Abstract
This work aimed to investigate the production of prodigiosin by S. marcescens UCP 1549 in solid-state fermentation (SSF), as a sustainable alternative for reducing the production costs and environmental impact. Thus, different agro–industrial substrates were used in the formulation of the prodigiosin production medium, obtaining the maximum yield of pigment (119.8 g/kg dry substrate) in medium consisting of 5 g wheat bran, 5% waste soybean oil and saline solution. The pigment was confirmed as prodigiosin by the maximum absorbance peak at 535 nm, Rf 0.9 in TLC, and the functional groups by infrared spectrum (FTIR). Prodigiosin demonstrated stability at different values of temperature, pH and NaCl concentrations and antimicrobial properties, as well as not show any toxicity. These results confirm the applicability of SSF as a sustainable and promising technology and wheat bran as potential agrosubstrate to produce prodigiosin, making the bioprocess economic and competitive for industrial purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide demand for microbial pigments has increased in recent years due to the biotechnological potential of applying these biomolecules in several industries, in addition to its advantages, when compared to the synthetic origin (Narsing et al. 2017; Ramesh et al. 2019; Venil et al. 2020a). In this context, prodigiosin is a natural red pigment, produced mainly by the Gram-negative bacteria Serratia marcescens, which has aroused great interest due to its wide potential, as an antimicrobial, antimalarial, immunosuppressive, and antitumor agent (Li et al. 2018; Yip et al. 2019; Paul et al. 2020).
Despite of its promising properties, the large-scale production of prodigiosin is still limited due to the high cost of production, associated with low yield, and use of expensive substrates. Cheap agro–industrial byproducts and wastes have been used as alternative substrates to guarantee a profitable process of pigment production (Aruldass et al. 2014; Elkenawy et al. 2017; Venil et al. 2020b). In particular, in Brazil, S. marcescens UCP 1549, a bacterium isolated from the semiarid region of the state of Pernambuco, has shown excellent potential to produce prodigiosin, as demonstrated in the literature (Araújo et al. 2010; Lapenda et al. 2014; Montero-Rodríguez et al. 2016, 2018). However, the search for new strategies is justified, in order to increase productivity and reduce costs.
In this sense, one strategy that have gained researchers’ attention to obtain cost-effective industrial bioprocess is solid-state fermentation (SSF). This is a promising technology that has many advantages over traditional submerged fermentation (SmF), including increased production yield, easy aeration, reduced energy consumption, easy product extraction, less equipment space and decreased microbial contamination, more effectiveness, more recovery easy from an ecological point of view (Lizardi-Jiménez and Hernández-Martínez 2017; Costa et al. 2018; Sala et al. 2019).
SSF has been used in the production of pigments by different microorganisms on several substrates (Venil et al. 2017; Kaur et al. 2019; Zahan et al. 2020). However, few studies have been carried out to produce prodigiosin through SSF (Xu et al. 2011; Arivizhivendhan et al. 2015; Luti et al. 2018). Thus, the aim of this study was to evaluate the production of prodigiosin by S. marcescens UCP 1549 by SSF using different agro–industrial substrates. In addition, the stability, toxicity, and antimicrobial activity of the pigment were investigated.
Materials and methods
Microorganism
S. marcescens UCP 1549, originally isolated from semi-arid soil, was previously identified by morphological and molecular methodologies described by Araujo et al. (2017). The strain was kindly provided by the Culture Collection of the Catholic University of Pernambuco, Recife, Brazil, and it was registered in the World Federation for Culture Collections (WFCC). The bacterium was maintained in Luria Bertani (LB) solid medium (tryptone 10 g/L, yeast extract 5 g/L, NaCl 10 g/L and agar 15 g/L) at 5 °C.
Agro–industrial substrates
Six agro–industrial substrates were used in formulation of production media: wheat bran (WB), sugarcane bagasse (SCB), instant noodle waste (INW), tangerine peels (TP), pineapple peels (PAP) and pineapple crown (PAC). SCB was kindly donated by Usina Japungu, Santa Rita-PB, Brazil and INW was kindly provided by the instant noodle industry. WB, tangerines, and pineapples were bought at a local market in city of Recife-PE, Brazil. WB and INW did not receive any kind of pre-treatment. SCB was initially maintained at − 4 °C until its use, then it was thawed at room temperature, oven-dried at 70 °C for 24 h and ground in a blender. Tangerines and pineapples were washed, and the wastes were separated from the edible pulps, oven dried at 70 °C for 72 h and ground in a blender. Then, all substrates were sieved, and the fraction used was either that retained between 16 and 32 mesh sieves (opening of 1.0 and 0.5 mm, respectively). In addition, it was used waste soybean oil (WSO), kindly supplied by a local restaurant in the city of Recife (Pernambuco, Brazil). Elemental analysis (C, H, N and S) was carried out at Perkin-Elmer Series II2400 CHNS/O elemental analyzer to determine the carbon, nitrogen, hydrogen, and sulfur present in one gram of each substrates as was used to formulation of production media.
Preparation of inoculum
Stored culture of S. marcescens was first transferred to LB medium and incubated for 18 h at 28 °C. Then, two colonies were transferred to 50 mL of LB broth and incubated during 18 h at 28 °C and 150 rpm in an orbital shaker. Once the optical density at 600 nm reached 0.8–1.0, this culture was used as inoculum.
Solid-state fermentation
SSF was carried out in 250 mL Erlenmeyer flasks containing 5 g of each dry solid agrosubstrate separately. The flasks were autoclaved at 121 °C for 15 min and then, corresponding amount of impregnating solution inoculated with seed culture at 5% was mixed into solid substrates. The amount of impregnating solution for each dry agrosubstrate was defined as described by Camilios-Neto et al. (2011). The impregnating solution itself contained KH2PO4 3 g/L, K2HPO4 7 g/L, MgSO4.7H2O 0.2 g/L, (NH4)2SO4 1 g/L and 5% WSO according to Montero-Rodríguez et al. (2018). The inoculated flasks were incubated at 28 °C for 120 h, under static conditions.
Extraction and quantification of biomass produced by SSF
After the fermentation period, 50 mL of distilled water was added to each Erlenmeyer flask and contents were agitated for 1 h at 200 rpm and 30 °C on an orbital shaker. Then, the suspensions were filtered using cheesecloths and the liquid excess was squeezed out manually (Nalini and Parthasarathi 2014). This procedure was carried out three times and the extracts were collected and centrifuged for 20 min at 10,000 g. The pellets obtained were separated from the supernatants and washed three times with distilled water by centrifugation for 20 min at 10,000 g. Followed, the biomasses were frozen, subjected to lyophilisation and quantified by gravimetry (Montero-Rodríguez et al. 2018).
Extraction and quantification of pigment
The pigments produced were extracted from the lyophilized biomasses using the method used by Araújo et al. (2010) with modifications. Briefly, 1 g of biomass was subjected to solvents system chloroform: methanol of increasing polarity (2:1, 1:1 and 1:2, v/v) and pigment was evaporated to dryness and quantified by dry weight. Every step of extraction and the storage of pigment were carried out in the dark.
Characterization and identification of pigment
Preliminary identification of the crude pigment was performed after solubilization of the pigment in 95% ethanol and analysis by UV–Vis spectrophotometry, and the absorbance was determined in the range 400–700 nm. Prodigiosin production was confirmed by the presence of a maximum absorbance peak at 535 nm (Araújo et al. 2010). Subsequently, the red pigment was solubilized in 3 mL of methanol and subjected to purification by column exclusion chromatography (column 22 × 1 cm) filled with Sephadex LH-20 (activated at 800 °C for 1 h), as absorbent. The elution process was carried out by the solvent system chloroform: methanol (1:1, v/v) and then modified to chloroform: methanol: acetone (4:2:3, v/v), to the maximum removal of impurities (Lapenda et al. 2014). The red fraction was collected and subjected to thin layer chromatography (TLC). For this, the sample as applied to an aluminum foil sheet covered with silica gel and placed in glass cube containing the mixture chloroform–methanol (9:1, v/v) as mobile phase (Araújo et al. 2010; Priya et al. 2013). The retention factor (Rf) was calculated according to the formula Rf: distance traveled by the compound/ distance traveled by the solvent front and then, it was compared to the standard prodigiosin Rf referred in the literature (Krishna et al. 2011; Lapenda et al. 2015). The purified red pigment was submitted to Fourier transform infrared (FT-IR) spectroscopic analysis on the Shimadzu equipment, IR-TRACER 100, using an attenuated total reflection (ATR) accessory consisting of a mixed “diamond/ZnSe” crystal. The peaks obtained were compared with the literature to confirm the presence of prodigiosin.
Prodigiosin stability
The stability of the prodigiosin produced by S. marcescens was investigated following the methodology proposed by Perumal et al. (2009) and Velmurugan et al. (2011), with modifications. Briefly, glass test tubes containing 10 mL of the purified prodigiosin were incubated independently at different temperatures (0, 10, 50, 70 and 100 °C) for 10 min. After cooling to room temperature, absorbance was measured using a UV–visible spectrophotometer and percent stability was calculated. Another set of tubes containing 10 mL of the ethanolic extract was adjusted to pH 2, 4, 6, 8, 10, 12 and 14, homogenized for 10 min and the absorbance measured. In addition, test tubes containing 10 mL of extract were amended with 0.1%, 0.2%, 0.5%, 1% and 5% (v/v) salt solution (NaCl) and kept at rest for 1 h to determine stability. Stability (%E) was calculated according to equation below:
where A0 is pigment absorbance before treatment and A1 is absorbance after treatment. Absorbance of the pigment was measured spectrophotometrically at 535 nm.
Toxicity of prodigiosin
The phytotoxicity of purified prodigiosin produced by S. marcescens were investigated for seeds of cabbage (Brassica oleracea), lettuce (Lactuca sativa), onion (Allium cepa) and cucumber (Cucumis sativus). The test is based on the determination of three variables: the percentage of seed germination (SG%), the percentage of root growth (RG%) and the percentage of the germination index (GI%), according to Tiquia et al. (1996). Initially the seeds were washed with sterile distilled water and disinfected in a 1% sodium hypochlorite solution and washed again to remove excess hypochlorite. Then, ten seeds from each plant were transferred separately to sterile Petri dishes containing Whatman no. 1 filter paper moistened with prodigiosin solutions of concentrations of 0.1, 0.5 and 1%, and incubated at 28 °C for 120 h. the experiment was carried out in triplicate and after the incubation period, SG %, RG % and GI % were determined. In addition, toxicity test using Artemia salina microcrustacean was carried out in three stages: incubation, exposure to the substance and counting the number of live and dead nauplii after exposure to the compound in 48 h (ABNT 2016). Initially, A. salina eggs were transferred to a container with 100 mL of marine solution and incubated at 30 °C for 48 h. After this period when the larvae of A. salina hatched, 20 nauplii were transferred to 20 mL volume flasks, containing a solution of prodigiosin in concentrations of 0.01, 0.1, 1, 10 and 100 mg/l, and incubated at 25 °C for 48 h. The bioassay was based only on the percentage of dead organisms in relation to the total number (20 larvae) in 5 ml of an aqueous solution containing synthetic marine salt (33.3 g/l) and 5 ml of the different concentrations of prodigiosin samples. After 48 h of incubation, the surviving organisms were quantified and the 50% lethal concentration (LC50) of the samples was determined (Mc Laughlin et al. 1995; Jan and Khan 2016). The analyses were performed in duplicates.
Antimicrobial activity
Antimicrobial activity of purified prodigiosin was determined to quantify the growth inhibition of bacterial strains: Klebsiella pneumoniae (UCP 1574), Staphylococcus aureus (UCP 1576), Enterococcus faecalis (UCP 1577) and Escherichia coli (UCP 1578), according to the M07-A6 standard from CLSI. The tests were carried out according to the microdilution method in 96-well plates (Elshikh et al. 2016). The strains were seeded in microdilution in the 96-well plates, where 100 µl of the prodigiosin solution with a final concentration of 0.0115 g/mL was added to the first column and successive dilutions were made with Muller Hinton broth (MHB) and in the other columns 50 µl of the MHB medium was added, in sequence the inoculum of the bacteria K. pneumoniae, S. aureus, E. faecalis and E. coli was added. Previously, the strains were kept at 37 °C overnight, and then adjusted with sterile distilled water to an optical density of 625 nm with absorbance between 0.08 and 0.1 (0.5 McFarland; 107–108 CFU/mL). Then the 96-well plate was incubated at 37 °C for 24 h. After this period, resazurin (indicator of cell viability) was added at a concentration of 0.0005 g/L in 3.5 mL of sterile distilled water. After the period of 2–4 h the wells were observed in blue, indicating the minimum inhibitory concentration (MIC) of prodigiosin.
Results and discussion
Production of pigment by Serratia marcescens in solid-state fermentation
Various differential and selective media have been used for growth of prodigiosin-producing microorganism (Borić et al. 2011; Lapenda et al. 2015; Rakh et al. 2017). However, due to the high cost of synthetic components, there is a need to design new and inexpensive medium to enhance the biosynthesis of this pigment. In this sense, the use of agrosubstrates would provide a profitable alternative to reducing production costs of prodigiosin. Several agricultural products and byproducts such as corn steep liquor, cassava wastewater, brown sugar and peanut oil cake have been successfully utilized for its production (Araújo et al. 2010; Aruldass et al. 2014; Bhagwat and Padalia 2020). In addition, SSF is an alternative technology to the production of prodigiosin by SmF, which has some economic and operational disadvantages, but there are still little researches involving the production of this pigment by SSF (Xu et al. 2011; Arivizhivendhan et al. 2015; Xia et al. 2016; Majumdar et al. 2020).
In this context, in the present study different agrosubstrates were investigated for the production of biomass and prodigiosin by SSF. As shown in Table 1, the growth of the bacteria in all evaluated substrates was verified. However, the highest biomass production was found in WB (219.62 g/kg dry substrate) and INW (308.80 g/kg dry substrate). According to the elemental composition of the substrates (Table S1), the C/N ratio in WB is 15.6 while in INW is 25.1, which justifies the higher biomass yield, when compared with the other substrates.
On the other hand, according to the results presented in Table 1, pigment production was verified in all tested substrates, except for SCB. However, the presence of red pigment was found only in WB medium (Figure S1). The yellowish and greenish tones in the pigments extracted from the biomass grown in INW and TP media, and PAP and PAC media, respectively, suggested the possible impregnation of the components of the substrates to the bacterial biomass, which ended up being extracted in the extraction process. In addition, the isolated pigments were subjected to UV–Vis spectrophotometry, and the pigment produced in WB medium was the only one that showed the maximum absorbance peak at 535 nm, suggesting the presence of prodigiosin (Figure S2).
The yield of red pigment obtained in medium containing WB (119.80 g/kg dry substrate) was higher than those previously reported using SSF (Table 2), indicating the suitability of this agrosubstrate for prodigiosin production. Also, Table 3 exhibits a comparison of our results in SSF with those previously obtained in the literature by S. marcescens strains using SmF, demonstrating the effectiveness of solid-state culture for obtaining prodigiosin.
Identification of red pigment produced by S. marcescens UCP 1549 in SSF
UV–Vis spectrophotometric analysis of the red pigment produced by S. marcescens UCP 1549 in WB showed the maximum absorbance peak at 535 nm (Fig. 1), indicating the presence of prodigiosin (Patil et al. 2011; Suryawanshi et al. 2014). In addition, the Rf = 0.9 determined by TLC confirmed the correspondence of the red pigment with prodigiosin, in agreement with previous report of Araújo et al. (2010), Priya et al. (2013) and Phatake and Dharmadhikari (2016).
On the other hand, Fig. 2 shows the absorption spectrum of the partially purified red pigment after being analyzed by ATR–FTIR spectroscopy, which showed very strong absorption bands at 2922.70 cm−1 (aromatic C–H) and 1014.71 cm−1. In the fingerprint region of the pigment, it was characterized by bands of medium intensity: 1707.36 cm−1 (C = O). The wide NH absorption band was evident at 3288.96 cm−1. The spectrum obtained is similar to that of prodigiosin, as shown in the literature (Patil et al. 2011; Aruldass et al. 2014).
Relative stability of prodigiosin
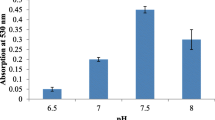
The potential application of natural pigments in various industrial fields depends on the stability against variables or extreme conditions of temperature, pH, and salinity (Velmurugan et al. 2011). In this sense, Fig. 3 illustrates the effects of temperature, pH and NaCl concentration on the absorbance spectrum of prodigiosin produced by S. marcescens UCP 1549.
As evidenced, prodigiosin showed stability when it was subjected to different values of temperature and salinity. However, in the case of pH, it showed less stability in alkaline conditions (pH 10–14), where there was a color change from pink to yellow, and consequently, a decrease in absorbance at 535 nm. Yuan et al. (2005) obtained similar results when they verified the stability of prodigiosin produced by Pseudomonas sp. at pH 2 and 5, while the pigment showed instability in alkaline conditions. The color change can be attributed to protonation/dissociation below/above the molecular dissociation constant of the pigment molecules. The presence/absence of color for a specific pigment is a function of pH due to ionization of aromatic—OH groups and tautomerism of –O (–) with = O. Changes in the relative proportions of dissociated/undissociated molecules (with respective colors) would produce the resulting coloration (Velmurugan et al. 2011).
The stability of pigments of natural origin such as the prodigiosin produced by S. marcescens UCP 1549, confirms their potential for application as an alternative to synthetic dyes, in several industrial processes, where they are generally subjected to adverse conditions, such as high temperature and acidic pH.
Toxicity of prodigiosin
Use of plants in toxicity tests offers several advantages, among them low maintenance cost and rapid results, with a special benefit assessment of the potential eco-toxic compounds in terrestrial environments (Farré and Barceló 2003; Priac et al. 2017). In this study, the germination index (GI), which combines measures of relative seed germination and relative root elongation (Santos et al. 2018; Pele et al. 2019), was used to evaluate the phytotoxic effect of prodigiosin produced by S. marcescens UCP 1549 on seeds of cabbage (Brassica oleracea), lettuce (Lactuca sativa), onion (Allium cepa) and cucumber (Cucumis sativus) (Table 4).
Considering that a GI value of 80% is commonly used as an indicator of the absence of phytotoxicity (Boutin, White and Carpenter 2010), the results obtained in the present study indicate that the tested concentrations of prodigiosin did not have an inhibitory effect on the elongation of the roots or in the germination of seeds in most of the analyzed vegetables. Only the 1% concentration of prodigiosin showed a decrease in the lettuce germination rate (67.9%). Interestingly, in the remaining plant species tested, there was the growth of abundant secondary roots, especially cabbage, with values of GI values above 200%. To our knowledge, this is the first study containing phytotoxic effect of prodigiosin produced by S. marcescens, indicating that is a nontoxic compound.
In addition, Fig. 4 displays the results of the toxicity test involving A. salina microcrustacean. All tested prodigiosin concentrations demonstrated extremely low toxicity to A. salina after 24 h of exposure. LC50 values were not calculated due to the low percentage of mortality (less than 50%). For example, 82.3% of the microcrustaceans remained alive at a concentration of 100 mg/l, whereas, at lower concentrations (0.1–10 mg/L) more than 90% remained alive, demonstrated the low degree of toxicity of the prodigiosin. The control (containing only seawater and A. salina) indicated that the larvae were not affected, thereby validating the conditions of the experiment. According to Cavalcante et al. (2000), lethality assays allow the assessment of general toxicity and should therefore be considered essential to preliminary tests involving the study of compounds with potential biological activity.
Antimicrobial activity of prodigiosin
Several studies report prodigiosin as a potent antimicrobial agent, with antagonistic effects against Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecalis, Gallionella sp., Bacillus subtilis and B. pumilus (Gulani et al. 2012; Stankovic et al. 2014; Herráez et al. 2019). Various mechanisms can explain the way through which prodigiosin affects the growth of bacterial cells. Kamble and Hiwarale (2012) proposed three: cleavage of bacterial DNA, cell cycle inhibition and modulation of pH.
In present study, the antimicrobial activity of prodigiosin produced by S. marcescens UCP 1549 was evaluated in different bacterial strains and Table 5 shows the MIC values obtained for each one. As observed, prodigiosin displayed effective antimicrobial action against the four tested pathogens; however, Gram-positive bacteria (S. aureus and E. faecalis) were more susceptible to pigment that Gram negative strains (K. pneumoniae and E. coli). Previously, Balasubramaniam et al. (2019) also reported prodigiosin with a higher activity against Gram-positive bacteria as compared with that of Gram-negative bacteria. In addition, the MIC values found in this study were lower than those reported in previous study (Ji and Kim 2019), indicating the excellent potential of prodigiosin produced by S. marcescens UCP 1549 for drug development.
Conclusions
S. marcescens UCP 1549 can efficiently convert low-cost agrosubstrates, with emphasis on WB, which has low C/N ratio, into prodigiosin in SSF. The obtained pigment proved to be non-toxic, stable at high temperature and NaCl concentration, as well as at low pH. In addition, the prodigiosin exhibited inhibitory activity against four different bacterial strains. The results in this paper demonstrated that the formulation of alternative culture medium containing new and different agrosubstrates is an efficient eco-friendly strategy to produce prodigiosin with high value and industrial applicability.
Availability of data and materials
Not applicable.
References
Araújo HWC, Fukushima K, Takaki GMC (2010) Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a low cost substrate. Molecules 15:6931–6940. https://doi.org/10.3390/molecules15106931
Araújo HWC, Andrade RFS, Montero-Rodríguez D et al (2017) Biochemical identification of molecular newly isolated pigmented bacterium, and improved production of biosurfactant. Afr J Microbiol Res 11:945–954. https://doi.org/10.5897/AJMR2016.8340
Arivizhivendhan KV, Mahesh M, Regina Mary R et al (2015) Bioactive prodigiosin isolated from Serratia marcescens using solid state fermenter and its bactericidal activity compared with conventional antibiotics. J Microb Biochem Technol 7:305–312. https://doi.org/10.4172/1948-5948.1000230
Aruldass CA, Venil CK, Zakaria ZA et al (2014) Brown sugar as a low-cost medium for the production of prodigiosin by locally isolated Serratia marcescens UTM1. Int Biodeter Biodegr 95:19–24. https://doi.org/10.1016/j.ibiod.2014.04.006
Balasubramaniam B, Alexpandi R, Darjily DR (2019) Exploration of the optimized parameters for bioactive prodigiosin mass production and its biomedical applications in vitro as well as in silico. Biocatal Agric Biotechnol 22:101385. https://doi.org/10.1016/j.bcab.2019.101385
Bhagwat A, Padalia U (2020) Optimization of prodigiosin biosynthesis by Serratia marcescens using unconventional bioresources. J Genet Eng Biotechnol 18:26. https://doi.org/10.1186/s43141-020-00045-7
Borić M, Danevčič T, Stopar D (2011) Prodigiosin from Vibrio sp. DSM 14379; a new UV-protective pigment. Microb Ecol 62:528–536. https://doi.org/10.1007/s00248-011-9857-0
Boutin C, White AL, Carpenter D (2010) Measuring variability in phytotoxicity testing using crop and wild plant species. Environ Toxicol Chem 29:327–337. https://doi.org/10.1002/etc.30
Camilios-Neto D, Bugay C, Santana-Filho AP et al (2011) Production of rhamnolipids in solid-state cultivation using a mixture of sugarcane bagasse and corn bran supplemented with glycerol and soybean oil. Appl Microbiol Biotechnol 89:1395–1403. https://doi.org/10.1007/s00253-010-2987-3
Cavalcante MF, Oliveira MCC, Velandia JR et al (2000) Síntese de 1,3,5-triazinas substituídas e avaliação da toxicidade frente a Artemia salina leach. Quím Nova 2:20–22. https://doi.org/10.1590/S0100-40422000000100005
Chávez-Castilla LR, Aguilar O (2016) An integrated process for the in situ recovery of prodigiosin using micellar ATPS from a culture of Serratia marcescens. J Chem Technol Biotechnol 91:2896–2903. https://doi.org/10.1002/jctb.4906
Costa JAV, Treichel H, Kumar V et al (2018) Advances in solid-state fermentation. In: Ashok P, Christian L, Carlos RS (eds) Current developments in biotechnology and bioengineering. Elsevier, Amsterdâm, pp 1–17. https://doi.org/10.1016/B978-0-444-63990-5.00001-3
Elkenawy NM, Yassin AS, Elhifnawy HN et al (2017) Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol Rep 14:47–53. https://doi.org/10.1016/j.btre.2017.04.001
Elshikh M, Ahmed S, Funston S et al (2016) Rezazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett 38:1015–1019. https://doi.org/10.1007/s10529-016-2079-2
Farré M, Barceló D (2003) Toxicity testing of wastewater and sewage sludge by biosensors, bioassays and chemical analysis. TrAC Trends Anal Chem 22:299–310. https://doi.org/10.1016/S0165-9936(03)00504-1
Gulani C, Bhattacharya S, Das A (2012) Assessment of process parameters influencing the enhanced production of prodigiosin from Serratia marcescens and evaluation of its antimicrobial, antioxidant and dyeing potentials. Mal J Microbiol 8:116–122
Herráez R, Mur A, Merlos A et al (2019) Using prodigiosin against some Gram-positive and Gram-negative bacteria and Trypanosoma cruzi. J Venom Anim Toxins Incl Trop Dis 25:1–8. https://doi.org/10.1590/1678-9199-jvatitd-2019
Jan S, Khan MR (2016) Protective effects of Monotheca buxifolia fruit on renal toxicity induced by CCI, in rats. BMC Complement Altern Med 16:289. https://doi.org/10.1186/s12906-016-1256-0
Ji K, Kim YT (2019) Antimicrobial activity of prodigiosin from Serratia sp. PDGS 120915 against intestinal pathogenic bacteria. Microbiol Biotechnol Lett 47:459–464. https://doi.org/10.4014/mbl.1901.01006
Kamble KD, Hiwarale VD (2012) Prodigiosin production from Serratia marcescens strain obtained from farm soil. Int J Environ Sci Technol 3:631–638. https://doi.org/10.6088/ijes.2012030131061
Kaur P, Ghoshal G, Jain A (2019) Bio-utilization of fruits and vegetables waste to produce β-carotene in solid-state fermentation: characterization and antioxidant activity. Process Biochem 76:155–164. https://doi.org/10.1016/j.procbio.2018.10.007
Krishna GJ, Basheer SM, Elyas KK et al (2011) Prodigiosin from marine bacterium: production, characterization and application as dye in textile industry. Int J Biotechnol Biochem 7:155–191
Lapenda JC, Maciel CCS, Xavier HS et al (2014) Production and toxicological evaluation of prodigiosin from Serratia marcescens UCP/WFCC1549 on Mannitol Solid Medium . Int J Appl Res Nat Prod 7:32–38
Lapenda JC, Silva PA, Vicalvi MC et al (2015) Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J Microbiol Biotechnol 31:399–406. https://doi.org/10.1007/s11274-014-1793-y
Li D, Liu J, Wang X et al (2018) Biological potential and mechanism of prodigiosin from Serratia marcescens subsp. lawsoniana in human choriocarcinoma and prostate cancer cell lines. Int J Mol 19:3465. https://doi.org/10.3390/ijms19113465
Lin C, Jia X, Fang Y et al (2019) Enhanced production of prodigiosin by Serratia marcescens FZSF02 in the form of pigment pellets. Electr J Biotechnol 40:58–64. https://doi.org/10.1016/j.ejbt.2019.04.007
Liu W, Yang J, Tian J et al (2021) An in situ extractive fermentation strategy for enhancing prodigiosin production from Serratia marcescens BWL1001 and its application to inhibiting the growth of Microcystis aeruginosa. Biochem Eng J 166:107836. https://doi.org/10.1016/j.bej.2020.107836
Lizardi-Jiménez MA, Hernández-martínez R (2017) Solid state fermentation (SSF): diversity of applications to valorize waste and biomass. 3 Biotechol 7:44. https://doi.org/10.1007/s13205-017-0692-y
Luti KJ, Yonis RW, Mahmoud ST (2018) An application of solid state fermentation and elicitation with some microbial cells for the enhancement of prodigiosin production by Serratia marcescens. ANJS 21:98–105. https://doi.org/10.22401/JNUS.21.2.15
Majumdar S, Paul I, Dey S et al (2020) Biotransformation of paper mill sludge by Serratia marcescens NITDPER1 for prodigiosin and cellulose nanocrystals: a strategic valorization approach. Biochem Eng J 164:107766. https://doi.org/10.1016/j.bej.2020.107766
Mc Laughlin JL, Saizarbitoria TC, Anderson JE (1995) Tres biosensayos simples para químicos de productos naturales. Rev Soc Venez Quím 18:13–18
Montero-Rodríguez D, Andrade RFS, Rubio-Ribeaux D et al (2016) A low-cost solid fermentation medium for potential prodigiosin production by Serratia marcescens UCP/WFCC 1549. In: Méndez-Vilas A (ed) Microb in the spotlight: recent progress in the understanding of beneficial and harmful microorganisms. Brown Walker Press, Florida, pp 312–315
Montero-Rodríguez D, Andrade RFS, Rubio-Ribeaux D et al (2018) Suitability of wheat bran as promising substrate for coproduction of prodigiosin and biosurfactant by Serratia marcescens UCP/WFCC 1549. In: Méndez-Vilas A (ed) Explore Microorganisms: Recent Advanced Applied Microbiology. BrownWalker Press, Florida, pp 149–153
Naik C, Srisevita JM, Shushma KN et al (2012) Peanut oil cake: a novel substrate for enhanced cell growth and prodigiosin production from Serratia marcescens CF-53. J Res Biol 2:549–557
Nalini S, Parthasarathi R (2014) Production and characterization of rhamnolipids produced by Serratia rubidaea SNAU02 under solid-state fermentation and its application as biocontrol agent. Bioresour Technol 173:231–238
Narsing Rao MP, Xiao M, Li WJ (2017) Fungal and bacterial pigments: secondary metabolites with wide applications. Front Microbiol 8:1113. https://doi.org/10.3389/fmicb.2017.01113
Nguyen VB, Chen SP, Nguyen TH et al (2020) Novel efficient bioprocessing of marine chitins into active anticancer prodigiosin. Mar Drugs 18:15. https://doi.org/10.3390/md18010015
Patil CD, Patil SV, Salunke BK et al (2011) Prodigiosin produced by Serratia marcescens NMCC46 as a mosquito larvicidal agent against Aedes aegypti and Anopheles stephensi. J Parasitol Res 109:1179–1187. https://doi.org/10.1007/s00436-011-2365-9
Paul T, Bandyopadhyay TK, Mondal A et al (2020) A comprehensive review on recent trends in production, purification, and applications of prodigiosin. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00928-2
Pele MA, Rubio-Ribeaux D, Viera ER et al (2019) Conversion of renewable substrates for biossurfactant production by Rhizopus arrhizus UCP 1607 and enhancing the removal of diesel oil from marine soil. Electr J Biotechnol 38:40–48. https://doi.org/10.1016/j.ejbt.2018.12.003
Perumal K, Stalin V, Chandrasekarenthiran S et al (2009) Extraction and characterization of pigment from Sclerotinia sp. and its use in dyeing cotton. Textile Res J 79:1178–1187. https://doi.org/10.1177/0040517508087680
Phatake YB, Dharmadhikari SM (2016) Physical parameters optimization for enhancement of prodigiosin production by using Serratia spp. World J Pharm Med Res 2:40–48
Priac A, Badot PM, Crini G (2017) Treated wastewater phytotoxicity assessment using Lactuca sativa: focus on germination and root elongation test parameters. C R Biol 340:188–194. https://doi.org/10.1016/j.crvi.2017.01.002
Priya KA, Satheesh S, Ashokkumar B et al (2013) Antifouling activity of prodigiosin from estuarine isolate of Serratia marcescens CMST 07. In: Velu R (ed) Microbiol Res Agroecosystem Management. Springer, India, pp 11–21. https://doi.org/10.1007/978-81-322-1087-0_2
Rakh R, Dalvi SM, Musle BB et al (2017) Production, extraction and characterization of red pigment produced by Serratia rubidaea JCM 1240T isolated from soil. Int J Curr Microbiol App Sc 6:143–154
Ramesh C, Vinithkumar NV, Kirubagaran R et al (2019) Multifaceted applications of microbial pigments: current knowledge, challenges and future directions for public health implications. Microorganisms 7:186. https://doi.org/10.3390/microorganisms7070186
Sala A, Barrena R, Artola A et al (2019) Current developments in the production of fungal biological control agents by solid-state fermentation using organic solid waste. J Crit Rev Environ Sc Technol 49:655–694. https://doi.org/10.1080/10643389.2018.1557497
Santos APP, Silva MDS, Costa EVL et al (2018) Production and characterization of a biosurfactant produced by Streptomyces sp. DPUA 1559 isolated from lichens of the Amazon region. Braz J Med Biol Res. https://doi.org/10.1590/1414-431x20176657
Stankovic N, Senerovic l, Ilic-Tomic T, et al (2014) Properties and applications of undecylprodigiosin and other bacterial prodigiosins. Appl Microbiol Biotechnol 98:3841–3858. https://doi.org/10.1007/s00253-014-5590-1
Suryawanshi RK, Patil CD, Borase H et al (2014) Studies on production and biological potential of prodigiosin by Serratia marcescens. Appl Biochem Biotechnol 173:1209–1221. https://doi.org/10.1007/s12010-014-0921-3
Tiquia SM, Tam NFY, Hodgkiss IJ (1996) Effects of composting on phytotoxicity of spent pig-manure sawdut litter. Environ Pollut 93:249–256. https://doi.org/10.1016/S0269-7491(96)00052-8
Velmurugan P, Hur H, Balachandar V et al (2011) Monascus pigment production by solid-state fermentation with corn cob substrate. J Biosci Bioeng 112:590–594. https://doi.org/10.1016/j.jbiosc.2011.08.009
Venil CK, Yusof NZB, Ahmad WA (2017) Solid state fermentation utilizing agro-industrial waste for microbial pigment production. In: Dhanarajan A (ed) Sustainable Agriculture towards Food Security. Springer, Singapore, pp 375–381. https://doi.org/10.1007/978-981-10-6647-4_20
Venil CK, Dufossé L, Renuka DP (2020a) Bacterial pigments: sustainable compounds with market potential for pharma and food industry. Front Sustain Food Syst 4:100. https://doi.org/10.3389/fsufs.2020.00100
Venil CK, Devi PR, Ahmad WA (2020b) Agro-Industrial Waste as Substrates for the Production of Bacterial Pigment. In: Zakaria Z, Boopathy R, Dib J (eds) Valorisation of Agro-industrial Residues–Volume I: Biological Approaches Appl Environ Sci Eng Sustain Future. Springer, Cham, pp 149–162
Xia Y, Wang G, Lin X et al (2016) Solid-state fermentation with Serratia marcescens Xd-1 enhanced production of prodigiosin by using bagasse as an inertia matrix. Ann Microbiol 66:1239–1247. https://doi.org/10.1007/s13213-016-1208-4
Xu F, Xia S, Yang Q (2011) Strategy for obtaining inexpensive prodigiosin production by Serratia marcescens. In: 3rd international conference on chemical. Biol Environ Eng. IACSIT Press, Sigapore, 20: p 32–36
Yip CH, Yarkoni O, Ajioka J et al (2019) Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl Microbiol Biotechnol 103:1667–1680. https://doi.org/10.1007/s00253-018-09611-z
Yuan B, Qingping DU, Chuanghua CAI et al (2005) Study on the extraction and stability of pigments from a marine bacterium Pseudomonas sp. Mar Sci Bulletin. 6:1–6
Zahan KA, Ismail NS, Ring LC et al (2020) Monascorubin production by Penicillium minioluteum ED24 in a solid-state fermentation using sesame seed cake as substrate. Mater Today 31:127–135. https://doi.org/10.1016/j.matpr.2020.01.347
Acknowledgements
This work was financially supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FACEPE (Fundação de Amparo à Ciência e Tecnologia de Pernambuco). The authors are grateful to NPCIAMB (Núcleo de Pesquisas em Ciências Ambientais e Biotecnologia) of Catholic University of Pernambuco for the use of the laboratories and to LABMAQ (Laboratório Multiusuário de Análises Químicas) of Chemistry Department of the Federal Rural University of Pernambuco by chemical analysis.
Funding
This work was financially supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FACEPE (Fundação de Amparo à Ciência e Tecnologia de Pernambuco).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflicts of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
dos Santos, R.A., Rodríguez, D.M., da Silva, L.A.R. et al. Enhanced production of prodigiosin by Serratia marcescens UCP 1549 using agrosubstrates in solid-state fermentation. Arch Microbiol 203, 4091–4100 (2021). https://doi.org/10.1007/s00203-021-02399-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02399-z