Abstract
Interaction with the immune system is one of the most recently established nonclassic effects of vitamin D (VitD). For many years, this was considered to be limited to granulomatous diseases in which synthesis of active 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) or calcitriol is known to be increased. However, recent reports have supported a role for 1,25(OH)2D3 in promoting normal function of the innate and adaptive immune systems. Crucially, these effects seem to be mediated not only by the endocrine function of circulating calcitriol but also via paracrine (i.e., refers to effects to adjacent or nearby cells) and/or intracrine activity (i.e., refers to a hormone acting inside a cell) of 1,25(OH)2D3 from its precursor 25(OH)D3, the main circulating metabolite of VitD. The ability of this vitamin to influence human immune responsiveness seems to be highly dependent on the 25(OH)D3 status of individuals and may lead to aberrant response to infection or even to autoimmunity in those who are lacking VitD. The potential health significance of this has been underlined by increasing awareness of impaired status in populations across the globe. This review will examine the current understanding of how VitD status may modulate the responsiveness of the human immune system. Furthermore, we discuss how it may play a role in host resistance to common pathogens and how effective is its supplementation for treatment or prevention of infectious diseases in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to the traditional role of vitamin D (VitD) in skeletal homeostasis, there has been a great deal of interest about its activity in regulating several other facets of human health. VitD induces differentiation and inhibits proliferation of various normal and cancer cells, while evidence suggests for different roles of VitD and of its active metabolites in a large number of tissues [1]. There has also been a great deal of interest in the potential role of VitD in host resistance to infections, which came as a result of the following four findings [2–6]:
-
1.
The immune system is able to produce the enzyme (cytochrome 27B1 (CYP27B1) = 25-hydroxyvitamin D3 1-α-hydroxylase) that converts the circulating form of VitD (25-hydroxyvitamin D3 (25(OH)D3)) to its active form (1,25-dihydroxyvitamin D3 (1,25(OH)2D3)) or calcitriol;
-
2.
The majority of immune system cells possesses VitD receptors (VDR) mainly after they have been stimulated;
-
3.
1,25(OH)2D3 production in the immune system led to the induction of antibacterial products such as cathelicidin which in turn inhibited replication of Mycobacterium tuberculosis in vitro;
-
4.
And finally, impaired VitD status is common to many population across the globe and could contribute to the increased burden of common infectious diseases across the world.
The potential immunomodulatory roles of VitD are furthermore of interest when we consider that inflammation significantly contributes to ethiopathogenesis of osteoporosis [7]. Moreover, infections are an important cause of morbi-mortality after hip fractures, and it has been demonstrated that VitD supplementation reduced hospital re-admission due to severe infections in this often severely 25(OH)D3-deficient population [8].
This association of VitD with infectious disorders had been suggested in fact for more than a century. Then, the practice of treating subjects with tuberculosis (TB) in a sun-exposed open-air mountains location [9], impressively described by Thomas Mann in The Magic Mountain [10], resulted in a double benefit on photocutaneous synthesis of 25(OH)D3 (i.e., lower concentrations of ozone and increased sunlight exposure) [11]. The Nobel Prize of medicine was for the matter given to N. Finsen who demonstrated that UV light was beneficial in treating lupus vulgaris, a devastating skin condition caused by M. tuberculosis [12]. Even earlier, the first clinical investigation using rational methodology was conducted in 1848 at the Brompton Hospital for Consumption and Diseases of the Chest to investigate the use of cod liver oil in the treatment of TB in London [13]. Thus, 542 patients with phthisis treated with cod liver oil were compared with 535 patients who received standard treatment alone. Although there were no important differences between the two groups in the number of patients who improved (cod liver oil, 63 % vs. control, 61 %), in 18 % of the group receiving cod liver oil the disease progression stopped compared with only 5 % in the control group. In addition, 33 % of patients given standard treatment alone deteriorated or died, compared with only 19 % of those given cod liver oil. One of the most striking effects was an increase in the patient’s weight (70 % gained weight, 21 % lost, and 9 % remained stationary). Subsequently, the use of cod liver oil was widely practised in the late nineteenth and twentieth centuries and death rates from M. tuberculosis infection in the UK declined steadily in the following years [13, 14]. While this practice even continues to the present day, cod liver oil is not a good dietary source of VitD. Although it is very rich with approximately 1,360 IU/tablespoon [15], it also contains retinol (i.e., vitamin A) that recently has been positively correlated with the risk for low bone mineral density and fractures [16].
The immunomodulatory role of 1,25(OH)2D3 was initially proposed more than 25 years ago [3]. This was based on the findings that monocytes/macrophages from individuals with granulomatous diseases synthesize calcitriol from the circulating precursor 25(OH)D3 [17, 18]. More recently, a clearer picture of 1,25(OH)2D3 as determinant of immune responsiveness has been obtained. Indeed calcitriol via endocrine (i.e., refers to substances that affect the activity of another part of the body via their direct secretion into the bloodstream), paracrine (i.e., refers to effects localized to adjacent or nearby cells), and/or intracrine (i.e., refers to a hormone that acts inside a cell) mechanisms [19, 20], may modulate the two arms of the immune system (i.e., innate and adaptive immunity) [3, 21–23].
Finally, answers to the question of whether VitD status may regulate immunity and its deficiency was associated with the occurrence of infectious diseases began to arise from two entirely different sources [24]: in vitro analysis of the immunomodulatory actions of 25(OH)D3 and 1,25(OH)2D3 [3], and from epidemiological observation of the link between VitD deficiency and susceptibility to infectious diseases [1, 4, 5, 25].
This review will examine the ways by which VitD status may interfere with the responsiveness of the human immune system. Furthermore, we discuss how the VitD status may play a role in host resistance to common pathogens and how effective is its supplementation for treatment or prevention of infectious diseases in humans.
VitD: a helpful immunomodulator
Host defence to pathogens: the art of war
The purpose of the human immune system is to recognize invading foreign organisms, prevent their spread, and ultimately clear them from the body. It is an extraordinarily complex system that relies on an elaborate and dynamic communication network using soluble mediators (i.e., cytokines), implicating billions of cells that patrol the body and interact with antigens in order to deal with all pathogens [26]. In response to microbial attacks, the first line of host defence is represented by innate immunity. Its mechanisms are fast but neither specific nor fixed in their mode of action. While the innate immune system is effective in stopping most pathogens at early stages of invasions, it responds to foreign antigens in a generic way without conferring long-lasting and protective immunity. Once microbes have breached epidermal and/or mucosal, they are recognized by pattern-recognition receptors such as toll-like receptors (TLR) [27]. TLRs are single, membrane-spanning, noncatalytic receptors that recognize structurally conserved molecules derived from pathogens and thereby activate innate immune cells (consisting mainly of monocytes, macrophages, natural killer cells, and dendritic cells (DCs)). DCs are heterogeneous in terms of their location, phenotype, and function. They express different types of soluble mediators (i.e., cytokines and chemokines) which also exert complementary by modulating T cell responses [28]. Typically myeloid DCs are valuable antigen-presenting cells (APCs) [29] and plasmacytoid DCs are more closely associated with immune tolerance [30]. Whether all innate immunity cells are able to identify and remove foreign substances present in organs, tissues, into the blood and lymph stream, they not only interact with pathogens but also with each other. In addition, they modulate the adaptive immune response by regulating timing, type, and amount of cytokines [31].
The functioning of adaptive immunity is slower to start but powerful enough to terminate almost all infections that elude the innate immune system. The adaptive immune system is composed of highly specialized, systemic cells and processes that eliminate antigens or prevent pathogenic growth. It is highly adaptable to antigens responding in a very specific way that finally confers long-lasting and protective immunity [32]. This sophisticated system of defence stimulates B and T cells, resulting in humoral (i.e., antibody) and cell-mediated immune response, respectively. The antibody-mediated immunity is usually dedicated to protection against extracellular pathogens, toxins and unfortunately can also induce autoimmunity. The cell-mediated immune system protects more against intracellular infections and cancer development.
The primary interaction with the adaptive immune system is made through a process known as antigen presentation [26]. The subsequent activation and differentiation of naive B cells are induced by antigen and CD4+ T-helper cells (Th) while naive T cells are activated by contact with APCs. In turn, activation of naïve Th leads to the generation of Th subgroups with distinct cytokine profiles, mainly represented by Th1 (i.e., interleukin (IL)-2, interferon (IFN)-γ, and tumour necrosis factor (TNF)-α) and Th2 (i.e., IL-3, IL-4, IL-5, and IL-10). They respectively support cell-mediated (Th1) and humoral immunity (Th2) [33, 34]. More recently, a third subgroup of IL-17 secreting T cells (Th17) has been identified. It plays a crucial role in combating certain pathogens and in autoimmune diseases [35–37]. In addition, a regulatory T cell (Threg) subgroup suppresses immune responses of other cells. It is considered as an important “self-checkpoint” built into the immune system to prevent excessive reactions [38, 39]. Finally, Th22 is involved in specific epidermal immunity [40] and Th9 in defending against helminth infections [41]. Upon stimulation, naive B cells differentiate into memory and antibody-secreting B cells (i.e., long-lived plasma cells). Long-term immunity is assumed by memory B and T cells (i.e., CD4+ and CD8+ T cells) into the blood and lymph nodes, as well as long-lived plasma cells and memory T cells in the bone marrow [42].
How does VitD interact with immune cells?
VitD regulates skeletal and calcium homeostasis through the binding of 1,25(OH)2D3 to VDR localized in the nucleus of various cells [1, 20, 43]. The VDR, also known as nuclear receptor subfamily 1, group I, member 1, is a member of the nuclear receptor family of transcription factors [44]. Upon activation by calcitriol, the VDR binds to hormone response elements on DNA sequences resulting in expression or transrepression of specific gene products [45, 46]. Downstream targets of this nuclear receptor are involved in the mineral metabolism but in a variety of other metabolic pathways. An activated VDR modulates the transcription of at least 913 genes and downstream gene products and regulates potent anti-proliferative and pro-differentiative [43, 47].
The finding that the majority of immune system cells (i.e., macrophages, B and T lymphocytes, neutrophils and DCs) possess VDR, mainly after activation, produced the idea that calcitriol could have pleiotropic effects on immune cells [20, 48]. In addition, some VDR transcription-independent actions play also key roles in regulating the immune system [20, 49].
VitD and innate immunity
Target cells such as monocytes/macrophages and DCs not only express VDR but also the VitD-activating enzyme or CYP27B1. As depicted in Fig. 1, these cells can directly utilize circulating 25(OH)D3 for intracrine activity that promotes antibacterial responses to pathogens (i.e., cathelicidin (hCAP) and defensin β2) [20]. Indeed, in addition to phagocytosis of pathogens, these cells also sense pathogen-associated molecular patterns through their TLRs. The latter upregulates the expression of genes that code for the VDR and CYP27B1 [23]. In turn, 1,25(OH)2D3 enhances the antibacterial potential by interacting with the promoter of the hCAP [50]. It so promotes microbial killing in phagocytic vacuoles and chemioattraction for neutrophils and monocytes [51]. However, in order to activate hCAP and enhance macrophage functions, it is necessary to reach sufficient levels of circulating 25(OH)D3 [52].
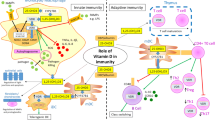
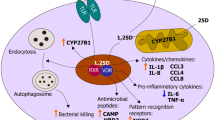
This figure depicts how VitD may influence the innate immune response. VitD derived from the action of sunlight on the epidermis (VitD3 only) or obtained from diet (VitD2 or D3) is metabolized at first in the liver to form 25-hydroxyvitamin D (25(OH)D 3 ), the main circulating form of VitD. Monocytes/macrophages and DC expressing the VitD-activating enzyme CYP27B1 (25-hydroxyvitamin D3 1-α hydroxylase) and the VitD receptor (VDR) can utilize 25(OH)D3 for intracrine responses via local conversion to active 1,25(OH)2D3. In monocytes/macrophages, intracrine synthesis of 1,25(OH)2D3 promotes antibacterial responses to pathogens. In DCs, this intracrine activity inhibits DC maturation and thereby modulates helper T cell (Th) functions. The Th responses may also be modulated in a paracrine fashion, with DC-generated 1,25(OH)2D3 acting on VDR-expressing Th cells. Intracrine immune effects of 25(OH)D3 also occur in CYP27B1/VDR-expressing epithelial cells. Neutrophils, which do not appear to express CYP27B1, are likely to be affected by circulating levels of active VitD synthesized by the kidneys. VDR-expressing Th are potential targets for systemic 1,25(OH)2D3 as well. Similarly, epithelial cells are able to respond in an intracrine fashion to 25OHD as well as to 1,25(OH)2D3 to promote antibacterial responses (adapted from Hewison [2])
Moreover, it appears that this action of 1,25(OH)2D3 may be dependent on the presence of IFN-γ [53, 54] suggesting a link with adaptive immunity [55].
Similarly, epithelial cells, trophoblasts, and decidual cells are able to respond to an intracrine hydroxylation of 25(OH)D3 to promote antibacterial responses but can also respond to systemic 1,25(OH)2D3 [52]. Strikingly, calcitriol also inhibits TLRs expression so inducing a state of hypo-responsiveness to pathogen-induced molecular cascades. This serves as a negative feedback mechanism, thus preventing excessive TLR activation and inflammation at a later stage of infections [56]. Other cells, such as neutrophils, do not appear to express CYP27B1 and are therefore likely to be regulated by circulating levels of calcitriol produced by the kidneys (i.e., through endocrine effect) [57].
VitD and adaptive immunity
The tissue-specific synthesis of calcitriol from 25(OH)D3 has been shown to be important for both T and B cells immune responses as presented in Fig. 2. Overall, the effect of calcitriol on the cell-mediated immune system includes modulating of T cell antigen receptor (TCR), decreasing Th1/Th17 CD4+ cells and cytokines, increasing Threg, downregulating T cell-driven immunoglobulin (Ig)-G production, and inhibiting DCs differentiation [21]. Indeed, in DCs, intracrine activation of 25(OH)D3 inhibits DCs maturation, thereby permitting induction and modulation of an initial CD4+ T cell response [58]. In this scenario, by acting as an inhibitor of maturation, 1,25(OH)2D3 inhibits T cells proliferation [17]. Thus, the calcitriol signalling represses the transcription of genes encoding Th1 [59, 60] and Th17 [21] cytokines in order to polarize the CD4+ T cells responses toward a more regulatory Th2 [61] or Threg phenotypes [3, 62]. These two phenotypes are considered key components of VitD capacity to suppress Th1-driven autoimmunity responses [17, 62]. Th17 cells also play a crucial role in combating certain pathogens (i.e., Candida albicans, Cryptococcus neoformans, Helicobacter pylori, Klebsiella pneumoniae, M. tuberculosis, and Staphylococcus [35]), but they have also been linked to tissue damage and inflammation [36, 63]. However, the precise role played by VitD in regulating Th17 cells is not yet completely elucidated [3]. Thus, CD4+ T cell responses to 25(OH)D3 are mediated in a paracrine fashion; DC-generated 1,25(OH)2D3 acting on VDR-expressing CD4+ T cells as depicted by Fig. 1. In summary, it seems that 1,25(OH)2D3 helps maintain self-tolerance by damping overly zealous adaptive immune system responses while enhancing protective innate responses [21]. However, the mechanism by which variations in VitD status may influence T cell functionalities is less clear. In other words, are there direct effects on T cells by systemic 1,25(OH)2D3, indirect effects via DC expression of CYP27B1 and intracrine synthesis of calcitriol, or direct effects following synthesis of 1,25(OH)2D3 by CYP27B1-expressing DCs or monocytes and/or finally intracrine conversion of 25(OH)D3 by T cells themselves? Moreover, the direct effect of 1,25(OH)2D3 on the function and differentiation of naive T cells is poorly known [3]. While naive T cells are known to express very little or even no VDR, a recently published study has shown that 1,25(OH)2D3 might directly modulate the TCR [64]. This was observed through a complex pathway leading via the alternative mitogen-activated protein kinase p38 to successive induction of VDR and phospholipase C gamma 1 (PLC-γ1), which are required for subsequent classical TCR signalling and T cell activation.
Overview of immunomodulatory actions of 25(OH)D3 and 1,25(OH)2D3 on monocytes and macrophages, dendritic cells, effector, and memory T and B lymphocytes. All these cells possess the enzymes to perform the hydroxylation steps to generate 1,25(OH)2D3. Through endocrine, paracrine, and intracrine mechanisms, 1,25(OH)2D3 binds to the vitamin D receptor (VDR) to induce a range of effects (adapted from Di Rosa et al. [3] and Van Bell et al. [4])
Despite the fact that for many years VDR was considered to be constitutively expressed on human B-cells and could be upregulated during cell activation, the ability of 1,25(OH)2D3 to suppress B cells proliferation and Ig production was initially considered to be an indirect effect mediated via CD4+ T cells [3]. However, recent in vitro studies have observed that B cells were capable of intracrine responses to 1,25(OH)2D3. Moreover, the finding that VDR expression in B cells was regulated by 1,25(OH)2D3 suggested that calcitriol might also exert differential effects on activated versus resting B cells and have different effects in individuals with different levels of serum 1,25(OH)2D3 [22]. Indeed, the inhibitory effects of 1,25(OH)2D3 mediated by the upregulation of the VDR, as presented in Fig. 2, in fact require a threshold level of VDR engagement for the antiproliferative effect to become apparent. Interestingly, CYP27B1 mRNA was found also expressed by resting B cells and could be further induced by stimulation but not by 1,25(OH)2D3. However, CYP24A1 (i.e., 1,25(OH)2D3 24-hydroxylase, the enzyme that inactivates calcitriol) was found significantly upregulated following the incubation of human B cells with 1,25(OH)2D3, suggesting that the activity of VitD3 on B cells might be influenced not only by VDR expression but also by the capacity to degrade the active molecule. In contrast to the VDR, CYP24A1 was not altered by B cell activation, demonstrating that human B cells can respond to 1,25(OH)2D3 directly. The increased susceptibility of activated B cells to many of the effects of 1,25(OH)2D3 might reflect the up-regulation of VDR but not CYP24A1 by these cells [22]. The precursor, 25(OH)D3, had similar effects on purified B-cells compared with the active form, but at higher concentrations [3]. Finally, 1,25(OH)2D3 limits ongoing B cell proliferation and modulates B cell responses. Its effects on plasma cell and memory cell differentiation however result from the suppression of ongoing B cell proliferation [65], which is required before the differentiation steps can occur [66]. These effects appear to be particularly significant in conditions in which there is a diffuse B-cell activation, such as systematic lupus erythematosus and other autoimmune diseases [67] that lends to further support the role played by VitD in B cell-related autoimmune disorders [2, 4]. Other B cells targets modulated by 1,25(OH)2D3 include IL-10 [68] and CCR10 [69], suggesting that B cell responses to VitD are extended to regulating allergic immune response [70] and mucosal immunity [4, 21].
In summary, it is now clear that effects of VitD on monocytes, macrophages, DCs, and lymphocytes are not constrained by the metabolic pathways associated with classical endocrine actions of 1,25(OH)2D3. Instead, it is now important to also consider intracrine and paracrine pathways that are subject to a distinct set of modulatory signals, and which may also be influenced by disease-specific dysregulation [19]. However, it is also important to mention that most of these data resulted from in vitro studies carried out in cell-cultures in which exogenous 1,25(OH)2D3 was added at levels above the physiological serum range. Finally, this raises the question of whether VitD effectively interferes at physiological concentrations with host immune cells. This could also suggest that intracell synthesis of 1,25(OH)2D3 and intracrine activity might be more effective in achieving these responses [3]. Conversely, some of these data are also supported by studies on VDR and CYP27B1 knock-out mice which presented an increased number of mature DCs and aberrant DCs trafficking [2].
Mechanisms by which VitD may enhance anti-microbial immunity
While it has been demonstrated that 1,25(OH)2D3 enhanced the bactericidal activity of human macrophage against M. tuberculosis [71], its exact role in determining pathogenesis and immune response to diverse pathogens has gained interest [3]. Thus, recent studies, for the most part conducted in animal models and ex vivo human cells, have provided new insight into the involvement of VitD deficiency and its supplementation in the anti-microbial response to various bacterial (e.g., H. pylori, Pseudomonas aeruginosa, Bordetella bronchoseptica, Salmonella, and Shigella) [72–76] and viral infections (e.g., Influenzae, Respiratory Syncytial Virus (RSV), Human Immunodeficiency Virus (HIV), hepatitis C virus (HCV)) [75, 77–80] and even in immune response to vaccination (e.g., influenza, hepatitis B, measles, rubella, and Mycobacterium bovis bacillus Calmette–Guérin vaccinations) [50, 81–84].
Our current understanding of the immunomodulatory role of VitD may help to suggest potential mechanisms by which it could act on host immunity against pathogens [85]. Based on the ability of 25(OH)D3 and 1,25(OH)2D3 to modulate innate and adaptive immune response (i.e., suppression of the oxidative burst, Th1-mediated and B cell immune responses), calcitriol would be expected to impair the host defences to pathogens. Indeed, as previously described, the Th1/Th17 immune responses are protective during infections [34, 35]. Furthermore, 1,25(OH)2D3 would be predicted to increase Threg cells [38, 39]. The evidence provided by studies conducted in VDR knock-out, wild-type, and VitD-deficient mice do not support either a beneficial or harmful effect of 1,25(OH)2D3 on host immunity to Listeria monocytogenes [86, 87], Leishamania major [88], M. bovis [89], M. tuberculosis [23], C. albicans [90], Herpes simplex [90], Shistosoma mansoni [85, 91], and Bordetella pertussis [85] that require either Th1/Th17 or Th2-mediated immune response [85].
However, the role played by calcitriol in regulating innate immunity and its ability to induce antibacterial peptides, such as cathelicidin or β-defensin [23], suggests a less straightforward outcome of any in vivo changes of 25(OH)D3 and/or 1,25(OH)2D3 and resulting effects on host resistance to infections [92, 93]. Both autophagy and calcitriol-mediated innate immunity have been shown to confer protection against intracellular M. tuberculosis infection [94]. In monocytes/macrophages, this occurs primarily in response to activation of TLRs, that induces expression of VDR and localized synthesis (i.e., intracrine) of 1,25(OH)2D3 from circulating 25(OH)D3 [23, 51, 95]. In turn, 1,25(OH)2D3 enhances cathelicidin synthesis by interacting with the promoter hCAP [72, 95]. This has been observed in vivo and ex vivo in human studies. Adams et al. [96] has demonstrated in 50 individuals with low bone mineral density and otherwise healthy that supplementation with 50,000 UI of VitD2 twice weekly for 5 weeks increased production of antibacterial hCAP following TLR activation of monocytes; 38 % of them were VitD insufficient. While baseline 25(OH)D3 status and VitD supplementation had no effect on circulating levels of hCAP, ex vivo changes in hCAP were measured. Under these VitD-“insufficient” conditions, the TLR2/1 ligand 19 kDa lipopeptide or the TLR4 ligand bacterial lipopolysaccharide and monocytes showed respectively a 5.0- and 5.5-fold increase, expression of VitD-activating CYP27B1 but decreased expression of hCAP mRNA (10.0- and 30.0-fold, both p < 0.001). Following TLR stimulation with 19 kDa bacterial lipopeptide, expression of hCAP not only correlated with 25(OH)D3 levels in serum culture supplements (p < 0.0001) but was significantly enhanced by exogenous 25(OH)D3 and with serum from in vivo VitD-supplemented subjects. Further studies have demonstrated that human cathelicidin served as a mediator of VitD-induced autophagy [21]. Finally, effective innate immunity against many bacterial pathogens requires macrophage responses that up regulate phagocytosis and direct antimicrobial pathways [97]. With respect to antibacterial effects, it is interesting to note that cathelicidin can exhibit antiviral properties [98], so that its induction by VitD may enhance protection against diseases such as influenza [50]. While many reports demonstrated the effect of VitD on the increased expression of antimicrobial peptides [99], the effect of either VitD or 1,25(OH)2D3 on the specific peptides against influenza infection has not been tested in vitro or in vivo [50].
The induction of cathelicidin by 25(OH)D3 and 1,25(OH)2D3 has been reported in several human cell types outside the classical immune system such as keratinocytes [100], gastrointestinal [101, 102] and bronchial epithelial cells [72], myeloid cell lines [51], decidual [103], and trophoblastic cells [104]. Thus, this innate antibacterial effects may be common to many human tissues and are therefore likely to influence a wide range of disease scenarios [2, 93]. Cells of gastrointestinal surfaces, including epithelial cells and lamina macrophages, which are also constantly exposed to luminal bacteria, play key roles in normal intestinal development and innate immunity. For example, the Paneth cells, known to secrete antimicrobial peptides, are regulated by VDR (see Fig. 2) [101, 102]. Studies in VDR−/− mice demonstrated increased bacterial loads in the intestine [105]. Recent endoscopic studies in humans have demonstrated that β-defensin was secreted in the gastric mucosa after infection by H. pylori [74]. It may therefore constitute a major aspect of immune defence against this pathogen at the mucosal surface. Furthermore, induction of cathelicidin by stimulated 1,25(OH)2D3 in cystic fibrosis bronchial epithelial cells causes an increased antibacterial activity against P. aeruginosa and Bordetella bronchiseptica [72]. Similarly, human biliary epithelial cells present an intense immunoreactivity for cathelicidin and the VDR [106]. Thus, some battles against pathogens at mucosal surfaces seem also regulated by VitD and VDR [21, 102]. Finally, it appears that optimal VitD status may have key immunomodulatory properties in infectious disease settings, and this is by downregulating excessive and therefore toxic cytokine responses while allowing the clearance of various microbial species through the production of antimicrobial peptides [3] (see Fig. 2).
Association between VitD serum levels and susceptibility to infections
VitD serum levels: what is the target?
The concentration of 25(OH)D3 in the serum is thought to be the best indicator of VitD status. It is a stable circulating metabolite of VitD and its concentration is close to around 1,000-fold higher that of 1,25(OH)2D3. 25(OH)D3 has a half life of 1–2 months in contrast to 1,25(OH)2D3 which is several hours [5]. While there is now overwhelming and compelling scientific and epidemiologic data demonstrating the beneficial effects of VitD supplementation on skeletal homeostasis and bone health, the 25(OH)D3 that is optimal for these health outcomes is still debated [2]. In the 2011 report of the Institute of medicine (IOM), a serum level of 50 nmol/L (20 ng/mL) 25OHD3 was suggested as sufficient to optimize bone mineral density as a marker of skeletal health for most populations in the USA and Canada [107]. This report was endorse by many organization such as the American Society for Bone and Mineral Research, and the cautious recommendations of the IOM have been supported by others [108, 109]. However, this support was not universal [110], and the IOM proposal received a more hostile reception from many researchers in the world of VitD [111–114]. A key underlying cause of this dichotomy of opinion was the remarkable increase of data highlighting nonclassical effects of calcitriol, and the health consequences this may have in humans with impaired VitD status [2]. Thus, for example, the International Osteoporosis Foundation recommends that the human body requires a blood level of 25(OH)D3 above 75 nmol/L (i.e., 30 ng/mL) for maximum health [115]. This choice is mainly driven by a concentration associated with good bone health and a close estimate for maximal parathyroid hormone suppression and decreasing the likelihood of secondary hyperparathyroidism [5, 15]. Consequently, a serum concentration of <50 nmol/L (i.e., <20 ng/L) is considered as deficient and between 50 and 75 nmol/L (i.e., 20 to 32 ng/L) as insufficient. It has been estimated that for every 100 IU of VitD ingested that the blood level of 25(OH)D3 increases by 2.5 nmol/L (i.e., 1 ng/mL).
In the lack of agreed consensus on the optimal level for VitD status in many countries, there are no up-to-date guidelines to define deficiency and insufficiency [55]. Theoretically achieve, a blood level above 75 nmol/L requires the ingestion of 3,000 IU of VitD a day [6]. There is evidence, however, that when the blood levels of 25(OH)D3 are less than 15 ng/mL, the body is able to more efficiently use VitD to raise the blood level to about 50 nmol/L (i.e., 20 ng/mL). To raise the blood level of 25(OH)D3 above 20 ng/mL and to reach the minimum 75 nmol/L requires the ingestion of at least 1,000 IU of VitD a day for adults [1]. One important note is that current target serum concentrations and dosage recommendations for VitD resolves around its application in bone health, while the effective dose eliciting an effect on the immune system in vivo remains to be determined [5].
Association between VitD serum levels and susceptibility to infections
Ginde et al. [75], in a large cohort study of 18,883 participants aged 12 years or older (secondary analysis of the third National Health and Nutrition Examination Survey), has observed that serum 25(OH)D3 levels were inversely associated with recent upper respiratory tract infections (URTI). With a median serum 25(OH)D3 of 29 ng/mL (i.e., ≈72 nmol/L—interquartile range, 21–37 ng/mL), recent URTI were reported in 24 % of participants with 25(OH)D3 levels of <10 ng/mL, 20 % with levels of 10 to <30 ng/mL, and 17 % with levels of ≥30 ng/mL (i.e., ≥75 nmol/l; p < .001). The rate of recent URTI was 19 % (95 % confidence interval (95 % CI, 18–20 %)) in the population studied. After adjusting for demographics and clinical factors (season, body mass index, smoking history, asthma, and chronic obstructive pulmonary disease (COPD)), lower levels were still independently associated with recent URTI. Compared with 25(OH)D3 levels of ≥30 ng/mL (i.e., 75 nmol/L), the odds ratio (OR) for developing a recent URTI was 1.36 (95 % CI, 1.01–1.84) and 1.24 (95 % CI, 1.07–1.43) for levels <10 and <30 ng/mL, respectively. This association between 25(OH)D3 levels and URTI seemed even stronger in individuals with asthma and COPD (OR, 5.67 and 2.26, respectively), two diseases in which 25(OH)D3 deficiency is linked to accelerated decline in lung function, increased inflammation, and further reduced immunity [116, 117]. Although 25(OH)D3 levels of <30 ng/mL (i.e., <75 nmol/L) and URTI were more frequent during the winter season, this inverse association was present throughout the year [75]. Similar results were observed in a population of North American children with mild-to-moderate persistent asthma in which VitD insufficiency was common and associated with higher odds of severe exacerbation over a 4-year period [118]. Conversely, in patients with severe COPD, baseline 25(OH)D3 levels were not predictive of subsequent acute exacerbation [119]. Plasma concentrations were measured at baseline in the 973 participants on entry to a 1-year study designed to determine if daily azithromycin decreased the incidence of acute exacerbations. A total of 33 % were insufficient (≥20 ng/mL but <30 ng/mL); 32 % were deficient (<20 ng/mL); and 8.4 % had severe deficiency (<10 ng/mL). However, within this very vulnerable population many other confounding risk factors for infection can explain this lack of significance such as deficiencies in other micronutrients or decreasing in the epithelial ciliary clearance and cough reflex [50, 120].
Serum levels of 25(OH)D3 below 30 ng/mL (i.e., 75 nmol/L) seem to also play a role in TB prevalence and susceptibility to active disease [121]. The incidence of virus infection typically peaks in the winter months when cutaneous VitD synthesis is lower [122]. Indeed, recent epidemiological evidence suggests that throughout the world influenza infection also occurs mainly during the month following the winter solstice, when circulating 25(OH)D3 levels reach the nadir [123]. In children with inadequate serum levels, infections seem particularly of viral origin. Concordantly, several studies have pointed out to the potential protective role of adequate serum 25(OH)D3 concentrations against influenza and RSV infections [77].
While 25(OH)D3 and 1,25(OH)2D3 related pathways have been mainly studied in relation to the host response to influenza infections, they interestingly seem to play a role in the control of HIV infection as well. Indeed, an increased prevalence of VitD deficiency in HIV-infected individuals has been noted in comparison with uninfected hosts [78]. More interestingly, shorter survival times were associated with abnormally low serum calcitriol while positive impacts on the CD4+ T-cell counts were observed after VitD supplementation [98, 124]. These results indicate that serum 1,25(OH)2D3 is correlated with the degree of immune deficiency during HIV infection; low serum levels being associated with increased incidence of acquired immunodeficiency syndrome events [125, 126]. In laboratory models of HIV infection, pretreatment of human monocytes and macrophages with 1,25(OH)2D3 prevented HIV infection in certain cell lines [127] but increased viral replication in some others [128]. More recently, Bergman et al. demonstrated that epithelial expression of cathelicidin contributed to the local protection against HIV-1 infection by inhibiting directly the replication of the virus [98]. However, while serum 25(OH)D3 levels of 30 ng/mL (i.e., 75 nmol/L) or more are necessary for optimal induction of host defence against pathogens, higher levels (i.e., 40 ng/mL or 100 nmol/L) do not seem to provide additional benefit [50, 75, 129].
Beyond serum levels of 25(OH)D3
Studies of VDR polymorphisms in humans support the hypothesis that variability in VitD status and host genes encoding VitD-responsive elements affects the immune response. A large number of single-nucleotide polymorphism (SNP) have been identified for the VDR gene as very recently reviewed by Di Rosa et al. [3] and Hewison [2]. Children with ff genotype (i.e., homozygous for the presence of the Fok1 site; “f” yields a VDR with three times more amino acids than the “F” form, but apparently less active [130]) had an increased risk of acquiring acute lower respiratory tract infections, predominantly RSV [131]. This genotype is also more common in patients with TB [132, 133] and has been implicated in down-regulating IL-12 and IFN production [134]; nevertheless, others were unable to replicate these observations [135, 136]. In a recent randomized control-trial in which high doses of VitD were administered, no effect on sputum conversion time was observed when assessed in relation to Fok1 genotype [137] while other VDR SNPs appeared to influence response to VitD supplementation [135, 136].
Genetic polymorphisms within the VitD system are not only restricted to VDR genes. While SNPs of the CYP27B1 gene have been shown to affect susceptibility to autoimmune diseases [2, 4], the best characterized inherited variations are provided by the gene coding for the VitD-binding protein (DBP) [138]. In a study conducted by Chun et al. [139], differences in serum concentration and genotype of DBP played a pivotal role in modulating the capacity of 25(OH)D3 to target monocytes. Three common allelic forms of the DBP are present in the serum at varying concentrations (Gc1F > Gc1S > Gc2) and exhibit varying affinities for 25(OH)D3 and 1,25(OH)2D3 (Gc1F > Gc1S > Gc2). DBP influences renal synthesis of calcitriol by facilitating glomerular re-absorption of 25(OH)D3, subsequent metabolised by kidney CYP27B1. This effect is more important for high-abundance/affinity Gc1F. DBP also transports VitD metabolites to peripheral target cells such as monocytes where however its actions appear to be the opposite of those observed in the kidney. Indeed, the intracrine conversion of 25(OH)D3 to 1,25(OH)2D3 usually induces antibacterial responses (e.g., enhanced production of hCAP). In monocytes, responses to 25(OH)D3 are more pronounced in the presence of low affinity forms of DBP (i.e., Gc2 and Gc1S) compared with high-affinity Gc1F [2]. This finally suggests that monocytes respond more to “free” rather than DBP-bound 25(OH)D3. The link between DBP genotype and host defence in humans has only been investigated in one study. In this case control study, Martineau et al. have recruited 534 adult and children with tuberculosis and 400 healthy controls in UK, Brazil, and South Africa [140]. The Gc2/2 genotype was strongly associated with susceptibility to active TB, compared with Gc1/1 genotype (OR, 2.81 (95 % CI, 1.19–6.66); p = .009). This association was preserved if serum 25(OH)D3 was <20 nmol/L (i.e., 8 ng/mL; p = .01) but not if serum 25(OH)D3 was ≥20 nmol/L.
Translating the role of VitD in protecting against infectious diseases
In parallel to the evidence pertaining to the immune-modifying effect of VitD, there has been a particular interest on the possible role of VitD supplementation as adjunctive therapy in settings of infection [5, 25]. All controlled human trials of VitD therapy for infection identified through a literature search are presented in Table 1. Of the 20 studies identified [79, 80, 137, 141–157], only two were not randomized controlled studies [146, 147]. They demonstrated substantial heterogeneity in baseline patient demographics, sample size, VitD status and VitD intervention strategies. Some are conducted in paediatric populations [144, 145, 147, 151, 153, 154] whereas other in postmenopausal women [148], hemodialysis [156], HIV- [153, 154] or hepatitis C virus (HVC) [79, 80] infected patients or in the elderly population [146, 149]. Sample size ranged from 24 to 3,444 individuals. Cholecalciferol was usually favoured over ergocholecalciferol in all but one study [142].
VitD supplementation and bacterial infectious diseases
Seven human trials of VitD replacement as treatment [141–144] or prevention [137, 145, 146] of bacterial disease have attempted to translate the mechanism of mediated macrophage activation to the human host. Four were conducted in TB-infected patients [137, 141, 143, 144] and yielded mixed results (see Table 1). The outcome of a preventive study conducted by Martineau et al. was encouraging [142]; the administration of a single dose of 100,000 IU of ergocholecalciferol to purified protein derivative-positive contacts of active TB cases improved their immunological control of bacilli Calmette-Guérin [25]. While Morcos et al. [144] and Nursyam et al. [143] reported faster resolution of TB symptoms and higher rates of sputum conversion respectively, both studies however failed to report baseline and follow-up 25(OH)2D3 levels, leaving uncertainly about the adequacy of repletion in each case. The most rigorously designed study was conducted by Wejse et al. [141]. It demonstrated no clear benefit of adjunctive vitamin therapy in TB treatment. However, although VitD was administered at doses higher than the total of 300,000 IU given to the intervention group, particular attention to potential confounding factors affecting 25(OH)2D3 levels in the control group would have been done; they remained surprisingly similar at baseline, 2 months, and 8 months [25]. Increased exogenous intake of VitD or independent effects improving nutritional status within the control group could probably contributed to affect the statistical power of the study.
More recently, Manaseki-Holland et al. have reported no beneficial effect of VitD supplementation in preventing the incidence of first episodes of pneumonia in 3,046 infants aged 1–11 months [145]. One of the most important issue to be considered relates to the generalisability of study results is that severe malnutrition was common in the study population and participants might therefore have been at high risk of deficiencies in other micronutrients such as calcium and vitamin A, both of which could modify effects of VitD supplementation; results of this study cannot necessarily be applied to better nourished populations [158]. Finally, one study was conducted in elderly women nursing home resident receiving VitD supplementation of 40 IU/day for two decades compared with subjects receiving no supplementation [146]. Although the study reported a lower incidence of infection with H. pylori, its primarily retrospective design, limited sample size, poor repletion potential of the very low vitamin dose, and failure to document baseline and follow-up 25(OH)2D3 status remain conflicting the potential value of VitD as adjunctive therapy in bacterial infections.
VitD supplementation and viral infectious diseases
Ten studies have been performed to help to translate information from laboratory and animal models of viral infection in setting of VitD deficiency. Most of them were randomized controlled trials concerning outcomes related to pneumonias [145] and URTI [147–152] of which one focusing specifically on influenza [148]. HIV [153, 154] and hepatitis C [79] infections were also investigated. Similarly to bacterial infections, they also yielded mixed results (see Table 1).
Indeed, currently, clinical studies assessing the potential benefits of VitD supplementation in reducing the occurrence of URTI have not been conclusive in adult populations [147–150, 152] while it seems to be protective in children [147, 151]. A same picture is observed in preventing influenza infections. As presented in Table 1, this could be explained by an inadequacy dosage of the VitD supplementation leading to a lack of effect on serum 25(OH)D3 concentrations [5]. In the study conducted by Laaksi et al. [152], only 29 % of the treatment group attained concentration > 80 nmol/L. Moreover in a post hoc analysis, Aloia et al. have found a decline in the rates of self-reported influenza and cold symptoms in individuals taking the supplementation [148]. Although 2,000 IU cholecalciferol significantly increased 25(OH)D3 concentrations in the trial conducted by Li-Ng et al. [150], negative results were unexpectedly attributed to lower GM-CSF, IFN-γ, IL-4, IL-8, and IL-10 levels in the intervention group as compared with those assigned to the control group [159].
To evaluate, in a randomized fashion, the impact of VitD supplementation on CD4 count in HIV-infected patients, Kakalia et al. randomly assigned children to receive no or VitD supplementation [154]. At baseline, only 15 % of children were VitD sufficient (i.e., 25(OH)D3 of ≥75 nmol/L) and their immune capacity was preserved. While VitD supplementation significantly improved serum 25(OH)D3 levels to reach desirable levels, even in doses as high as 1,600 IU/day, it did not impact the CD4 count. Similar results were reported by Arpadi et al. [153]. However, because of the potential antagonistic relationship between antiretroviral therapy and VitD metabolism and particularly its effect on 1,25(OH)2D3 rather than 25(OH)D3 [25].
To examine whether adding VitD improved the HCV viral response to antiviral therapy, two randomized controlled trials have been conducted in chronic HCV genotype 1 [79] and genotype 2–3 individuals [80]. In the two trials, individuals were treated with pegylated α-interferon combined with ribavirin (PEG/RBV) and were randomly assigned to receive no or oral VitD supplementation (2,000 IU/day) for 48 [79] and 36 weeks [80] respectively. In both studies, to the treatment group VitD was given for 12 [79] and 4 [80] weeks before the initiation of antiviral treatment in order to reach serum 25(OH)D3 levels at >30 ng/mL (i.e., > 75 nmol/L). Moreover, due to possible difference in VitD status and alanine aminotransferase levels, the study populations were stratified according to ethnic group (i.e., Russian/Jewish/Arab). Finally, adding VitD to conventional PEG/RBV therapy for treatment-naïve patients with chronic HCV genotypes 1, 2, and 3 infections significantly improved the viral response. Moreover, low 25(OH)D3 levels were predictive of negative treatment outcome. Despite these positive results, before to suggest routine testing of VitD levels prior to combination therapy and replacement during treatment for chronic HCV, additional studies in other ethnic and larger populations, and taking into account genetic polymorphisms within VDR are needed. Indeed, it is well known that people of African and Hispanic descent, for example, are less likely to respond to standard antiviral therapy [160] probably due to a polymorphism of the IL28B gene [161] but the impact of diet on liver fibrosis and on response to IFN therapy has been also reported [162].
VitD supplementation and other pathogens
Intervention trials in other pathogens such as fungi, protozoa and other parasites are very limited [25]. One trial, conducted by Snyman et al. [155] has however demonstrated the beneficial impact of VitD supplementation on levels of activated eosinophils and Shistosoma-specific antibodies in adolescent under or not antiparasitic therapy. By contrast, no evident clinical benefit was observed.
Finally, the current body of evidence supports the view that VitD supplementation holds promises as risk-modifying intervention in tuberculosis, influenza and viral upper respiratory illnesses. But it also supports the need for further controlled studies because research is still fraught with considerable methodological and epidemiological challenges. Not only the optimal regimen of VitD supplementation remains to be determined, but future trials in this area may benefit from studies that include larger populations taking into account variation in ethnicity, geographical location and seasonal variability in VitD status and considering more aggressive supplementation regimen (i.e., total dose ≈2,000 IU daily for 8–12 weeks) to achieve the target serum 25(OH)D3 level of 75 nmol/L [5]. Some of the aforementioned studies also included nonspecific outcomes and inadequate documentation of the effectiveness of the selected repletion regimen in terms of improving the VitD status of the host. Thus, measurement and reporting of prerepletion and postrepletion serum 25(OH)D3 levels are also an essential component of future studies in this field [25].
VitD and immune response to vaccination
Consecutively to the potent effects of VitD on cytokine production and regulation of immunity, the influence of its serum levels on the immune response to vaccination and its role as a potential vaccine-enhancing agent have been investigated [157, 163]. Indeed, with specific regard to vaccination, it is of special interest that on activation via TLRs by vaccine antigen and/or adjuvants DCs upregulate CYP27B1. In an animal model locally produced 1,25(OH)2D3 induced migration of these DCs from the site of vaccination to nondraining lymphoid organs, where they could stimulate antigen-specific T and B cells to mount a strong and persistent antibody response to diphtheria vaccination [164, 165]. Thus, while 1,25(OH)2D3 blunt B cell functions, it would then paradoxically stimulate vaccine effectiveness through its effects on innate immunity.
Thus, retrospectively, in groups of patients with chronic kidney disease, prostate cancer, VitD deficiency was an independent and significantly negative predictor of poor antibody formation after hepatitis B vaccination [81]. Conversely, repleting VitD status was associated with more frequent serological responses to influenza vaccine [166]. Moreover, studies have demonstrated a strong inverse association between IFN-γ responses to M. tuberculosis purified protein and VitD concentrations in infants [84].
The addition of 1,25(OH)2D3 to a variety of vaccine preparations increased immunity to H. simplex virus, tetanus toxoid, hepatitis B surface antigen, and HIV glycoprotein 160 [157, 163]. Co-administration of calcitriol with trivalent influenza vaccine in mice enhanced both mucosal and systemic antibody response, and the animals’ ability to neutralize live influenza virus instilled in the nose [167, 168]. However, when 1.0 μg of 1,25(OH)2D3 was intramuscularly co-administered with influenza vaccine at the site adjacent to vaccination in a randomized control-study conducted in 175 healthy human volunteers (18–49 years of age), humoral immunity was not enhanced [157]. Serum influenza hemagglutination inhibition (HAI) titres were used to assess the ability of the host immune response to neutralize the infectivity of the virus [169]. However three important limitations are drawn from this study. First, the subjects were young and healthy and since the serum 25(OH)D3 status were not measured at baseline, it is most probable that those individuals were neither VitD deficient nor insufficient. This reinforces the idea, as previously mentioned with respect to the host defence against pathogens, that higher than optimal levels (i.e., 40 ng/mL or 100 nmol/L) do not seem to provide an additional benefit [50, 129]. Second, significant pre-vaccination HAI titres were measured in nearly all subjects. This indicates that the subjects had considerable immunity to three vaccine influenza strains before their vaccination. Since a clear inverse relationship exists between pre-immunization serum HAI and antibody response after vaccination this could have masked the potential vaccine-enhancing effect of 1,25(OH)2D3 [50]. Third, vitamin supplementation and vaccine were simultaneously administered; the delay between the two injections was too limited to expect any beneficial biological effects on DCs, on macrophages, or on cells composing the adaptive immunity. This is true even if the active form of VitD was considered in the study, because the endocrine activation pathway, as depicted in Fig. 1 does not seem to be the main mechanism by which calcitriol modulates innate immunity. More recently, the influence of VitD on influenza vaccine immunogenicity in HIV was assessed using data from a phase 3, randomized trial conducted during the 2008–2009 influenza season [170]. Thirty-three percent of participants were on supplemental VitD at baseline. Neither seroconversion nor seroprotection rates were predicted by VitD use for any of the three vaccine strains. Finally, one controlled study is ongoing to determine the increase in specific cell-mediated immune response from herpes zoster vaccination in nursing home residents after 4 months of high-dose compared with standard-dose VitD3 supplementation (http://clinicaltrials.gov/ct2/show/NCT01262300—final data collection and primary outcome measure, March 2013).
Interestingly, specific allelic variations and haplotypes of the VDR genes seem to also modulate the immune response to vaccines. Using a tag SNP approach, 745 healthy children were genotyped for the 391 polymorphisms in their VDR genes [82]. Significant associations between multiple VDR SNPs/haplotypes and measles-specific IL-2, IL-6, IL-10, IFN-α, IFN-γ, IFNλ-1, and TNF-α cytokine secretions were found, suggesting that several allelic variations and haplotypes in the VDR genes influence adaptive immune responses to measles vaccine. Similarly, Ovsyannikova et al. [83] genotyped 714 healthy children for 148 candidate SNP markers and presented evidence that, after two doses of rubella-containing vaccine, polymorphisms in VDR but also in innate immunity-related genes can influence adaptive cytokine responses to rubella vaccine.
Conclusions
In conclusion, although implications of 25(OH)D3 and 1,25(OH)2D3 synthesis in the maintenance of host immune defence against pathogens and immune homeostasis are still under active investigation, the current idea of their function as “more than just a key player in bone formation” seems reasonable. This review demonstrates that immune cells are not only targets for 1,25(OH)2D3 but are also able to locally activate this hormone from circulating 25(OH)D3 arguing for intracrine, paracrine, and endocrine activities of VitD.
Current epidemiological data support that VitD insufficiency/deficiency increases susceptibility to various pathogens. However, the underlying mechanisms are yet to be clarified and further investigate. Complementarily, the role of inherited polymorphism in DBP, CYP27B1, and VDR should be also further elucidated. Finally, although evidence about the skeletal functions of VitD support the need of maintaining 25(OH)D3 at the desirable serum level (i.e., ≥75 nmol/L), despite the major research advances that have been made over the last 5 years our understanding of the nonclassical actions of VitD is far from complete. On the basis of the clinical trials reviewed and even though results from bench and translational studies are very promising, the strongest evidence supports further research, clinical studies, and larger population-based epidemiological analyses in order to validate the health benefits of optimized serum 25(OH)D3 status.
References
Holick MF (2012) Vitamin D: extraskeletal health. Rheum Dis Clin North Am 38:141–160
Hewison M (2012) An update on vitamin D and human immunity. Clin Endocrinol 76:315–325
Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L (2011) Vitamin D3: a helpful immuno-modulator. Immunology 134:123–139
Van Belle TL, Gysemans C, Mathieu C (2011) Vitamin D in autoimmune, infectious and allergic diseases: a vital player? Best Pract Res Clin Endocrinol Metab 25:617–632
Khoo LA, Chai L, Koenen H, Joosten I, Netea M, van der Ven A (2012) Translating the role of vitamin D3 in infectious diseases. Crit Rev Microbiol 38:122–135
Wimalawansa SJ (2012) Vitamin D in the new milemium. Curr Osteoporos Rep 10:4–15
Lang PO, Mitchell WA, Lapenna A, Pitts D, Aspinall R (2010) Immunological pathogenesis of main aged-related disease and frailty: role of immunosenescence. Eur Geriatr Med 1:112–121
Bischoff-Ferrari HA, Dawson-Hughes B, Platz A, Orav EJ, Stähelin HB, Willett WC, Can U, Egli A, Mueller NJ, Looser S, Bretscher B, Minder E, Vergopoulos A, Theiler R (2010) Effect of high-dosage cholecalciferol and extended physiotherapy on complications after hip fracture: a randomized controlled trial. Arch Intern Med 170:813–820
Hart PH, Gorman S, Finlay-Jones JJ (2011) Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol 11:584–596
Mann T (1924) The magic mountain (Der Zauberberg). S. Fisher Verlag, Berlin
Chesney RW (2010) Vitamin D and the magic mountain: the anti-infectious role of the vitamin D. J Pediatr 156:698–703
Tan SY, Lindsey K (2011) Medicine in stamps: Niels Finsen (1860–1904): gift of light. Singapore Med J 52:778
Green M (2011) Cod liver oil and tuberculosis. BMJ 343:d7505. doi:7510.1136/bmj.d7505
Bryder L (1987) Below the magic mountain—a social history of tuberculosis in twentieth century Britain, vol. 7. Clarendon Press, Oxford
Biesalski HK (2011) Vitamin D recommendations—beyond deficiency. Ann Nutr Metab 59:10–16
Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Ando F, Shimokata H, Yano M (2011) Dietary patterns of antioxidant vitamin and carotenoid intake associated with bone mineral density: findings from post-menopausal Japanese female subjects. Osteoporos Int 22:143–152
Van Etten E, Mathieu C (2005) Immunoregulation by 1,25-di-hydroxy1,25(OH)2D3: basic concepts. J Steroid Biochem Mol Biol 97:93–101
Papapoulos SE, Clemens TL, Fraher LJ, Lewin IG, Sandler LM, O’Riordan JL (1979) 1,25 di-hydroxycholecalciferol in the pathogenesis of the hypercalcemia of sarcoidosis. Lancet 1:627–630
Hewison M (2012) Vitamin D and immune function: autocrine, paracrine or endocrine? Scand J Clin Lab Invest Suppl 243:92–102
White JH (2012) Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord 13:21–29
Sun J (2010) Vitamin D and mucosal immune function. Curr Opin Gastroenterol 26:591–595
Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE (2007) Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179:1634–1647
Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773
Hewison M (2010) Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin N Am 39:365–379
Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V (2009) Vitamin D for treatment and prevention of infectious diseases: a systemic review of randomized controlled trials. Endocr Pract 15:438–449
Dempsey PW, Vaidya SA, Cheng G (2003) The art of war: innate and adaptive immune responses. Cell Mol Life Sci 60:2604–2621
Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17:1–14
Andorini L, Penna G, Giarratana N, Roncari A, Amuchastegui S, Daniel KC, Uskokovic M (2004) Dendritic cells as key target for immunomodulation by vitamin D receptor ligands. J Steroid Biochem Mol Biol 90:437–441
Liu YJ (2005) IPC: professional type I interferon-producing cells and plasmacytoid dendritic cell precursor. Annu Rev Immunol 23:275–306
Steinman RM, Hawiger D, Nussenzweig MC (2003) Tolerogenic dendritic cells. Annu Rev Immunol 21:685–711
Abdelsadik A, Trad A (2011) Toll-like receptors on the fork roads between innate and adaptive immunity. Hum Immunol 72:1188–1193
Rodríguez RM, López-Vázquez A, López-Larrea C (2012) Immune systems evolution. Adv Exp Med Biol 739:237–251
Abbas AK, Murphy KM, Sher A (1996) Functional diversity of helper T lymphocytes. Nature 383:1357–1366
Romagnani S (2006) Regulation of the T-cell response. Clin Exp Allergy 36:1357–1366
Peck A, Mellins ED (2010) Precarious balance: Th17 cells in host defense. Infect Immun 78:32–38
Korn T, Oukka M, Kuchroo V, Betteli E (2007) Th17 cells: effector cells with inflammatory properties. Semin Immunol 19:362–371
Tang J, Zhou R, Luger D et al (2009) Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th-17 effector response. J Immunol 167:4974–4980
Penna G, Adorini L (2000) 1 alpha,25-dihydroxyvitamin D-3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164:2405–2411
Jeffery LE, Burke F, Mura M et al (2009) 1,25-dihydroxyvitamin D(3) and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol 183:5458–5467
Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A (2009) Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119:3573–3585
Ma CS, Tangye SG, Deenick EK (2010) Human Th9 cells: inflammatory cytokines modulate IL-9 production through the induction of IL-21. Immunol Cell Biol 88:621–623
Braciale TJ, Sun J, Kim TS (2012) Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol 12:295–305
Carlberg C, Seuter S, Heikkinen S (2012) The first gemone-wide view of vitamin D receptor locations and their mechanistic implications. Anticancer Res 32:271–282
Moore DD, Kato S, Xie W, Mangelsdorf DJ, Schmidt DR, Xiao R, Kliewer SA (2006) International Union of Pharmacology. LXII. The NR1H and NR1I receptors: constitutive androstane receptor, pregnene X receptor, farnesoid X receptor alpha, farnesoid X receptor beta, liver X receptor alpha, liver X receptor beta, and vitamin D receptor. Pharmacol Rev 58:742–759. doi:710.1124/pr.1158.1124.1126
Chawla A, Repa J, Evans RM, Mangelsdorf DJ (2001) Nuclear receptors and lipid physiology: opening hte X-files. Science 294:1866–1870
Carlberg C, Polly P (1998) Gene regulation by 1,25(OH)2D3. Crit Rev Eukariot Gene Expr 8:19–42
Aranda A, Pascual A (2001) Nuclear hormon receptors and gene expression. Physiol Rev 81:1269–1304
Wang Y, Zhu J, DeLuca HF (2012) Where is the vitamin D receptor? Arch Biochem Biophys 523:123–133
Nagy L, Szanto A, Szatmari I, Széles L (2012) Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol Rev 92:739–789
Lang PO, Samaras D (2012) Aging adults and seasonal influenza: does the vitamin D status (h)arm the body? J Aging Res, 806198 pages. doi:806110.801155/802012/806198
Gombart AF, Borregaard N, Koeffler HP (2005) Human cathelicidin antimicrobial peptide (CAMP) is a direct target of the vitamin D receptor and is strongly upregulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–1077
Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Törmä H, Stahle M (2005) Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol 124:1080–1082
Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, Teles R, Montoya D, Iyer SS, Bruns H, Lewinsohn DM, Hollis BW, Hewison M, Adams JS, Steinmeyer A, Zügel U, Cheng G, Jo EK, Bloom BR, Modlin RL (2011) Vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages. Sci Trans Med 3:104ra102
Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, Keegan C, Krutzik SR, Adams JS, Hewison M, Modlin RL (2010) T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci USA 107:22593–22598
Battersby AJ, Kampmann B, Burl S (2012) Vitamin D in early childhood and the effect on immunity to Mycobacterium tuberculosis. Clin Dev Immunol 2012:430972
Sadeghi K, Wessner B, Laggner U et al (2006) 1,25(OH)2D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol 36:361–370
van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP (2006) Evidence for auto/paracrine actions of vitamin D in bone: 1 alpha hydroxylase expression and activity in human bone cells. FASEB J 13:2417–2419
Hewison M, Freeman L, Hughes SV et al (2003) Dufferential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol 170:5382–5390
Alroy I, Towers T, Freedman LP (1995) Transcriptional repression of interleukin-2 gene by 1,25(OH)2D3: direct inhibition NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol 15:5789–5799
Mahon BD, Wittke A, Weaver V, Cantorna MT (2003) The targets of vitamin D depend on the differentiation and activation status of CD4-positive T-cells. J Cell Biochem 89:922–932
Bansal ASHF, Sumar N, Patel S (2012) T helper cell subsets in arthritis and the benefits of immunomodulation by 1,25(OH)2 vitamin D. Rheumatol Int 32:845–852
Ooi JH, Chen J, Cantorna MT (2012) Vitamin D regulation of immune function in the gut: why do T cells have vitamin D receptors? Mol Aspects Med 33:77–82
Kamen DL, Wu S, Rhee KJ, Albesiano E et al (2009) A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 15:1016–1022
Kamen DL, Tangpricha V (2010) Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med 88:441–450
Adams JS, Gacad MA (1985) Characterization of 1 alpha-hydroxylation of 1,25(OH)2D3 sterol by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med 161:755–765
Vernino L, McAnally LM, Ramberg J, Lipsky PE (1992) Generation of nondividing high-rate Ig-secreting plasma-cells in cultures of human B-cells stimulated with anti-Cd3-acti- vated T-cells. J Immunol 148:404–410
Grammer AC, Lipsky PE (2003) B cell abnormalities in systemic lupus erythematosus. Arthritis Res Ther 5:S22–S27
Heine G, Niesner U, Chang HD, Steinmeyer A, Zügel U, Zuberbier T, Radbruch A, Worm M (2008) 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur J Immunol 38:2210–2218
Shirakawa AK, Nagakubo D, Hieshima K, Nakayama T, Jin Z, Yoshie O (2008) 1,25-dihydroxyvitamin D3 induces CCR10 expression in terminally differentiating human B cells. J Immunol 180:2786–2795
Jones AP, Tulic MK, Rueter K, Prescott SL (2012) Vitamin D and allergic disease: sunlight at the end of the tunnel? Nutrients 4:13–28
Chun RFAJ, Hewison M (2011) Immunomodulation by vitamin D: implications for TB. Expert Rev Clin Pharmacol 4:583–591
Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G (2007) Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D3. J Cyst Fibros 6:403–410
Wu S, Liao AP, Xia Y, Li YC, Li JD, Sartor RB, Sun J (2010) Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol 177:686–697
Wehkamp J, Schauber J, Stange EF (2007) Defensins and cathelicidins in gastrointestinal infections. Curr Opin Gastroenterol 23:32–38
Ginde AA, Mansbach JM, Camargo CA Jr (2009) Association between serum 25-hydroxyvitamin D and upper respiratory tract infection in the third national Health and Nutrition Examination survey. Arch Intern Med 169:384–390
von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C (2010) Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 11:344–349
Grant WB (2008) Variation in Vitamin D production could possibly explain the seasonality of childhood respiratory infection in Hawaii. Pediatr Infect Dis J 27:853
Rodríguez M, Daniels B, Gunawardene S, Robbins GK (2009) High frequency of vitamin D deficiency in ambulatory HIV-positive patients. AIDS Res Hum Retroviruses 25:9–14
Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N (2011) Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol 17:5184–5190
Nimer A, Mouch A (2012) Vitamin D improves viral response in hepatitis C genotype 2–3 naïve patients. World J Gastroenterol 18:800–805
Zitt E, Sprenger-Mähr H, Knoll F, Neyer U, Lhotta K (2012) Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine 30:931–935
Ovsyannikova IG, Haralambieva IH, Vierkant RA, O’Byrne MM, Jacobson RM, Poland GA (2012) Effects of vitamin A and D receptor gene polymorphisms/haplotypes on immune responses to measles vaccine. Pharmacogenet Genomics 22:20–31
Ovsyannikova IG, Dhiman N, Haralambieva IH, Vierkant RA, O’Byrne MM, Jacobson RM, Poland GA (2010) Rubella vaccine-induced cellular immunity: evidence of associations with polymorphisms in the Toll-like, vitamin A and D receptors, and innate immune response genes. Hum Genet 127:207–221
Lalor MK, Floyd S, Gorak-Stolinska P, Weir RE, Blitz R, Branson K, Fine PE, Dockrell HM (2011) BCG vaccination: a role for vitamin D? PLoS One 6:e16709
Bruce D, Ooi JH, Yu S, Cantorna MT (2010) Vitamin D and host resistance to infection? Putting the cart in front of the horse. Exp Biol Med (Maywood) 235:921–927
Helming L, Böse J, Ehrchen J, Schiebe S, Frahm T, Geffers R, Probst-Kepper M, Balling R, Lengeling A (2005) 1alpha,25-dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood 106:4351–4358
Bruce D, Whitcomb JP, August A, McDowell MA, Cantorna MT (2009) Elevated non-specific immunity and normal Listeria clearance in young and old vitamin D receptor knockout mice. Int Immunol 21:113–122
Ehrchen J, Helming L, Varga G, Pasche B, Loser K, Gunzer M, Sunderkötter C, Sorg C, Roth J, Lengeling A (2007) Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J 21(12):3208–3218
Waters WR, Palmer MV, Nonnecke BJ, Whipple DL, Horst RL (2004) Mycobacterium bovis infection of vitamin D-deficient NOS2−/− mice. Microb Pathog 36:11–17
Cantorna MT, Hullett DA, Redaelli C, Brandt CR, Humpal-Winter J, Sollinger HW, Deluca HF (1998) 1,25-dihydroxyvitamin D3 prolongs graft survival without compromising host resistance to infection or bone mineral density. Transplantation 66:828–831
Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT (2003) A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 17:2386–2392
Gombart AF (2009) The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol 4:1151–1165
Lagishetty V, Liu NQ, Hewison M (2011) Vitamin D metabolism and innate immunity. Mol Cell Endocrinol 347:97–105
Rook GA, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J, Stanford J (1986) Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology 57:159–163
Wang TT, Nestel FP, Bourdeau V et al (2004) Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912
Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M (2009) Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 182:4289–4295
Montoya D, Cruz D, Teles RM, Lee DJ, Ochoa MT, Krutzik SR, Chun R, Schenk M, Zhang X, Ferguson BG, Burdick AE, Sarno EN, Rea TH, Hewison M, Adams JS, Cheng G, Modlin RL (2009) Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe 6:343–353
Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Söderlund J (2007) The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res 5:410–415
Leikina E, Delanoe-Ayari H, Melikov K, Cho MS, Chen A, Waring AJ, Wang W, Xie Y, Loo JA, Lehrer RI, Chernomordik LV (2005) Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat Immunol 6:995–1001
Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zügel U, Bikle DD, Modlin RL, Gallo RL (2007) Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117:803–811
Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, Hollis B, DeLuca HF, Adams JS, Hewison M (2008) Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology 149:4799–4808
Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK (2009) Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6:231–243
Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M (2006) Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod 75:816–822
Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, Hewison M (2009) Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod 80:398–406
Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M (2010) Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology 151:2423–2432
D’Aldebert E, Biyeyeme Bi Mve MJ, Mergey M, Wendum D, Firrincieli D, Coilly A, Fouassier L, Corpechot C, Poupon R, Housset C, Chignard N (2009) Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology 136:1435–1445
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58
Slomski A (2011) IOM endorses vitamin D, calcium only for bone health, dispels deficiency claims. JAMA 305(453–454):456
Reid IR, Avenell A (2011) Evidence-based policy on dietary calcium and vitamin D. J Bone Miner Res 26:452–454
Hollis BW (2011) Short-term and long-term consequences and concerns regarding valid assessment of vitamin D deficiency: comparison of recent food supplementation and clinical guidance reports. Curr Opin Clin Nutr Metab Care 14:598–604
Heaney RP, Holick MF (2011) Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res 26:455–457
Grant WB (2011) The Institute of Medicine did not find the vitamin D-cancer link because it ignored UV-B dose studies. Public Health Nutr 14:745–746
Grant WB, Boucher BJ (2011) Requirements for vitamin D across the life span. Biol Res Nurs 13:120–133
Hollis BW, Wagner CL (2011) The vitamin D requirement during human lactation: the facts and IOM’s ‘utter’ failure. Public Health Nutr 14:748–749
Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N (2010) IOF position statement: vitamin D recommendations for older adults. Osteoporos Int 21:1151–1154
Sundar IK, Rahman I (2011) Vitamin D and susceptibility of chronic lung diseases: role of epigenetics. Front Pharmacol 2:50. doi:10.3389/fphar.2011.0050
Herr C, Greulich T, Koczulla RA, Meyer S, Zakharkina T, Brandscheidt M, Eschmann R, Bals R (2011) The role of vitamin D in pulmonary disease: COPD, astham, infection, and cancer. Respir Res 12:31
Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, Weiss ST, Litonjua AA, Childhood Asthma Management Program Research Group (2010) Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 126:52.e55–58.e55
Kunisaki KM, Niewoehner DE, Connett JE, COPD Clinical Research Network (2012) Vitamin D levels and risk of acute exacerbations of chronic obstructive pulmonary disease: a prospective cohort study. Am J Respir Crit Care Med 185:286–290
Diamond G, Legarda D, Ryan LK (2000) The innate immune response of the respiratory epithelium. Immunol Rev 173:27–38
Nnoaham KE, Clarke A (2008) Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol 37:113–119
Pfleiderer M, Löwer J, Kurth R (2001) Cold-attenuated live influenza vaccines, a risk-benefit assessment. Vaccine 20:886–894
Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E (2006) Epidemic influenza and vitamin D. Epidemiol Infect 134:1129–1140
Haug CJ, Müller F, Rollag H, Aukrust P, Degré M, Frøland SS (1996) The effect of 1,25-vitamin D3 on maturation of monocytes from HIV-infected patients varies with degree of immunodeficiency. APMIS 104:539–548
Mehta S, Mugusi FM, Spiegelman D, Villamor E, Finkelstein JL, Hertzmark E, Giovannucci EL, Msamanga GI, Fawzi WW (2011) Vitamin D status and its association with morbidity including wasting and opportunistic illnesses in HIV-infected women in Tanzania. AIDS Patient Care STDS 25:579–585
Terrier B, Carrat F, Geri G, Pol S, Piroth L, Halfon P, Poynard T, Souberbielle JC, Cacoub P (2011) Low 25-OH vitamin D serum levels correlate with severe fibrosis in HIV-HCV co-infected patients with chronic hepatitis. J Hepatol 55:756–761
Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, Sweep FC, Hermus AR, Bosch ME, Burger DM, Bravenboer B, Koopmans PP, Van Der Ven AJ (2008) Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses 24:1375–1378
Connor RI, Rigby WF (1991) 1 alpha,25-dihydroxyvitamin D3 inhibits productive infection of human monocytes by HIV-1. Biochem Biophys Res Commun 176:852–859
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28
Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, Tonai T, Nishisho T, Mori S, Takeda E (1997) A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res 12:915–921
Janssen R, Bont L, Siezen CL, Hodemaekers HM, Ermers MJ, Doornbos G, van’t Slot R, Wijmenga C, Goeman JJ, Kimpen JL, van Houwelingen HC, Kimman TG, Hoebee B (2007) Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis 196:826–834
Gao L, Tao Y, Zhang L, Jin Q (2010) Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis 14:15–23
Zhang HQ, Deng A, Guo CF, Wang YX, Chen LQ, Wang YF, Wu JH, Liu JY (2010) Association between FokI polymorphism in vitamin D receptor gene and susceptibility to spinal tuberculosis in Chinese Han population. Arch Med Res 41:46–49
Lemire JM (1995) Immunomodulatory actions of 1,25-dihydroxy1,25(OH)2D3. J Steroid Biochem Mol Biol 53:599–602
Søborg C, Andersen AB, Range N, Malenganisho W, Friis H, Magnussen P, Temu MM, Changalucha J, Madsen HO, Garred P (2007) Influence of candidate susceptibility genes on tuberculosis in a high endemic region. Mol Immunol 44:2213–2220
Lewis SJ, Baker I, Davey Smith G (2005) Meta-analysis of vitamin D receptor polymorphisms and pulmonary tuberculosis risk. Int J Tuberc Lung Dis 9:1174–1177
Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Woodward NJ, Venton TR, Barnes KE, Mullett CJ, Coussens AK, Rutterford CM, Mein CA, Davies GR, Wilkinson RJ, Nikolayevskyy V, Drobniewski FA, Eldridge SM, Griffiths CJ (2011) High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 377:242–250
Arnaud J, Constans J (1993) Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum Genet 92:183–188
Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M (2010) Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab 95:3368–3376
Martineau AR, Leandro AC, Anderson ST, Newton SM, Wilkinson KA, Nicol MP, Pienaar SM, Skolimowska KH, Rocha MA, Rolla VC, Levin M, Davidson RN, Bremner SA, Griffiths CJ, Eley BS, Bonecini-Almeida MG, Wilkinson RJ (2009) Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J 35:1106–1112
Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M (2009) Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 179:843–850
Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ (2007) A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 176:208–213
Nursyam EW, Amin Z, Rumende CM (2006) The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones 38:3–5
Morcos MM, Gabr AA, Samuel S, Kamel M, Baz M el, Beshry M el, Michail RR (1998) Vitamin D administration to tuberculous children and its value. Boll Chim Farm 137:157–164
Manaseki-Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, Walraven G, Chandramohan D (2012) Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 379:1419–1427
Kawaura A (2006) Inhibitory effect of long term 1alpha-hydroxyvitamin D3 administration on Helicobacter pylori infection. J Clin Biochem Nutr 38:103–106
Rehman PK (1994) Sub-clinical rickets and recurrent infection. J Trop Pediatr 40:58
Aloia JF, Li-Ng M (2007) Re: epidemic influenza and vitamin D [with author reply]. Epidemiol Infect 135:1095–1096
Avenell A, Cook JA, Maclennan GS, Macpherson GC (2007) Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing 36:574–577
Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, Berbari N (2009) A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect 137:1396–1404
Urashima M, Segawa T, Okazaki M, Wada Y, Ida H (2010) Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 91:1255–1260
Laaksi I, Ruohola JP, Mattila V, Auvinen A, Ylikomi T, Pihlajamäki H (2010) Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Infect Dis 202:809–814
Arpadi SM, McMahon D, Abrams EJ, Bamji M, Purswani M, Engelson ES, Horlick M, Shane E (2009) Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics 123:e121–e126, Erratum in: Pediatrics. 2009 May;2123(2005):1437
Kakalia S, Sochett EB, Stephens D, Assor E, Read SE, Bitnun A (2011) Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. J Pediatr 159:951–957
Snyman JR, de Sommers K, Steinmann MA, Lizamore DJ (1997) Effects of calcitriol on eosinophil activity and antibody responses in patients with schistosomiasis. Eur J Clin Pharmacol 52:277–280
Moe SM, Zekonis M, Harezlak J, Ambrosius WT, Gassensmith CM, Murphy CL, Russell RR, Batiuk TD (2001) A placebo-controlled trial to evaluate immunomodulatory effects of paricalcitol. Am J Kidney Dis 38:792–802
Kriesel JDSJ (1999) Calcitriol (1,25-dihydroxy-vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine 17:1883–1888
Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ (2012) Bolus-dose vitamin D and prevention of childhood pneumonia. Lancet 379:1373–1375
Yusupov E, Li-Ng M, Pollack S, Yeh JK, Mikhail M, Aloia JF (2010) Vitamin D and serum cytokines in a randomized clinical trial. Int J Endocrinol. Doi:10.1155/2010/305054
Rodriguez-Torres M, Jeffers LJ, Sheik MY, Rossaro L, Ankoma-Sey V, Hamzeh FM, Martin P, Latino Study Group (2009) Peginterferon alfa-2a and Ribavirin in Latino and non-Latino whites with hepatitis C. N Eng J Med 360:257–267
Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB (2009) Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461:399–401
Loguercio C, Frederico A, Massarone M, Torella R, Blanco Cdel V, Persico M (2008) The impact of diet on liver fibrosis and on response to interferon therapy in patients with HCV-related chronic hepatatis. Am J Gastroenterol 103:3159–3166
Daynes RA, Enioutina EY, Butler S, Mu HH, McGee ZA, Araneo BA (1996) Induction of common mucosal immunity by hormonally immunomodulated peripheral immunization. Infect Immun 64:1100–1109
Enioutina EY, Bareyan D, Daynes RA (2008) TLR ligands that stimulate the metabolism of vitamin D3 in activated murine dendritic cells can function as effective mucosal adjuvants to subcutaneously administered vaccines. Vaccine 26:601–613
Enioutina EY, Bareyan D, Daynes RA (2009) TLR-induced local metabolism of vitamin D3 plays an important role in the diversification of adaptive immune responses. J Immunol 182:4296–4305
Chadha MK, Fakih M, Muindi J, Tian L, Mashtare T, Johnson CS, Trump D (2011) Effect of 25-hydroxyvitamin D status on serological response to influenza vaccine in prostate cancer patients. Prostate 71:368–372
Daynes RA, Araneo BA, Hennebold J, Enioutina E, Mu HH (1995) Steroids as regulators of the mammalian immune response. J Invest Dermatol 105(1 Suppl):14S–19S
Daynes RA, Araneo BA (1994) The development of effective vaccine adjuvants employing natural regulators of T-cell lymphokine production in vivo. Ann NY Acad Sci 730:144–161
Lang PO, Govind S, Mitchell WA, Siegrist CA, Aspinall R (2011) Vaccine effectiveness in older individuals: what has been learned from the influenza-vaccine experience. Ageing Res Rev 10:389–395
Cooper C, Thorne A, Canadian HIV Trials Network Ctn Influenza Vaccine Research Group (2011) Vitamin D supplementation does not increase immunogenicity of seasonal influenza vaccine in HIV-infected adults. HIV Clin Trials 12:275–276
Conflicts of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lang, P.O., Samaras, N., Samaras, D. et al. How important is vitamin D in preventing infections?. Osteoporos Int 24, 1537–1553 (2013). https://doi.org/10.1007/s00198-012-2204-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-2204-6