Abstract
Recently two algorithms have become available to estimate the 10-year probability of fracture in patients suspected to have osteoporosis on the basis of clinical risk factors: the FRAX algorithm and QFractureScores algorithm (QFracture). The aim of this study was to compare the performance of these algorithms in a study of fracture patients and controls recruited from six centers in the United Kingdom and Ireland. A total of 246 postmenopausal women aged 50–85 years who had recently suffered a low-trauma fracture were enrolled and their characteristics were compared with 338 female controls who had never suffered a fracture. Femoral bone mineral density was measured by dual-energy X-ray absorptiometry, and fracture risk was calculated using the FRAX and QFracture algorithms. The FRAX algorithm yielded higher scores for fracture risk than the QFracture algorithm. Accordingly, the risk of major fracture in the overall study group was 9.5% for QFracture compared with 15.2% for FRAX. For hip fracture risk the values were 2.9% and 4.7%, respectively. The correlation between FRAX and QFracture was R = 0.803 for major fracture and R = 0.857 for hip fracture (P ≤ 0.0001). Both algorithms yielded high specificity but poor sensitivity for prediction of osteoporosis. We conclude that the FRAX and QFracture algorithms yield similar results in the estimation of fracture risk. Both of these tools could be of value in primary care to identify patients in the community at risk of osteoporosis and fragility fractures for further investigation and therapeutic intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Osteoporosis is a common condition characterized by low bone mass and an increased risk of low-trauma fracture [1, 2]. Currently, the diagnosis of osteoporosis is based on bone densitometry, and the disease is defined to exist when bone mineral density (BMD) values at the spine or hip fall 2.5 standard deviations (SD) or more below normal values in young healthy individuals. Although BMD values can be measured conveniently and noninvasively by dual-energy X-ray absorptiometry (DXA), not all physicians have access to this test. In addition, many patients who suffer fragility fractures do not have osteoporosis as defined by DXA [3], demonstrating that complementary approaches are required to develop new techniques to better identify patients at risk of fragility fractures. Reflecting this fact, a recent report by the World Health Organization (WHO) has recommended that research be conducted into the use of alternative technologies to DXA [4].
Osteoporosis is a multifactorial disease, and many clinical risk factors (CRFs) for susceptibility to the disease have been identified [5–11], some of which increase the risk of fracture independently of BMD [12]. This has led to the development of algorithms to assess fracture risk on the basis of CRFs in the absence of BMD measurements. The first of these to be developed was the FRAX algorithm, which used data from nine prospective population-based cohorts (190,000 patient-years) from Europe, North America, Australia, and Japan [13] and validated the performance in 11 independent population-based cohorts (1.2 million person-years) [14]. The FRAX algorithm is country-specific as fracture rates vary considerably in different countries [15].
A limitation of the FRAX tool is that for several of the CRFs, such as corticosteroid use, alcohol, and smoking, no account is taken of the magnitude of exposure and that no information is collected on falls, an important risk factor for fragility fracture [16, 17]. In order to address this and other issues, the QFractureScores algorithm (QFracture) was developed to estimate fracture risk based on CRFs alone [18]. The QFracture tool was developed using data from a prospective cohort study of 1,183,663 females and 1,174,232 males in the United Kingdom (15.9 million person-years), and the validation cohort was composed of 642,153 females and 633,764 males [18]. QFracture uses many of the CRFs included in FRAX in addition to other variables that influence fracture risk (Table 1). Unlike FRAX, the QFracture algorithm does not incorporate BMD or previous fractures but does include more detailed information on dose response for variables like alcohol intake and smoking habit. The age range has also been extended in the QFracture algorithm (30–85 years) to allow for assessment of younger patients [18].
The aim of this study was to compare the performance of the FRAX and QFracture algorithms in identifying patients who suffered fractures in a case–control study of postmenopausal women recruited from the United Kingdom and Ireland.
Materials and Methods
Cases and controls were recruited as part of a multicenter study in Ireland and the United Kingdom. Subject recruitment by geographical region was as follows: England n = 237, Ireland n = 88, Scotland n = 195, and Wales n = 64. The study received ethical approval (MREC 07/Q1704/1), and all subjects gave written informed consent to participate. All participants were Caucasian women aged 50–85 years who were at least 5 years postmenopausal. To fully assess the performance of both tools in the identification of patients at risk of future fracture, participants included subjects who had recently suffered a fracture (cases) as well as individuals who had never suffered a fracture (controls). All fracture cases had suffered a low-trauma fracture at the hip, spine, humerus, pelvis, or wrist after the age of 45 years, whereas controls were subjects who had never sustained a fracture during adulthood (age > 18 years). We excluded subjects who were receiving treatment for osteoporosis, those on corticosteroids, and those with a secondary cause of osteoporosis such as malabsorption, chronic liver disease, renal failure, and malignant disease.
FRAX scores were calculated manually from the FRAX Web site (www.shef.ac.uk/FRAX), with double data entry in 10% of subjects. The UK version of FRAX was used for all subjects as an Irish version of FRAX is not currently available. The 10-year probabilities of major osteoporotic and hip fracture with and without BMD were recorded for FRAX. Values for QFracture were assessed using the published algorithm (Web version 1) as implemented at www.qfracture.org, and the 10-year probabilities of major osteoporotic and hip fracture were recorded.
Statistical analysis was performed using SPSS software V17 (Microsoft, San Diego, CA). Descriptive statistics are presented as mean values and SDs. Variables were tested for normality using the Kolmogorov-Smirnov test. Depending on the normality of the distribution, Pearson’s or Spearman’s correlation coefficients were calculated to examine the relationship between variables. Student’s t-test or the Mann-Whitney U-test was used, as appropriate, for comparisons of two groups. Receiver operating curve (ROC) analysis was performed to calculate sensitivity, specificity, and area under the curve (AUC) values.
Results
Characteristics of the Study Sample
Characteristics of the study population are summarized in Table 2. There was a significant difference in age (P ≤ 0.01), history of falls (P ≤ 0.0001), and femoral BMD/T score (P ≤ 0.0001) between the fracture cases and controls. The most common fracture type was wrist fracture (n = 173, 70%), followed by fractures of the humerus (n = 30, 12%), hip (n = 22, 9%), and spine (n = 21, 9%).
A history of falls was recorded in 39% of the total sample (n = 227), and 32% of subjects had a family history of osteoporosis (n = 188). A diagnosis of osteoporosis was confirmed in 15% of the sample (n = 87), and 57% were identified as osteopenic (n = 333). Body mass index (BMI) ranged from 15.8–49.3 kg/m2 with 2% underweight (n = 10), 32% normal weight (n = 188), 40% overweight (n = 234), and 26% obese (n = 152). Current smokers comprised 15% of the sample (n = 87), and 33% were ex-smokers (n = 190), with 9% of subjects (n = 50) reporting an alcohol intake of ≥14 U/week.
Absolute Fracture Risk
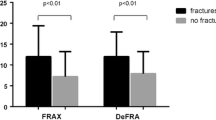
The estimated absolute fracture risks are summarized in Table 3. The risks generated by the QFracture algorithm were consistently lower than those calculated by the FRAX algorithm (excluding a BMD measurement). In the total study sample the mean major fracture risk for QFracture was 9.5% (range 1.7–37.0%) vs. 15.2% (3.4–49.0%) for FRAX. Similarly, for hip fracture risk the values were 2.9% (range 0.2–29.6%) and 4.7% (0.2–36.0%), respectively. Overall, however, there was a significant correlation between the risks calculated by QFractureScores and FRAX, as demonstrated in Fig. 1. The correlation was r = 0.803 for major fracture and r = 0.857 for hip fracture (P ≤ 0.0001).
The FRAX algorithm can also produce absolute risks including a BMD measurement; therefore, these risks were also calculated in this data set. Major fracture risk for the fracture cases was 18.7 ± 8.2 compared with 10.7 ± 5.3 for the control subjects (P < 0.0001). Hip fracture risk for the cases was 5.3 ± 6.2 compared with 2.4 ± 3.4 for the control subjects (P < 0.0001). Overall, when the risks calculated for FRAX (excluding BMD) were compared with FRAX (including BMD) (n = 584), a significant difference was observed for estimated major fracture (P ≤ 0.05) and hip fracture (P ≤ 0.0001) risks.
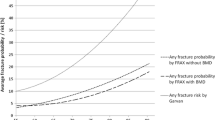
Sensitivity and specificity for prediction of osteoporosis (as defined by femoral BMD) were calculated for QFracture and FRAX (excluding BMD). Both algorithms yielded high specificity but poor sensitivity (Table 4, Fig. 2) for prediction of osteoporosis as defined by DXA.
Discussion
The use of CRFs to estimate 10-year probability of fracture is a significant advance in the management of osteoporosis. The aim of this study was to compare the performance of the FRAX and QFracture algorithms in a case–control study of postmenopausal women. In this study there was a significant difference in age (66 vs. 68 years, P ≤ 0.01), history of falls (69 vs. 158, P ≤ 0.0001), and femoral T score (−1.2 vs. −1.7, P ≤ 0.0001) between the control and fracture groups, respectively. This was not unexpected as these risk factors are among the strongest for fracture [19, 20]; however, the history of falls data was striking. A history of falls was recorded in 39% of the total study sample. Fall history is included in the QFracture tool [18] but not in the FRAX tool. This might explain why QFractureScores had greater specificity for prediction of major fractures and hip fractures than the FRAX score, although this was counterbalanced by a poorer sensitivity.
The absolute fracture risk values calculated in this study are broadly in agreement with previous published findings, where QFracture predicted lower risks than FRAX [18, 21]. We think that the lower scores with the QFracture algorithm are likely due to the fact that this tool does not take previous fracture into account, which is a strong risk factor for future fracture [22]. Reflecting this fact, the differences in estimates of fracture risk between FRAX and QFracture were much greater in the cases than in the controls (Table 3). It is not possible to determine from this cross-sectional study, however, whether fracture risk is underestimated by QFracture or overestimated by FRAX. Although the algorithms differed, there were similarities between estimates, with an overall correlation between FRAX and QFracture of r = 0.803 for major fracture and r = 0.857 for hip fracture (P ≤ 0.0001). Although both algorithms yielded high specificity for the detection of osteoporosis as defined by DXA, sensitivity was poor.
At present, there is no universally accepted policy for population screening in the United Kingdom to identify individuals with osteoporosis or those at high risk of fracture [23], which has been driven by the cost–benefit equation using the current diagnostic tools available. A potential advantage of QFracture is the collection of data on many more risk factors than FRAX, but an important limitation is that it does not take previous fracture into account. Most of the variables assessed in the QFracture algorithm may have already been gathered in patients’ electronic general practice record in the United Kingdom or could be collected by a clinician during a standard consultation [18]. The FRAX score is also easy and quick to calculate and can be used with or without the inclusion of a femoral neck BMD measurement [21]. Some studies have indicated that FRAX does not offer significant advantages over less complex models that also incorporate BMD [24, 25].
Both FRAX and QFracture are platform technologies, which theoretically could be upgraded as new validated risk indicators become available [26]. However, these additional risk factors would need to be validated on the original populations.
This study has some limitations, including its retrospective nature, case–control design, and relatively small sample size. However, to the best of the authors’ knowledge, this is the first study to compare the FRAX and QFracture tools in an independent sample.
There has been considerable debate as to how FRAX should be used in routine clinical practice. The procedure implemented on the UK National Osteoporosis Guideline Group Web site suggests that patients at high risk of fracture on the basis of FRAX should be treated without recourse to DXA, although this remains controversial since the vast majority of randomized controlled trials of osteoporosis therapies have focused on patients with osteoporosis as defined by DXA [27–29]. Until further evidence emerges to demonstrate that targeting patients for therapy on the basis of absolute fracture risk is effective, it could be that the optimal use of FRAX and/or QFracture might be as a prescreening tool to identify patients who should be referred for DXA.
References
Melton LJ, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL (1998) Bone density and fracture risk in men. J Bone Miner Res 13:1915–1923
Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I et al (2000) Long-term risk of osteoporotic fracture in Malmö. Osteoporos Int 11:669–674
Schuit SC, Van der Klift M, Weel AE, De Laet C, Burger H et al (2004) Fracture incidence and association with bone mineral density in elderly mean and women: the Rotterdam study. Bone 34:195–202
Organisation World Health (2004) WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level. WHO, Brussels
Kanis JA, Johnell O, De Laet C, Johansson H, Oden A et al (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382
Kanis JA, Johansson H, Oden A, Johnell O, De Laet C et al (2004) A family history of fracture and fracture risk: a meta-analysis. Bone 35:1029–1037
Kanis JA, Johansson H, Oden A, Johnell O, de Laet C et al (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19:893–899
Kanis JA, Johnell O, Oden A, Johansson H, De Laet C et al (2005) Smoking and fracture risk: a meta-analysis. Osteoporos Int 16:222–228
Kanis JA, Johansson H, Johnell O, Oden A, De Laet C et al (2005) Alcohol intake as a risk factor for fracture. Osteoporos Int 16:737–742
Kanis JA, Johansson H, Oden A, De Laet C, Johnell O et al (2005) A meta-analysis of milk intake and fracture risk: low utility for case finding. Osteoporos Int 16:799–804
De Laet C, Kanis JA, Odén A, Johanson H, Johnell O et al (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16:1330–1338
De Laet C, Odén A, Johansson H, Johnell O, Jönsson B et al (2005) The impact of the use of multiple risk indicators for fracture on case-finding strategies: a mathematical approach. Osteoporos Int 16:313–318
Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O et al (2005) Assessment of fracture risk. Osteoporos Int 16:581–589
Kanis JA, Oden A, Johnell O, Johansson H, De Laet C et al (2007) The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int 18:1033–1046
Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A et al (2002) International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res 17:1237–1244
Ward KD, Klesges RC (2001) A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int 68:259–270
Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A et al (2008) Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med 121:406–418
Hippisley-Cox J, Coupland C (2009) Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ 339:b4229
Johnell O, Kanis JA, Oden A, Johansson H, De Laet C et al (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194
Cummings SR, Nevitt MC (1994) Non-skeletal determinants of fractures: the potential importance of the mechanics of falls. Study of Osteoporotic Fractures Research Group. Osteoporos Int 4:67–70
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
Black DM, Arden NK, Palermo L, Pearson J, Cummings SR (1999) Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. J Bone Miner Res 14:821–828
Compston J, Cooper A, Cooper C, Francis R, Kanis JA et al (2009) Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas 62:105–108
Ensrud KE, Lui LY, Taylor BC, Schousboe JT, Donaldson MG et al (2009) A comparison of prediction models for fractures in older women: is more better? Arch Int Med 169:2087–2094
Pluskiewicz W, Adamczyk P, Franek E, Leszczynski P, Sewerynek E et al (2010) 10-year probability of osteoporotic fracture in 2012 Polish women assessed by FRAX and nomogram by Nguyen et al.—conformity between methods and their clinical utility. Bone 46:1661–1667
Kanis JA, Oden A, Johanson H, Borgstrom F, Strom O et al (2009) FRAX and its applications to clinical practice. Bone 44:734–743
Siris ES, Simon JA, Barton IP, McClung MR, Grauer A (2008) Effects of risedronate on fracture risk in postmenopausal women with osteopenia. Osteoporos Int 19:681–686
Rogers A, Glover SJ, Eastell R (2009) A randomised, double-blinded, placebo-controlled, trial to determine the individual response in bone turnover markers to lasofoxifene therapy. Bone 45:1044–1052
Finkelstein JS, Wyland JJ, Lee H, Neer RM (2010) Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 95:1838–1845
Acknowledgments
The authors gratefully acknowledge the financial support of the Enterprise Ireland Innovation Partnership grant board. The authors also sincerely acknowledge the assistance of all the participating subjects.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cummins, N.M., Poku, E.K., Towler, M.R. et al. Clinical Risk Factors for Osteoporosis in Ireland and the UK: A Comparison of FRAX and QFractureScores. Calcif Tissue Int 89, 172–177 (2011). https://doi.org/10.1007/s00223-011-9504-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-011-9504-2