Abstract
Summary
This study revealed the change in the paravertebral muscles in patients with osteoporotic vertebral fracture. Increased pain is likely to be the driver for reduced activity, reduced activities of daily living, and consequent increase in fat infiltration of the paravertebral muscles, assumed to be secondary to reduced activity level or, conversely, partial immobilization.
Introduction
To reveal the time courses and impact of the paravertebral muscles (PVMs) on the healing process of osteoporotic vertebral fractures and risk factors for PVM decrease.
Methods
Consecutive patients with symptomatic osteoporotic vertebral fractures were enrolled in 11 hospitals. At enrollment and 3- and 6-month follow-up, PVMs, including the multifidus and erector spinae, were examined using magnetic resonance imaging (MRI). The PVM cross-sectional area (CSA) and fat signal fraction (FSF) were measured at L3. Low back pain (LBP), activities of daily living (ADLs), and risk factors for PVM decrease at the 6-month follow-up were investigated. PVM decrease was defined as > 1 standard deviation decrease of the CSA or > 1 standard deviation increase of the FSF.
Results
Among 153 patients who completed the 6-month follow-up, 117 (92 women, 79%) had MRI of L3 at enrollment and 3- and 6-month follow-up (mean age at enrollment, 78.5 years). The CSA did not change 6 months from onset (p for trend = 0.634), whereas the FSF significantly increased (p for trend = 0.033). PVM decrease was observed in 30 patients (26%). LBP was more severe, and delayed union was more frequent in patients with PVM decrease (p = 0.021 mixed-effect model and p = 0.029 chi-square test, respectively). The risk factors for PVM decrease were ADL decline at the 3-month follow-up (adjusted odds ratio = 5.35, p = 0.026).
Conclusion
PVM decrease was significantly related to LBP and delayed union after osteoporotic vertebral fracture onset. ADL decline at the 3-month follow-up was a risk factor for PVM decrease. Therefore, restoring ADLs within 3 months after onset is important.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporotic vertebral fracture is a common disease with a prevalence of approximately 50% in 80-year-old Japanese female patients [1]. Osteoporotic vertebral fracture has a significant impact on the health status of elderly people and is a very important health issue in the aging society. Clinical osteoporotic vertebral fractures can result in a decline in the patients’ quality of life and survival rate [2, 3]. On the other hand, there is no universal treatment strategy for osteoporotic vertebral fractures. To decrease pain and immobility in acute vertebral fracture, the use of spinal orthoses becomes inevitable [4]. However, there is no apparent difference in the compression ratio, bony union rate, and clinical outcome according to the conservative treatment [5, 6]. Therefore, it is very important to reveal further the risk factors for poor outcome after osteoporotic vertebral fractures, and many studies have investigated factors related to poor outcome [5, 7, 8].
The paravertebral muscles (PVMs) have a substantial role in low back pain (LBP), sagittal balance, and quality of life [9,10,11]. Concomitant with the loss of muscle, aging also results in progressive bone loss, leading to bone fragility and increased risk for osteoporosis and fractures [12]. Regarding osteoporotic vertebral fractures, Sinaki et al. [13] demonstrated that individuals who performed spinal flexion and bending exercises increased their risk of developing further vertebral compression fractures. In contrast, another study showed that back strengthening exercises are helpful for decreasing the risk of further osteoporotic vertebral fractures [14]. Exercise can be beneficial for reducing the occurrence of vertebral fracture, improving disequilibrium, and decreasing the risk of falls [15]. However, the change in the PVMs after osteoporotic vertebral fracture injury remains unclear. The PVMs may also have a substantial role in the healing process of fractured vertebrae and patient’s outcome. A better understanding of the change in the PVMs after osteoporotic vertebral fractures is critical to the development of more effective strategies to improve osteoporotic vertebral fracture treatment. However, the natural course of changes in the PVMs following osteoporotic vertebral fractures is unclear. Kim et al. [16] demonstrated that osteoporotic patients with osteoporotic vertebral fractures showed reduction of CSA and increased intramuscular fat infiltration compared to those without osteoporotic vertebral fractures. So et al. [17] showed the increase of fatty infiltration at L3. However, they did not evaluate the time course after the onset of osteoporotic vertebral fractures. The purpose of this study was to reveal the time courses and impact of the PVMs on the healing process of osteoporotic vertebral fractures and the risk factors for the decrease.
Methods
This was a multicenter cohort study in 11 institutions [18]. From 2012 to 2015, 153 patients with symptomatic osteoporotic vertebral fractures were followed up for 6 months after the onset of osteoporotic vertebral fractures. The inclusion criteria were age of > 65 years, diagnosis of a recent osteoporotic vertebral fracture, and back pain onset within 2 weeks prior to presentation. The exclusion criteria were pathological fractures, multiple fractures, malignant cancer, dementia, and high-energy injury.
Demographic data included age, sex, and body mass index (BMI). BMI was computed as the ratio of the body mass in kg divided by the height in m2. The severity of pain was subjectively assessed by the patients with a visual analog scale (VAS), which was based on the average level of back pain that the patient had felt in the previous week. To evaluate the patients’ activities of daily living (ADLs), we used the criteria proposed by the long-term care insurance system of the Japanese Health and Welfare Ministry for evaluation of the degree of independence of disabled elderly individuals [8]. In rank J, although some disability is present, daily life is almost independent and patients can leave the home without assistance from other individuals; in rank A, patients live independently indoors but require assistance to leave the home; in rank B, patients require some assistance living indoors and spend most of the day in bed but are able to sit up, and in rank C, patients spend all day in bed and require assistance with urination/defecation, dressing, and eating. We divided the ranks into two groups, that is, J, A (dependent or requires assistance to leave home) and B, C (bedridden or nearly bedridden). Magnetic resonance imaging (MRI), radiographic examinations, VAS, and ADLs were evaluated at enrollment and at 1, 3, and 6 months after the onset.
During treatment, 30% of patients wore tailor-made hard corsets, 62% wore tailor-made elastic corsets, 4% wore ready-made elastic corsets, and 4% did not wear a corset. Mobilization to an erect position and ambulation were basically allowed immediately when the brace had been applied. Medication includes common analgesics, such as acetaminophen and non-steroidal anti-inflammatory drugs. Rehabilitation was performed during admission only for patients who were admitted. The rehabilitation focused on early activity, not according to any study protocol. No back-muscle exercise was prescribed during the study period.
Imaging assessment
For measuring the CSA and fatty infiltration of the lumbar paraspinal muscles, MRI was recommended because of the higher reliability and absence of radiation exposure compared to computed tomography [19].
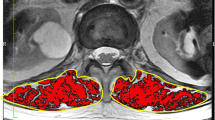
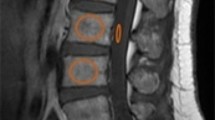
At the time of enrollment and at the 3- and 6-month follow-ups, the patients were examined using plain radiography and spine MRI. MRI was carried out with 1.5-T MRI scanners. The multifidus (MF) and erector spinae (ES) including the longissimus and iliocostalis were identified as the medial-to-lateral PVMs. The border between the MF and ES was scouted from the mammillary processes [20]. The cross-sectional area (CSA) was calculated by the average of the left and right PVM regions of interest of the axial T2-weighted image. The fat signal fraction (FSF) was calculated as the ratio of signal fat/CSA × 100. The CSA and FSF at the superior endplate of L3 were measured using a software program (Horos, version 2.0.1, Horos Project). PVM decrease was defined as > 1 standard deviation (SD) decrease in the CSA (0.9 cm2) or > 1 SD increase in the FSF (5.7%) at the 6-month follow-up compared with the values at the time of injury.
Plain radiographs were assessed on the sagittal view in both the supine and weight-bearing positions. The relative height of the anterior wall (%) was calculated by the following formula: [2 × affected vertebral height / (lower vertebral height + upper vertebral height)] × 100 [18]. When either the cranial or caudal adjacent vertebral body was deformed due to an old fracture, the vertical height of the anterior wall of the fractured vertebral body was divided by the vertical height of the anterior wall of the undeformed adjacent vertebral body. Delayed union was defined by a recognizable intravertebral cleft on plain radiography at the 6-month follow-up. Dual-energy x-ray absorptiometry was used to measure the bone mineral density (BMD) of the mean femoral neck at the time of enrollment.
Data analysis
Jonckheere’s trend test was used to evaluate the trend of the CSA and FSF of ES, MF, and total PVM. To establish whether there was a significant difference in the VAS score of back pain, CSA, and FSF of ES, MF, and total PVM between the PVM decrease and non-decrease groups, a restricted maximum likelihood, mixed-model regression was used. T test was used for continuous variables. Either the chi-square test or Fisher’s exact test was used to compare the categorical variables. The odds ratio (OR) of each variable for PVM decrease was calculated using a logistic regression model adjusted for age, sex, BMI, old osteoporotic vertebral fractures, admission, institution, and ADLs at each time point, or delayed union. Demographic data and those with a p value of < 0.1 in the univariate analysis were included in the multivariate analysis. The variable of delayed union was entered in the model, but that of angular motion between the supine and sitting positions was not because of multiple collinearity. Additionally, the variables of ADLs, VAS of back pain, and delayed union were separately inserted into the model because of multiple collinearity. Statistical test results were considered significant at p < 0.05. All p values were two-sided. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Among the 153 eligible patients, 40 patients were excluded because of the lack of MRI data including axial T2-weighted image at L3. As a result, 117 patients (92 women and 25 men) with a mean age of 78.5 years (standard deviation 7.4) were finally analyzed.
Regarding the trend of the CSA and FSF, there were no trends in the CSA of ES, MF, and total PVM (Table 1). On the other hand, the FSF in both ES and MF showed an increasing tendency from 42.3% ± 7.8% and 53.0% ± 8.9% to 44.2% ± 8.3% and 55.4% ± 9.3%, respectively, for 6 months (p = 0.078 and 0.055, respectively). The FSF of total PVM showed a significant increase from 47.7 to 49.8% for the period (p = 0.033). The mean change in the CSA and FSF of total PVM was − 0.2 cm2 ± 0.6 cm2 and 2.1% ± 3.6%, respectively. There were 30 patients with PVM decrease at the 6-month follow-up.
There were no differences in age, BMI, brace duration, BMD, and fractured level between the PVM decrease and non-decrease groups (Table 2). The male proportion was significantly lesser in the PVM decrease group (p = 0.023). Old osteoporotic vertebral fractures were more frequently observed in the PVM decrease group (50% vs. 28%, respectively, p = 0.042,). Table 3 shows that the VAS score of back pain was significantly higher in the PVM decrease group throughout the study period (p = 0.021). Compression ratio and kyphotic angle of fractured vertebrae were not different between the two groups. However, the angular motion between the supine and sitting positions was significantly higher in the PVM decrease group (p = 0.013). Delayed union was more frequently observed in the PVM decrease group than in the non-decrease group (33% vs. 14%, p = 0.029). Regarding ADLs, the status of “bedridden or nearly bedridden” was more frequently observed in the PVM decrease group at the 3-month follow-up (38% vs. 15%, p = 0.019), whereas no significant differences were found at the other time points (Table 4).
The results of the multivariate logistic regression model are listed in Table 5. Compared with the status of “dependent or requires assistance to leave home,” patients with the status of “bedridden or nearly bedridden” at the 3-month follow-up displayed a fourfold increase of the risk for PVM decrease (OR = 5.35; 95% CI, 1.23–23.34). Patients who had delayed union at 6-month follow-up also increased the OR for PVM decrease (OR = 4.12; 95% CI, 1.16–14.71). The admission significantly reduced the risk for PVM decrease (OR = 0.24; 95% CI, 0.06–0.97). VAS of back pain at the 6-month follow-up was associated with PVM decrease (OR = 1.24; 95% CI, 1.02–1.49). A typical case was shown in supplementary figure. The case showed the increase of the FSF at 3- and 6-month follow-up.
Discussion
This study is the first to reveal the natural course of the PVMs after the injury of osteoporotic vertebral fractures. The CSA did not change for 6 months, but the FSF significantly increased. CSA might be unlikely to reflect the early change in the PVMs compared with the FSF. This result may be consistent with the age effect of PVM change. Shahidi et al. [21] quantified changes in the CSA and FSF with age in men and women with lumbar spine pathology. There was no change in CSA with age in either sex (p > 0.05), but there was an increase in the FSF with age in the ES and MF muscles in both sexes (p < 0.001). Hebert et al. [10] described that fatty infiltration appears earlier in individuals with a low back disorder. In addition, a cross-sectional study [22] demonstrated that paraspinal fat infiltration, but not muscle CSA, was associated with disability and structural abnormalities in the lumbar spine. Therefore, the FSF might be more useful when observing PVM change. Fatty infiltration in the muscles could be associated with mechanisms, such as generalized disuse, chronic denervation, and aging [23].
LBP is reported as a risk factor for PVM decrease. Although Paalanne et al. [24] evaluated the PVMs of 554 young adults (18–21 years of age) and showed that the CSA of the lumbar paraspinal muscles did not correlate with symptom severity, a significantly higher fat content in the MF muscle was found in patients with chronic LBP than in asymptomatic volunteers [25]. D’hooge et al. [22] showed that fatty infiltration in the lean muscle tissue was increased, without alterations in muscle size or macroscopic fat deposition during remission of LBP, and they demonstrated the positive correlation between fatty infiltration and LBP episode frequency. In the current longitudinal study, patients with PVM decrease showed an increase in LBP. Moreover, Hori et al. [9] demonstrated that trunk muscle mass was significantly associated with quality of life and VAS score for LBP. Our study showed that back pain at the 6-month follow-up was associated with PVM decrease. However, the causality was unclear.
So et al. [17] demonstrated in a cross-sectional study that fat infiltration of low back extensor muscle was increased in patients with osteoporotic vertebral fractures. Kim et al. [16] also conducted a cross-sectional study to investigate the PVM influence on postmenopausal osteoporotic vertebral fractures. Postmenopausal osteoporotic vertebral fractures were associated with reduction of CSA and increased fat infiltration [16]. However, the causality of PVM decrease and osteoporotic vertebral fracture was unclear. The current study demonstrated that the FSF deteriorated from 47.7 to 49.8% for 6 months. This change is greater than one in the FSF of the PVMs, from 28.8 to 31.6%, during 9 years among individuals aged 40 years [26], while there has been no report revealing serial changes in the PVMs in the elderly. Our study suggests that the FSF increased after osteoporotic vertebral fractures, indicating that osteoporotic vertebral fractures result in PVM decline, although our study did not demonstrate whether PVM decline increased the risk of osteoporotic vertebral fracture or not.
Movement of vertebrae requires the PVMs to contract and work against the levers of an internal skeleton. Disuse or unweighting of the muscle-bone unit in immobilized individuals results in a dramatic loss of bone and muscle mass [12]. Muscular atrophy of extremities regularly occurs as a consequence of immobilization or disuse [27]. To avoid atrophy of supported back muscles, the use of spinal supports may need to be discontinued as soon as pain subsides [28]. Significant changes in muscle weight and fiber size are reported in the first week of immobilization. The patients with osteoporotic vertebral fractures are not able to move the spine causing disuse atrophy of the PVMs. Osteoporotic vertebral fractures also alter neuromuscular patterns in individuals who have sustained vertebral fracture compared to those who have no history of vertebral fracture, and this may be interpreted as one of the sequelae of vertebral fractures [29]. Our study showed that ADL decline (bedridden or nearly bedridden) for 3 months was associated with PVM decrease. However, ADL decline at the 6-month follow-up was not associated with PVM decrease. The reason might be that the patients with poor ADLs at the 6-month follow-up had poorer ADLs before injury, which might be linked to less change. On the other hand, patients who were admitted to the hospital had reduced risk for PVM decrease. This might indicate that rehabilitation prevented disuse atrophy despite immobilization by brace treatment for 3 months.
There are several limitations in this study. First, we did not check the other risk factors including low levels of vitamin D associated with high levels of parathyroid hormone, low levels of anabolic hormones in men and menopause in women, malnutrition, and frailty [22]. These factors could be at a higher risk for PVM reduction. Second, this study did not include controls who had no osteoporotic vertebral fractures. It was unclear that PVM decrease was dependent on aging or osteoporotic vertebral fractures. Third, nerve root compression was one of the risk factors for fat infiltration, but radiculopathy was not assessed. Finally, a prior episode of LBP was not assessed in this study. If chronic LBP was present, the course of LBP after osteoporotic vertebral fractures might be influenced by the other causes.
In conclusion, the FSF significantly increased for 6 months after osteoporotic vertebral fracture injury. ADL decline at the 3-month follow-up was a risk factor for PVM decline. LBP at the 6-month follow-up was higher in patients with PVM decline. Another treatment strategy should be considered in patients with ADL decline at the 3-month follow-up.
References
Horii C, Asai Y, Iidaka T, Muraki S, Oka H, Tsutsui S, Hashizume H, Yamada H, Yoshida M, Kawaguchi H, Nakamura K, Akune T, Tanaka S, Yoshimura N (2019) Differences in prevalence and associated factors between mild and severe vertebral fractures in Japanese men and women: the third survey of the ROAD study. J Bone Miner Metab 37(5):844–853. https://doi.org/10.1007/s00774-018-0981-5
Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ 3rd (1993) Population-based study of survival after osteoporotic fractures. Am J Epidemiol 137(9):1001–1005. https://doi.org/10.1093/oxfordjournals.aje.a116756
Tosteson AN, Gabriel SE, Grove MR, Moncur MM, Kneeland TS, Melton LJ 3rd (2001) Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int 12(12):1042–1049. https://doi.org/10.1007/s001980170015
Sinaki M, Pfeifer M, Preisinger E, Itoi E, Rizzoli R, Boonen S, Geusens P, Minne HW (2010) The role of exercise in the treatment of osteoporosis. Curr Osteoporos Rep 8(3):138–144. https://doi.org/10.1007/s11914-010-0019-y
Hoshino M, Tsujio T, Terai H, Namikawa T, Kato M, Matsumura A, Suzuki A, Takayama K, Takaoka K, Nakamura H (2013) Impact of initial conservative treatment interventions on the outcomes of patients with osteoporotic vertebral fractures. Spine (Phila Pa 1976) 38(11):E641–E648. https://doi.org/10.1097/BRS.0b013e31828ced9d
Kim HJ, Yi JM, Cho HG, Chang BS, Lee CK, Kim JH, Yeom JS (2014) Comparative study of the treatment outcomes of osteoporotic compression fractures without neurologicinjury using a rigid brace, a soft brace, and no brace a prospective randomized controlled non-inferiority trial. J Bone Joint Surg Am 96(23):1959–1966. https://doi.org/10.2106/JBJS.N.00187
Matsumoto T, Hoshino M, Tsujio T, Terai H, Namikawa T, Matsumura A, Kato M, Toyoda H, Suzuki A, Takayama K, Takaoka K, Nakamura H (2012) Prognostic factors for reduction of activities of daily living following osteoporotic vertebral fractures. Spine (Phila Pa 1976) 37(13):1115–1121. https://doi.org/10.1097/BRS.0b013e3182432823
Takahashi S, Hoshino M, Takayama K, Iseki K, Sasaoka R, Tsujio T, Yasuda H, Sasaki T, Kanematsu F, Kono H, Toyoda H, Nakamura H (2016) Predicting delayed union in osteoporotic vertebral fractures with consecutive magnetic resonance imaging in the acute phase: a multicenter cohort study. Osteoporos Int 27(12):3567–3575. https://doi.org/10.1007/s00198-016-3687-3
Hori Y, Hoshino M, Inage K, Miyagi M, Takahashi S, Ohyama S, Suzuki A, Tsujio T, Terai H, Dohzono S, Sasaoka R, Toyoda H, Kato M, Matsumura A, Namikawa T, Seki M, Yamada K, Habibi H, Salimi H, Yamashita M, Yamauchi T, Furuya T, Orita S, Maki S, Shiga Y, Inoue M, Inoue G, Fujimaki H, Murata K, Kawakubo A, Kabata D, Shintani A, Ohtori S, Takaso M, Nakamura H (2019) ISSLS PRIZE IN CLINICAL SCIENCE 2019: clinical importance of trunk muscle mass for low back pain, spinal balance, and quality of life-a multicenter cross-sectional study. Eur Spine J 28(5):914–921. https://doi.org/10.1007/s00586-019-05904-7
Hebert JJ, Kjaer P, Fritz JM, Walker BF (2014) The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: a 9-year population-based prospective cohort study. Spine (Phila Pa 1976) 39(17):1417–1425. https://doi.org/10.1097/BRS.0000000000000424
Yagi M, Hosogane N, Watanabe K, Asazuma T, Matsumoto M, Keio Spine Research Group (2016) The paravertebral muscle and psoas for the maintenance of global spinal alignment in patient with degenerative lumbar scoliosis. Spine J 16(4):451–458. https://doi.org/10.1016/j.spinee.2015.07.001
DiGirolamo DJ, Kiel DP, Esser KA (2013) Bone and skeletal muscle: neighbors with close ties. J Bone Miner Res 28(7):1509–1518. https://doi.org/10.1002/jbmr.1969
Sinaki M, Mikkelsen BA (1984) Postmenopausal spinal osteoporosis: flexion versus extension exercises. Arch Phys Med Rehabil 65(10):593–596
Sinaki M, Itoi E, Wahner HW, Wollan P, Gelzcer R, Mullan BP, Collins DA, Hodgson SF (2002) Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone 30(6):836–841. https://doi.org/10.1016/S8756-3282(02)00739-1
Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR (2005) Significant reduction in risk of falls and back pain in osteoporotic-kyphotic women through a spinal proprioceptive extension exercise dynamic (SPEED) program. Mayo Clin Proc 80(7):849–855. https://doi.org/10.4065/80.7.849
Kim JY, Chae SU, Kim GD, Cha MS (2013) Changes of paraspinal muscles in postmenopausal osteoporotic spinal compression fractures: magnetic resonance imaging study. J Bone Metab 20(2):75–81. https://doi.org/10.11005/jbm.2013.20.2.75
So KY, Kim DH, Choi DH, Kim CY, Kim JS, Choi YS (2013) The influence of fat infiltration of back extensor muscles on osteoporotic vertebral fractures. Asian Spine J 7(4):308–313. https://doi.org/10.4184/asj.2013.7.4.308
Takahashi S, Hoshino M, Takayama K, Iseki K, Sasaoka R, Tsujio T, Yasuda H, Sasaki T, Kanematsu F, Kono H, Toyoda H, Nakamura H (2017) Time course of osteoporotic vertebral fractures by magnetic resonance imaging using a simple classification: a multicenter prospective cohort study. Osteoporos Int 28(2):473–482. https://doi.org/10.1007/s00198-016-3737-x
Hu ZJ, He J, Zhao FD, Fang XQ, Zhou LN, Fan SW (2011) An assessment of the intra- and inter-reliability of the lumbar paraspinal muscle parameters using CT scan and magnetic resonance imaging. Spine (Phila Pa 1976) 36(13):868–874. https://doi.org/10.1097/BRS.0b013e3181ef6b51
Crawford RJ, Filli L, Elliott JM, Nanz D, Fischer MA, Marcon M, Ulbrich EJ (2016) Age- and level-dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. AJNR Am J Neuroradiol 37(4):742–748. https://doi.org/10.3174/ajnr.A4596
Shahidi B, Parra CL, Berry DB, Hubbard JC, Gombatto S, Zlomislic V, Allen RT, Hughes-Austin J, Garfin S, Ward SR (2017) Contribution of lumbar spine pathology and age to paraspinal muscle size and fatty infiltration. Spine (Phila Pa 1976) 42(8):616–623. https://doi.org/10.1097/BRS.0000000000001848
Teichtahl AJ, Urquhart DM, Wang Y, Wluka AE, Wijethilake P, O'Sullivan R, Cicuttini FM (2015) Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community-based adults. Spine J 15(7):1593–1601. https://doi.org/10.1016/J.SPINEE.2015.03.039
Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW (2006) Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders. Spine (Phila Pa 1976) 31(22):E847–E855. https://doi.org/10.1097/01.brs.0000240841.07050.34
Paalanne N, Niinimäki J, Karppinen J, Taimela S, Mutanen P, Takatalo J, Korpelainen R, Tervonen O (2011) Assessment of association between low back pain and paraspinal muscle atrophy using opposed-phase magnetic resonance imaging: a population-based study among young adults. Spine (Phila Pa 1976) 36(23):1961–1968. https://doi.org/10.1097/BRS.0b013e3181fef890
Mengiardi B, Schmid MR, Boos N, Pfirrmann CW, Brunner F, Elfering A, Holder J (2006) Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology 240(3):786–792. https://doi.org/10.1148/radiol.2403050820
Hebert JJ, Kjaer P, Fritz JM, Walker BF (2014) The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: a 9-year population-based prospective cohort study. Spine (Phila Pa 1976) 39(17):1417–1425. https://doi.org/10.1097/BRS.0000000000000424
Appell HJ (1990) Muscular atrophy following immobilisation. A review. Sports Med 10(1):42–58. https://doi.org/10.2165/00007256-199010010-00005
Walsh NE, Schwartz RK (1990) The influence of prophylactic orthoses on abdominal strength and low back injury in the workplace. Am J Phys Med Rehabil 69(5):245–250. https://doi.org/10.1097/00002060-199010000-00004
Briggs AM, Greig AM, Bennell KL, Hodges PW (2007) Paraspinal muscle control in people with osteoporotic vertebral fracture. Eur Spine J 16(8):1137–1144. https://doi.org/10.1007/s00586-006-0276-8
Funding
This study was funded by the Grant of Japan Orthopedics and Traumatology Research Foundation (Grant no. 270).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
This study was approved by the Ethics Committee of Osaka City University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1235 kb)
Rights and permissions
About this article
Cite this article
Takahashi, S., Hoshino, M., Takayama, K. et al. The natural course of the paravertebral muscles after the onset of osteoporotic vertebral fracture. Osteoporos Int 31, 1089–1095 (2020). https://doi.org/10.1007/s00198-020-05338-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05338-8