364-9
Olowe OM, Akanmu AO, Asemoloye MD (2020) Exploration of microbial stimulants for induction of systemic resistance in plant Disease management. Ann Appl Biol 177:282–293. https://doi.org/10.1111/aab.12631
Osaki H, Nakamura H, Sasaki A, Matsumoto N, Yoshida K (2006) An endornavirus from a hypovirulent strain of the violet root rot fungus, Helicobasidium mompa. Virus Res 118:143–149. https://doi.org/10.1016/j.virusres.2005.12.004
Palm ME (2001) Systematics and the impact of invasive fungi on agriculture in the United States: knowledge of the systematics of plant-inhabiting fungi is fundamental for making appropriate plant quarantine decisions and thereby safeguarding US plant resources. Bioscience 51:141–147. https://doi.org/10.1641/0006-3568(2001)051[0141:SATIOI]2.0.CO;2
Pang F, Solanki MK, Wang Z (2022) Streptomyces can be an excellent plant growth manager. World J Microbiol Biotechnol 38(11):193. https://doi.org/10.1007/s11274-022-03380-8
Pavela R, Vrchotová N (2008) Growth inhibitory effect of extracts from Reynoutria sp. plants against Spodoptera littoralis larvae. Agrociencia 42:573–584
Pedro, de Oliveira Mirelle, Takaki Teresa Cristina Castilho et al (2013) Synthesis characterization and antifungal activity of quaternary derivatives of chitosan on Aspergillus flavus Microbiological Research 168(1) 50–55. https://doi.org/10.1016/j.micres.2012.06.006
Pei DF, Wu QQ, Luo H, Paul NC, Deng JX, Zhou Y (2019) Diversity and antifungal activity of endophytes associated with Spiranthes sinensis (Orchidaceae, Magnoliophyta) in China. Int J Appl Microbiol Biotechnol Res 7:7–17. https://doi.org/10.33500/ijambr.2019.07.003
Peixoto Neto PDS, Azevedo JL, Araújo WD (2002) Microrganismos endofíticos. Biotecnologia Ciência & Desenvolvimento 29:62–77
Penha RO, Vandenberghe LP, Faulds C, Soccol VT, Soccol CR (2020) Bacillus lipopeptides as powerful pest control agents for a more sustainable and healthy agriculture: recent studies and innovations. Planta 251(3):1–15. https://doi.org/10.1007/s00425-020-03357-7
Peters LP, Prado LS, Silva FI, Souza FS, Carvalho CM (2020) Selection of endophytes as antagonists of Colletotrichum gloeosporioides in açaí palm. Biol Control 150:104350. https://doi.org/10.1016/j.biocontrol.2020.104350
Quehenberger J, Shen L, Albers S, Siebers B, Spadiut O (2017) Sulfolobus—a potential key organism in future biotechnology. Front Microbiol 8:2474. https://doi.org/10.3389/fmicb.2017.02474
Rana KL, Kour D, Yadav N, Yadav AN (2020) 10 - endophytic microbes in nanotechnology: current development, and potential biotechnology applications (chapter). Microbial endophytes (Book). Elsevier, pp 231–262. https://doi.org/10.1016/B978-0-12-818734-0.00010-3
Rani A, Saini KC, Bast F, Mehariya S, Bhatia SK, Lavecchia R, Zuorro A (2021) Microorganisms: a potential source of bioactive molecules for antioxidant applications. Molecules 26(4):1142. https://doi.org/10.3390/molecules26041142
Rao TB, Chopperla R, Methre R, Punniakotti E, Venkatesh V, Sailaja B, Reddy MR, Yugander A, Laha G, Madhav MS (2019) Pectin induced transcriptome of a Rhizoctonia solani strain causing sheath blight Disease in rice reveals insights on key genes and RNAi machinery for development of pathogen derived resistance. Plant Mol Biol 100:59–71. https://doi.org/10.1007/s11103-019-00843-9
Rigling D, Prospero S (2018) Cryphonectria parasitica, the causal agent of chestnut blight: invasion history, population biology and Disease control. Mol Plant Pathol 19:7–20. https://doi.org/10.1111/mpp.12542
Rojas-Solís D, Zetter-Salmón E, Contreras-Pérez M, del Carmen Rocha-Granados M, Macías-Rodríguez L, Santoyo G (2018) Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal Agric Biotechnol 13:46–52. https://doi.org/10.1016/j.bcab.2017.11.007
Roscetto E, Contursi P, Vollaro A, Fusco S, Notomista E, Catania MR (2018) Antifungal and anti-biofilm activity of the first cryptic antimicrobial peptide from an archaeal protein against Candida spp. clinical isolates. Sci Rep 8(1):17570. https://doi.org/10.1038/s41598-018-35530-0
Rotolo C, De Miccolis Angelini RM, Pollastro S, & Faretra F (2016) A TaqMan-based qPCR assay for quantitative detection of the biocontrol agents Bacillus subtilis strain QST713 and Bacillus amyloliquefaciens subsp. plantarum strain D747. BioControl 61:91-101
Saad AM, Salem HM, El-Tahan AM, El-Saadony MT, Alotaibi SS, El-Shehawi AM, Alkahtani MA, Ahmed AE, Swelum AA (2022) Biological control: an effective approach against nematodes using black pepper plants (Piper nigrum L). Saudi J Biol Sci 29(4):2047–2055. https://doi.org/10.1016/j.sjbs.2022.01.004
Sabra MA, Alaidaroos BA, Jastaniah SD, Heflish AI, Ghareeb RY, Mackled MI, El-Saadony MT, Abdelsalam NR, Conte-Junior CA (2022) Comparative effect of commercially available nanoparticles on soil bacterial community and Botrytis fabae caused brown spot: in vitro and in vivo experiment. Front Microbiol 13:934031. https://doi.org/10.3389/fmicb.2022.934031
Saeed EE, Sham A, Salmin Z, Abdelmowla Y, Iratni R, El-Tarabily KA, AbuQamar SF (2017) Streptomyces globosus UAE1, a potential effective biocontrol agent for black scorch Disease in date palm plantations. Front Microbiol 8:1455. https://doi.org/10.3389/fmicb.2017.01455
Sanogo S & Lujan P (2022) Seed, plant, and soil treatment with selected commercial Bacillus-based and Streptomyces-based biofungicides and chemical fungicides and development of Phytophthora blight on chile pepper. Arch. Phytopathol. Pflanzenschutz 55(3):331-343
Santos CMD, Ribeiro ADS, Garcia A, Polli AD, Polonio JC, Azevedo JL, Pamphile JA (2019) Enzymatic and antagonist activity of endophytic fungi from Sapindus saponaria L.(Sapindaceae). Acta Biol Colomb 24(2):322–330. https://doi.org/10.15446/abc.v24n2.74717
Sass G, Nazik H, Penner J, Shah H, Ansari SR, Clemons KV, Groleau MC, Dietl AM, Visca P, Haas H (2018) Studies of Pseudomonas aeruginosa mutants indicate pyoverdine as the central factor in inhibition of aspergillus fumigatus biofilm. J Bacteriol 200(1):e00345. https://doi.org/10.1128/JB.00345-17
Schardl CL, Phillips TD (1997) Protective grass endophytes: where are they from and where are they going? Plant Dis 81:430–438. https://doi.org/10.1094/PDIS.1997.81.5.430
Schoelz JE, Stewart LR (2018) The role of viruses in the phytobiome. Annu Rev Virol 5:93–111. https://doi.org/10.1146/annurev-virology-092917-043421
Selim HM, Gomaa NM, Essa AM (2016) Antagonistic effect of endophytic bacteria against some phytopathogens. Egypt J Bot 56(3):613–626. https://doi.org/10.21608/ejbo.2016.2721
Selim HM, Gomaa NM, Essa AM (2017) Application of endophytic bacteria for the biocontrol of Rhizoctonia solani (Cantharellales: ceratobasidiaceae) damping-off Disease in cotton seedlings. Biocontrol Sci Technol 27:81–95. https://doi.org/10.1080/09583157.2016.1258452
Shand RF, Leyva KJ (2008) Archaeal Antimicrobials: An Undiscovered Country, Archaea: New Models for Prokaryotic Biology (Edited by: Paul Blum). Caister Academic Press, U.K
Silva RN, Monteiro VN, Steindorff AS, Gomes EV, Noronha EF, Ulhoa CJ (2019) Trichoderma/pathogen/plant interaction in pre-harvest food security. Fungal Biol 123(8):565–583. https://doi.org/10.1016/j.funbio.2019.06.010
South KA, Peduto Hand F, Jones ML (2020) Beneficial bacteria identified for the control of Botrytis Cinerea in petunia greenhouse production. Plant Dis 104:1801–1810. https://doi.org/10.1094/PDIS-10-19-2276-RE
Stahr M, Quesada-Ocampo L (20200. Assessing the role of temperature, inoculum density, and wounding on disease progression of the fungal pathogen Ceratocystis fimbriata causing black rot in sweetpotato. Plant Dis 104: 930–937. https://doi.org/10.1094/PDIS-12-18-2224-RE
Tanapichatsakul C, Pansanit A, Monggoot S, Brooks S, Prachya S, Kittakoop P, Panuwet P, Pripdeevech P (2020) Antifungal activity of 8-methoxynaphthalen-1-ol isolated from the endophytic fungus diatrype palmicola MFLUCC 17–0313 against the plant pathogenic fungus athelia rolfsii on tomatoes. PeerJ 8:e9103. https://doi.org/10.7717/peerj.9103
Tortora ML, Díaz-Ricci JC, Pedraza RO (2011) Azospirillum brasilense siderophores with antifungal activity against Colletotrichum Acutatum. Arch Microbiol 193(4):275–286. https://doi.org/10.1007/s00203-010-0672-7
Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A (2016. Role of plant growth promoting rhizobacteria in agricultural sustainability—a review. Molecules 21(5):573. https://doi.org/10.3390/molecules21050573
Xiao X, Cheng J, Tang J, Fu Y, Jiang D, Baker TS, Ghabrial SA, Xie J (2014) A novel partitivirus that confers hypovirulence on plant pathogenic fungi. J Virol 88(17):10120–10133. https://doi.org/10.1128/JVI.01036-14
Xiao Y, Li HX, Li C, Wang JX, Li J, Wang MH, Ye YH (2013) Antifungal screening of endophytic fungi from Ginkgo biloba for discovery of potent anti-phytopathogenic fungicides. FEMS Microbiol Lett 339:130–136
Xie J, Jiang D (2014) New insights into mycoviruses and exploration for the biological control of crop fungal Diseases. Annu Rev Phytopathol 52:45–68. https://doi.org/10.1146/annurev-phyto-102313-050222
Yan Y, Yuan Q, Tang J, Huang J, Hsiang T, Wei Y, Zheng L (2018) Colletotrichum Higginsianum as a model for understanding host–pathogen interactions: a review. Int J Mol Sci 19(7):2142. https://doi.org/10.3390/ijms19072142
Yeh C, Wang C, Chen Y, Tsai S, Chung W (2021) Potential of a volatile-producing endophytic fungus nodulisporium sp. PDL-005 for the control of Penicillium Digitatum. Biol Control 152:104459. https://doi.org/10.1016/j.biocontrol.2020.104459
Yu X, Li B, Fu Y, Jiang D, Ghabrial SA, Li G, Peng Y, Xie J, Cheng J, Huang J (2010) A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc Natl Acad Sci 107(18):8387–8392. https://doi.org/10.1073/pnas.0913535107
Yu X, Li B, Fu Y, Xie J, Cheng J, Ghabrial SA, Li G, Yi X, Jiang D (2013) Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc Natl Acad Sci 110(4):1452–1457. https://doi.org/10.1073/pnas.1213755110
Zhou T, Boland GJ (1998) Suppression of dollar spot by hypovirulent isolates of Sclerotinia homoeocarpa. Phytopathology 88(8):788–794. https://doi.org/10.1094/PHYTO.1998.88.8.788
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alamoudi, S.A. Using some microorganisms as biocontrol agents to manage phytopathogenic fungi: a comprehensive review. J Plant Pathol 106, 3–21 (2024). https://doi.org/10.1007/s42161-023-01542-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-023-01542-7