Abstract
Summary
Due to the lack of diagnostics in primary health care, over 75 % of osteoporotic patients are not diagnosed. A new ultrasound method for primary health care is proposed. Results suggest applicability of ultrasound method for osteoporosis diagnostics at primary health care.
Introduction
We lack effective screening and diagnostics of osteoporosis at primary health care. In this study, a new ultrasound (US) method is proposed for osteoporosis diagnostics.
Methods
A total of 572 Caucasian women (age 20 to 91 years) were examined using pulse-echo US measurements in the tibia and radius. This method provides an estimate of bone mineral density (BMD), i.e. density index (DI). Areal BMD measurements at the femoral neck (BMDneck) and total hip (BMDtotal) were determined by using axial dual-energy X-ray absorptiometry (DXA) for women older than 50 years of age (n = 445, age = 68.8 ± 8.5 years). The osteoporosis thresholds for the DI were determined according to the International Society for Clinical Densitometry (ISCD). Finally, the FRAX questionnaire was completed by 425 participants.
Results
Osteoporosis was diagnosed in individuals with a T-score −2.5 or less in the total hip or femoral neck (n = 75). By using the ISCD approach for the DI, only 28.7 % of the subjects were found to require an additional DXA measurement. Our results suggest that combination of US measurement and FRAX in osteoporosis management pathways would decrease the number of DXA measurements to 16 % and the same treatment decisions would be reached at 85.4 % sensitivity and 78.5 % specificity levels.
Conclusions
The present results demonstrate a significant correlation between the ultrasound and DXA measurements at the proximal femur. The thresholds presented here with the application to current osteoporosis management pathways show promise for the technique to significantly decrease the amount of DXA referrals and increase diagnostic coverage; however, these results need to be confirmed in future studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a skeletal disorder which manifests itself as an accelerated bone loss, deteriorates mechanical integrity of bone, and exposes individuals to an increased risk of fractures. It is estimated that 200 million people worldwide have osteoporosis [1]. Approximately 75 % of osteoporotic patients are not diagnosed and do not receive treatment for this pathological condition [2]. Post-menopausal women are associated with the highest risk of morbidity; up to 30 % of Caucasian women over 50 years of age suffer from osteoporosis [3]. The lifetime risk of an osteoporotic fracture after the age of 50 years has been estimated to be 40–53 and 13–22 % for women and men, respectively [4]. During the first year after a hip fracture, over 24 % of the patients at or over 65 years of age will die [5]. In both sexes, the highest mortality rates are associated with fractures of the proximal femur [6].
By the definition of the World Health Organization (WHO), osteoporosis is diagnosed when bone mineral density (BMD) is 2.5 standard deviations (SD) or more below the mean for young adults [3]. Diagnosis is based on the determination of BMD by dual-energy X-ray absorptiometry (DXA) at the one-third radius, femoral neck, total hip, or lumbar spine [7]; however, clinical practice guidelines recommend the use of central sites for diagnosis [8–10]. Currently, DXA is considered as the gold standard for osteoporosis diagnosis. Due to bulky instrumentation, ionizing radiation, and costs, it is not an optimal method for point-of-care diagnostics and cannot provide optimal solution for the management of osteoporosis. A true need to screen individuals with high risk of fracture or osteoporosis in primary health care exists to improve treatment coverage. Further, referrals to special care and costs due to traveling may be significantly reduced if indications for treatment could be established at primary care.
The International Osteoporosis Foundation and many national guidelines have suggested the use of the WHO fracture risk assessment tool FRAX® for fracture risk estimation and first-line osteoporosis screening in primary health care [11]. However, FRAX has been demonstrated to exhibit high specificity but low sensitivity for diagnosing osteoporosis [12]. Therefore, axial DXA measurement may be considered for subjects with intermediate FRAX scores. Guidelines for the application of FRAX are different in the UK (National Osteoporosis Guideline Group (NOGG)) and USA (National Osteoporosis Foundation (NOF)) [8, 9]. The UK approach has also been applied in Finnish current care recommendations [10].
Ultrasound methods have been proposed for screening and diagnosing osteoporosis ever since the introduction of the first quantitative ultrasound (QUS) parameters in 1984 [13]. Several commercial devices have been introduced for through-transmission measurement of the calcaneus, and manufacturers have presented device-specific T-score values, comparing the values of measured ultrasound parameters to those in young reference population. Due to poor correlation with central BMD measurements by DXA, the T-score values obtained using the two methods are different, and therefore, calcaneal ultrasound techniques cannot be recommended for the diagnostics of osteoporosis [14].
Another line of commercial ultrasound products includes axial transmission devices. These devices are based on transmission of an ultrasound pulse to the bone cortex, sound propagation along the cortical layer parallel to the long axis of the bone, and receiving it with another ultrasound transducer at a known distance. Similarly to the calcaneal devices, the low correlation (0.35–0.36) with central DXA hinders the use of the technique for diagnostics [15, 16].
Pulse-echo (PE) ultrasound methods have been introduced for a simple measurement of cortical thickness, known to typically decrease in osteoporosis [17, 18]. More recently, these methods have been applied to multi-site measurement of cortical thickness and, moreover, construction of a parameter called the density index (DI), an estimate for proximal femur BMD [19]. The DI has been shown to correlate significantly with the femoral neck BMD [19].
According to the International Society for Clinical Densitometry (ISCD) recommendations, the use of peripheral X-ray devices in the diagnosis of osteoporosis should be based on determination of 90 % sensitivity and specificity thresholds against axial DXA [20, 21]. The same approach has been proposed for the diagnostic use of ultrasound [22]. The aim of the present study is to determine the association between proximal femur BMD and novel pulse-echo ultrasound measurement and suggest the diagnostic thresholds for DI. Preliminary analyses on treatment pathway (UK with Finnish thresholds), including FRAX and ultrasound, were also evaluated.
Methods
Subjects
A total of 572 women (age 20 to 91 years) participated in the study. The subjects were tested at two locations, at Bone and Cartilage Research Unit (BCRU, Mediteknia, University of Eastern Finland, Kuopio, Finland; subjects 1, n = 226) and Kuopio University Hospital (KUH, Kuopio, Finland; subjects 2, n = 346). A subgroup of women aged over 50 years (subjects 3, n = 448, age = 68.8 ± 8.5 years, height = 159.6 ± 6.4 cm, weight = 67.5 ± 12.9 kg) was formed for the analysis of diagnostic thresholds for DI and DXA measurements (threshold group). No exclusion criteria were applied in selecting the subjects. The subjects 1 belonged to the population-based Osteoporosis Risk Factor and Prevention (OSTPRE) study [23]. The majority of the subjects 2 (n = 346) were referred for DXA examination at KUH due to clinical considerations for osteoporosis by a general practitioner (e.g., long periods of glucocorticoid treatment or other risk factors for osteoporosis). The study was approved by the local ethical committee, and written consent was obtained from each subject (Kuopio University Hospital Ethical Committee, permit 80/2008 and 39/2012).
Ultrasound measurements
Ultrasound measurements were conducted using two similar PE ultrasound device prototypes (Bindex BI-100, Bone Index Finland Ltd., Kuopio, Finland, Software v.2.0). Ultrasound measurements were done by an experienced nurse and a nurse student. The device consists of a pulser unit plugged into the USB port of a laptop and a focused ultrasound probe (3.0 MHz nominal center frequency) (Fig. 1a). US measurements were conducted at 1/3 of the length of the radius from the distal head and 1/3 of the length of the tibia from the proximal and distal heads, respectively (Fig. 1b). The length of the tibia was measured as the distance between the medial malleolus and knee joint space (top of the medial condyle) and at the radius by determining first the distance between the olecranon and styloid process of the ulna and second transferring mark on top of the radius. Apparent cortical thickness (Ct.Th.) was determined at the distal radius (Ct.Thrad) and at the proximal (Ct.Thprox) and distal (Ct.Thdist) tibia. The method for cortical thickness measurement has been described earlier in detail [17, 19]. A strict 30-min time slot was reserved in total for each patient for ultrasound and DXA examination. Standard BMD measurement typically required 15 min. The protocol for ultrasound measurements started from the radius, moving to the proximal tibia and finally the distal tibia. Five repetitions were made at each location. Due to the strict timetable, three subjects had no measurements at the proximal tibia and seven subjects had no measurements at the distal tibia. In total, all three sites were measured for 439 subjects out of 448 in the threshold group.
The density index, DI, an estimate for total hip BMD, is formed as a combination of patient characteristics (age, weight, and height) and cortical thickness measurements [19]. The ultrasound device provides two indices based on (1) a single-site measurement of cortical thickness (Ct.Thprox) (i.e., DI1) and (2) a three-site measurement of cortical (Ct.Thrad), (Ct.Thprox), and (Ct.Thdist) (i.e., DI3). The reproducibility of the DI1 was assessed by two different operators. A total of 16 subjects were measured for three times, relocating the measurement sites (sites were marked with a water-soluble marker pen and wiped clear before the next repeated measurement) according to the method previously described by Gluer et al. [24]. The two ultrasound prototype devices were calibrated by measuring three locations in 20 subjects with both devices. The two prototypes showed high linear correlation in cortical bone thickness values (r = 0.96, n = 60, p < 0.001), and the mean absolute error between the cortical bone thickness measurements was 0.2 mm.
DXA measurements
Axial DXA (Lunar Prodigy, GE Healthcare Ltd, Pollards Wood, UK) measurements of BMD, conducted along the guidelines of the manufacturer, were made in women over the age of 50 (subjects 3, n = 448, age = 68.8 ± 8.5 years). BMD values were recorded for the femoral neck (BMDneck) and total hip (BMDtotal). The subject was diagnosed with osteoporosis if the T-score at either the femoral neck or total hip was −2.5 or less. Finnish reference thresholds for osteoporosis were 0.684 g/cm2 for the femoral neck and 0.708 g/cm2 for the total hip. At both measurement sites (KUH and Mediteknia), the same manufacturer and model of DXA device was used. Cross-calibration was performed by measuring the spine phantom (DPA/QDR-1, Hologic Inc., Waltham, MA, USA) 10 times with both devices [25]. The difference between the measured mean values of phantom BMD was 0.2 %, considered to be negligible, and thus was not accounted for in analyses.
Threshold analyses
The diagnostic osteoporosis thresholds for the DI (90 % sensitivity and specificity thresholds for osteoporosis) were determined in accordance with the guidelines of the ISCD [20, 21] and the National Osteoporosis Society (NOS) [26]. The upper threshold for the DI was calculated so that 90 % of osteoporotic subjects were below the threshold. The lower threshold was calculated so that 90 % of healthy subjects were over the threshold. According to the guidelines, subjects who fall between the thresholds would require additional DXA measurement to verify diagnosis.
Osteoporosis intervention pathway and FRAX®
A total of 425 subjects (out of subjects 3, n = 448) completed the FRAX questionnaire during their examination visit. The 10-year risks of major osteoporotic and hip fractures were calculated based on body mass index (BMI) (FRAXBMI) and BMD (major osteoporotic fracture risk FRAXBMD,MAJOR, hip fracture risk FRAXBMD,HIP). The basic application of FRAX (i.e., the fracture risk score determined without BMD), as applied according to the National Osteoporosis Guideline Group (NOGG), involves classification of subjects in high, intermediate, or low risk groups based on age-dependent risk thresholds. Treatment would be recommended for those with high fracture risk. For those with intermediate fracture risk, reassessment is recommended after bone density scan (axial DXA).
According to the NOGG guidelines [9], FRAX would be used as a screening tool for case finding among men and women aged 50 years or more and treatment could be considered for individuals with FRAX scores above the country-specific intervention thresholds. In this study, a FRAXBMD score for major osteoporotic or hip fracture risk over the Finnish treatment threshold or osteoporosis at the femoral neck or hip was considered an indication for treatment to which the treatment paths (including FRAXBMI and US examination) were compared. In this approach, DI1 assessment was considered for those with an intermediate fracture risk by FRAXBMI (Fig. 2).
An approach for application of ultrasound method in the case-finding strategy of the National Osteoporosis Guideline Group (NOGG) for those who fall on the yellow category by FRAX. This approach showed 85.4 % sensitivity and 78.5 % specificity when compared with treatment decision obtained by FRAXBMD and DXA. Only 16 % out of the total 425 subjects would require additional DXA measurement
Statistical analyses
The Pearson’s correlation analysis was applied when associating normally distributed parameters. The statistical difference between the parameter values in the subjects in different groups was tested using the Mann-Whitney U test.
Results
Threshold analyses
In the threshold group (subjects 3, n = 448), a total of 75 subjects were diagnosed with osteoporosis using the Finnish reference database thresholds (T-score at or below −2.5 at the femoral neck (n = 66) or total hip (n = 42)). The BMD measurements at the total hip or femoral neck were significantly correlated (r = 0.89, n = 448). Among cortical thickness measurements, Ct.Thprox showed the highest correlation with BMDtotal (r = 0.54, p < 0.0001). For density indices, DI3 (cortical thickness measurements at three sites) showed a stronger correlation with BMDtotal (n = 439, r = 0.69, p < 0.0001) than DI1 (single-site measurement) (n = 445, r = 0.62, p < 0.0001). The average reproducibility (CVrms for two operators) of the DI1 and DI3 measurements were 2.5 and 2.1 %, respectively. Both DI1 and DI3 showed statistically significant differences between the osteoporotic (T-score ≤−2.5 at the hip or femoral neck) and healthy group (T-score >−2.5) (p < 0.01). The average values and standard deviations for osteoporotic and healthy subjects for DI1 were 0.781 ± 0.056 and 0.870 ± 0.079, respectively, and for DI3 0.797 ± 0.068 and 0.912 ± 0.089, respectively.
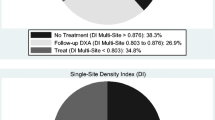
For DI1, the upper (90 % sensitivity) and lower (90 % specificity) thresholds were 0.844 and 0.779, respectively, and for DI3, 0.876 and 0.803, respectively (Fig. 3). By measuring DI3, 126 (28.7 %) of the subjects would fall between the thresholds and would therefore require an additional examination by axial DXA to verify diagnosis. Out of the 126 subjects between the thresholds, 82.5 % had an osteopenic/osteoporotic T-score (<−1.0) at either the femoral neck or total hip. With DI1, the number of subjects between the thresholds was 32.6 %.
Multi-site DI3 vs. minimum T-score (either femoral neck or total hip). Vertical dashed lines indicate T-score thresholds for osteoporosis (T-score −2.5) and osteopenia (T-score −1.0). Horizontal dashed lines indicate upper and lower thresholds for DI3. Significant correlation between DI3 and T-score minimum was observed (r = 0.65, p < 0.0001)
Cortical thickness analyses
Cortical thickness, as a function of patient age, decreased non-linearly at all locations. The correlation coefficients of the second-order polynomial fit between the cortical thickness and age at the distal radius and proximal and distal tibia were r = 0.60, r = 0.45, r = 0.21, respectively (n = 572). Average cortical thickness varied at different locations and was highest at the distal tibia (3.5 ± 0.8 mm), whereas the mean values at the distal radius (2.5 ± 0.5 mm) and proximal tibia (2.6 ± 0.7 mm) locations were similar. The difference in cortical thickness between the osteoporotic and healthy groups was significant at all locations (p < 0.05).
The average values and standard deviations of Ct.Thrad, Ct.Thprox, and Ct.Thdist for osteoporotic and healthy subjects were 2.1 ± 0.4 and 2.6 ± 0.5, 2.0 ± 0.5 and 2.7 ± 0.0.6, and 2.9 ± 0.7 and 3.6 ± 0.7, respectively.
Preliminary treatment pathway analyses
A total of 137 subjects were selected for treatment when the Finnish thresholds based on FRAXBMD,major, FRAXBMD,hip, or DXA T-score (≤−2.5 at the hip or femoral neck) were applied. The treatment decisions based on FRAXBMI (if all subjects between the thresholds receive DXA examination) showed high sensitivity (94.9 %) and specificity (86.8 %) with treatment decisions based on FRAXBMD,major/hip or DXA. However, the number of DXA examinations needed with the FRAXBMI approach was high, i.e., 244 subjects (57.0 %) or even 357 subjects (84 %) if DXA measurement is considered also for subjects with a high fracture risk FRAXBMI. Application of the ultrasound examination to the treatment pathway (Fig. 2.) showed a specificity of 78.5 % and sensitivity of 85.4 %. This approach would reduce the percentage of required DXA examinations down to 16 %. Table 1 shows the distribution of classified subjects by using FRAXBMI or by using FRAXBMI with the present ultrasound method.
Discussion
The density index revealed a statistically significant correlation with the proximal femur BMD, suggesting that the use of a new ultrasound testing in osteoporosis diagnosis can be effective, with 29–33 % of patients requiring diagnostic verification. The performance of DI seems better than that previously reported for subjects in need of diagnostic verification after peripheral DXA or calcaneal ultrasound measurement (39–50 and 56 %, respectively) [21, 22].
The majority of the subjects (78 %, excluding subjects 1 from OSTPRE cohort) included in threshold analyses were recruited from those who had been referred to DXA by a general practitioner on the suspicion of reduced BMD resulting from possible disease or based on other relevant factors or medical treatment. On the other hand, it should be noted that ultrasound examination for screening or diagnosis in primary health care could be performed when there is a reason to suspect osteoporosis. Therefore, the present results support well the intended use of the device and examination of the question at hand. This is in line with the recommendation of the ISCD for selecting subjects in the threshold analysis.
The treatment decisions based on FRAXBMI agree well with those obtained by DXA or FRAXBMD, as assessed in line with the Finnish thresholds. However, the high percentage (57 %) of additional DXA examinations needed diminishes the feasibility of this screening method. When the US measurement was performed on those with intermediate fracture risk, indicated by FRAX, the sensitivity and specificity were 85.4 and 78.5 %, respectively. This is due to the higher number of false positives in the approach where only FRAXBMI is applied.
The threshold values of density index are now suggested for use in Finnish Caucasian female population. This is particularly important as several studies have shown that T-scores determined with any other device than axial DXA cannot be used for the diagnosis of osteoporosis or osteopenia according to the WHO criteria [14, 22]. Since the introduction in 2005, the diagnostic thresholds for peripheral devices have been adopted by international and national societies for the management of osteoporosis [20, 22, 26]. The relationship to patient characteristics and cortical thicknesses in the derivation of DI may vary in populations with different ethnic backgrounds and should be addressed in future studies. Another limitation in the clinical use of DI is that there is no data showing its feasibility for follow-up of treatment. However, the use of the thresholds determined in comparison to DXA allows the investigated ultrasound method to pick up the same patients for treatment from which they can be assumed to benefit.
It is important to note that the present study involved BMD measurements at the proximal femur only. Therefore, the present thresholds apply for the detection of individuals with osteoporotic BMD values at the hip. The present threshold values do not present a definitive classification of osteoporosis but rather high or low probability for the presence of osteoporosis at the hip. Another important limitation is the fact that same population was first applied to develop DI and then used to assess the performance in different osteoporosis management pathways. However, these results suggest how the ultrasound method could be applied with current OP management scenarios. However, they do not completely validate the performance of the technique in these settings.
The present results suggest that ultrasound testing may be beneficial as a first-line screening method or after the FRAX questionnaire for those with an intermediate FRAX score and in need of bone density assessment. Application of US testing to the evaluated osteoporosis management guideline could reduce the required number of DXA examinations. The present results suggest that the performance of the DI is similar or better than those with the currently available peripheral DXA devices and substantially better than the other currently available ultrasound devices. The present thresholds for the application of DI to current osteoporosis management pathways show promise for the technique to decrease the amount of DXA referrals and increase diagnostic coverage; however, these results need to be confirmed in future studies.
References
Reginster JY, Burlet N (2006) Osteoporosis: a still increasing prevalence. Bone 38(2 Suppl 1):S4–S9
Nguyen TV, Center JR, Eisman JA (2004) Osteoporosis: underrated, underdiagnosed and undertreated. Med J Aust 50:S18–S22
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4(6):368–381
Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporos Int 16(Suppl 2):S3–S7
Keene GS, Parker MJ, Pryor GA (1993) Mortality and morbidity after hip fractures. BMJ 307(6914):1248–1250
Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA (1999) Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 353(9156):878–882
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42(3):467–475
National Osteoporosis Foundation (2013) Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC, National Osteoporosis Foundation
Compston J, Bowring C, Cooper A, Cooper C, Davies C, Francis R et al (2013) Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas 73:392–396
Osteoporosis [Internet]. Helsinki: The Finnish Medical Society Duodecim, Finnish Endocrine Society, Finnish Gynecological Association; 2014 [updated 24.04.2014; cited 30.5.2014]. Available from: http://www.kaypahoito.fi/web/kh/suositukset/suositus?id=hoi24065
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY (2012) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24(1):23–57
Cummins MN, Poku EK, Towler MR, O’Driscoll MO, Ralston SH (2011) Clinical risk factors for osteoporosis in Ireland and the UK: a comparison of FRAX and QFractureScores. Calcif Tissue Int 89(2):172–177
Langton CM, Palmer SB, Porter RW (1984) The measurement of broadband ultrasonic attenuation in cancellous bone. Eng Med 13(2):89–91
Nayak S, Olkin I, PhD, Liu H, Grabe M, Gould MK, Allen IE et al (2006) Meta-analysis: accuracy of quantitative ultrasound for identifying patients with osteoporosis. Ann Intern Med: 832–41
Damilakis J, Papadokostakis G, Perisinakis K, Maris T, Dimitriou P, Hadjipavlou A et al (2004) Discrimination of hip fractures by quantitative ultrasound of the phalanges and the calcaneus and dual X-ray absorptiometry. Eur J Radiol 50(3):268–272
Wang SF, Chang CY, Shih C, Teng MMH (1997) Evaluation of tibial cortical bone by ultrasound velocity in oriental females. Br J Radiol 70(839):1126–1130
Karjalainen J, Riekkinen O, Töyräs J, Kröger H, Jurvelin JS (2008) Ultrasonic assessment of cortical bone thickness in vitro and in vivo. IEEE Trans Ultrason Ferroelectr Freq Control 55(10):2191–2197
Wear KA (2003) Autocorrelation and cepstral methods for measurement of tibial cortical thickness. IEEE Trans Ultrason Ferroelectr Freq Control 50(6):655–660
Karjalainen J, Riekkinen O, Töyräs J, Hakulinen M, Kröger H, Rikkonen T et al (2012) Multi-site bone ultrasound measurements in elderly women with and without previous hip fractures. Osteoporos Int 23(4):1287
Hans DB, Shepherd JA, Schwartz EN, Reid DM, Blake GM, Fordham JN et al (2008) Peripheral dual-energy X-ray absorptiometry in the management of osteoporosis: the 2007 ISCD Official Positions. J Clin Densitom 11(1):188–206
Blake GM, Chinn DJ, Steel SA, Patel R, Panayiotou E, Thorpe J et al (2005) A list of device-specific thresholds for the clinical interpretation of peripheral x-ray absorptiometry examinations. Osteoporos Int 16:2149–2156
Krieg MA, Barkmann R, Gonnelli S, Stewart A, Bauer DC, Barquero L et al (2008) Quantitative ultrasound in the management of osteoporosis: the 2007 ISCD official positions. J Clin Densitom 11(1):163–187
Honkanen R, Tuppurainen M, Kröger H, Alhava E, Saarikoski S (1998) Relationships between risk factors and fractures differ by type of fracture: a population based study of 12,192 perimenopausal women. Osteoporos Int 8(1):25–31
Gluer CC, Blake GM, Lu Y, Blunt A, Jergas M, Genant HK (1995) Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 5:262
Shepherd JA, Lu Y, Wilson K, Fuerst T, Genant HK, Hangartner TN et al (2006) Cross-calibration and minimum precision standards for dual-energy X-ray absorptiometry: the 2005 ISCD Official Positions. J Clin Densitom 9(1):31–36
Patel R, Blake GM, Fordham JN, McCrea D, Ryan P (2011) Peripheral X-ray absorptiometry in the management of osteoporosis. National Osteoporosis Society - Practical Guides
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Janne P Karjalainen owns stocks and is an employee at Bone Index Finland Ltd. Ossi Riekkinen owns stocks and is an employee at Bone Index Finland Ltd. Juha Töyräs has no disclosures. Jukka S Jurvelin owns stocks at Bone Index Finland Ltd. Heikki Kröger owns stocks at Bone Index Finland Ltd.
Rights and permissions
About this article
Cite this article
Karjalainen, J.P., Riekkinen, O., Töyräs, J. et al. New method for point-of-care osteoporosis screening and diagnostics. Osteoporos Int 27, 971–977 (2016). https://doi.org/10.1007/s00198-015-3387-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3387-4