Abstract
Summary
We have examined the effect of oral monthly ibandronate on distal radius and tibia microarchitecture with high-resolution peripheral quantitative tomography compared with placebo, in women with osteopenia, and found that ibandronate did not significantly affect trabecular bone but improved cortical density and thickness at the tibia.
Methods
We have examined the effect of ibandronate on bone microarchitecture with peripheral high-resolution quantitative computed tomography (HR-pQCT) in a randomized placebo-controlled trial among 148 women with osteopenia. Patients received either oral 150 mg monthly ibandronate or placebo over 24 months. Bone microarchitecture was assessed at baseline, 6, 12, and 24 months, using HR-pQCT at the distal radius and tibia; areal bone mineral density (aBMD) was measured with DXA at the spine, hip, and radius.
Results
At 12 months, there was no significant difference in trabecular bone volume at the radius (the primary end point) between women on ibandronate (10.8 ± 2.5%) and placebo (10.5 ± 2.9%), p = 0.25. There was no significant difference in other radius trabecular and cortical microarchitecture parameters at 12 and 24 months. In contrast, at the tibia, cortical vBMD in the ibandronate group was significantly greater than in the placebo group at 6, 12, and 24 months, with better cortical thickness at 6, 12, and 24 months. With ibandronate, aBMD was significantly increased at the hip and spine at 12 and 24 months but at the radius was significantly superior to placebo only at 24 months. Most of the adverse events related to ibandronate were expected with bisphosphonate use, and none of them were serious.
Conclusion
We conclude that 12 months of treatment with ibandronate in women with osteopenia did not affect trabecular bone microarchitecture, but improved cortical vBMD at the tibia at 12 and 24 months, and preserved cortical thickness at the tibia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Areal bone mineral density (aBMD) measured by dual energy X-ray absorptiometry (DXA) predicts at most half of fragility fractures among postmenopausal women when using a T-score <−2.5 SDs as a threshold [1, 2]. Therefore, bone microarchitecture has been proposed as a better way to explore bone fragility. A noninvasive examination of bone microarchitecture—high-resolution peripheral quantitative tomography (HR-pQCT)—has been developed in recent years, with good reproducibility and low irradiation exposure [3]. Using HR-pQCT, a few case–control studies have shown an association between bone microarchitecture and prevalent fracture that could be independent of aBMD [4–7]. Prospective studies to demonstrate the association between bone microarchitecture and fragility fracture are ongoing.
In addition, BMD measurement does not adequately describe the impact of therapeutic interventions [8]. Variations in BMD explain a limited proportion of fracture risk reduction in response to treatments, and fracture risk can be reduced even without significant BMD variation [9].Therefore, much interest is currently directed at the investigation of other factors associated with bone strength and therapeutic response, including bone geometry, cortical and trabecular microarchitecture, and tissue composition. For instance, measures of trabecular bone structure improve the prediction of bone mechanical properties [10, 11]. Microarchitecture may also better assess the effects of drugs than BMD variation alone [12, 13].
To date, three clinical trials using HR-pQCT providing longitudinal in vivo assessment of bone microarchitecture in response to therapeutic intervention have been reported. In a randomized placebo-controlled trial, alendronate use was associated with increased cortical and trabecular volumetric density [volumetric BMD (vBMD)] and cortical thickness at the tibia [14]. In another trial, denosumab compared to both placebo and alendronate prevented the decline or improved architecture variables both at the radius and tibia [15]. In a placebo-controlled trial, risedronate has been able to maintain cortical vBMD, which was declining in the placebo group [16].
Because the different bisphosphonates have different pharmacodynamic potencies, with possible effects on microarchitecture that would be proportional to their potency, we have conducted a randomized placebo-controlled trial to test the effect of another bisphosphonate, ibandronate, on bone microarchitecture at the distal radius and tibia, as assessed with HR-pQCT, in postmenopausal women with osteopenia over 2 years of treatment.
Methods
Patients
This randomized placebo-controlled, double-blind, 2-year trial has been conducted in four clinical centers in France (Lyon, Paris, Saint-Etienne, and Toulouse) between January 2007 and March 2010. Ambulatory postmenopausal women who had a −2.5 < T-score < −1.0 at either the spine or hip and without spine or hip T-score less than −2.5 could be enrolled. These women were chosen in this BMD range to be at mild risk for fracture, given the necessity of having a placebo group. Inclusion criteria also included age between 55 and 75, at least 1 year postmenopause, and no history of bisphosphonate therapy. Exclusion criteria were the medical conditions precluding informed consent or trial compliance; grade 2 and 3 vertebral fractures; hip fracture, gastroduodenal and esophageal diseases; malignancies in the last 5 years; creatinine clearance <30 mL/min; uncorrected hypocalcemia, intolerance to ibandronate, calcium, or vitamin D; inability to stand for 1 h following drug swallowing; any treatment interfering with bone metabolism; and all conditions known to affect bone metabolism.
The Lyon ethics committee approved the study protocol on 7 May 2006. The study was conducted according to the International Conference on Harmonization Good Clinical Practice Guidelines. All subjects provided written informed consent prior to enrollment. Women whose BMD at two out of three regions of interest (spine, femoral neck, total hip) would decrease of more than 6% during the first year of the study, or who would sustain a fragility fracture, could stop the study drug to be offered an appropriate medication by the study investigators, without unblinding.
Subjects were randomized 1:1 to oral monthly ibandronate (Bonviva 150 mg; n = 72; Roche, Basel, Switzerland) or placebo (n = 76). Subjects at each study site were randomized to treatment using a central interactive voice response system. Randomization proceeded according to a minimization scheme with center and bone volume (BV)/trabecular volume (TV) at screening as minimization factors. Subjects and study sites were blinded to the treatment. The placebo and ibandronate tablets were identical. Patients had to write the dates of drug intake on the pill box and give it back to the investigators at each study visit. If the treatment had not been taken properly, this was specified in the clinical report form to calculate the adherence rate. In addition to ibandronate or placebo, all patients also received 500 mg of elemental calcium and 400 IU of vitamin D3 daily.
BMD and microarchitecture measurements
Areal BMD was measured at the spine, radius, and hip with Hologic machines (Discovery A in Lyon, Delphi W in St Etienne, QDR 1000 in Paris) and a Lunar DPX in Toulouse that were cross-calibrated between sites, before starting the study, with the European Spine Phantom. A local phantom was measured daily, and these quality control data were analyzed centrally (Dr Kolta, Paris, France).
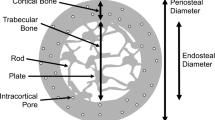
vBMD and microarchitecture were assessed at the nondominant distal radius and right distal tibia by HR-pQCT (XtremeCT; Scanco Medical, Bruttisellen, Switzerland). The four machines used in different clinical centers were not cross-calibrated. Measurements were made at the baseline, 6, 12, and 24 months of follow-up. The arm or leg of the patient was immobilized in a carbon fiber shell. An anteroposterior scout view was used to define the measured volume of interest (VOI). At each site, a stack of 110 parallel CT slices with an isotropic voxel size of 82 μm was obtained, thus delivering a 3D representation of approximately 9 mm in the axial direction. The most distal CT slice was placed 9.5 and 22.5 mm proximal to the endplate of the radius and tibia, respectively. Quality control was performed by daily scans of a phantom containing rods of hydroxyapatite (densities of 0, 100, 200, 400, and 800 mg/cm3) embedded in a soft tissue equivalent resin (QRM, Moehrendorf, Germany). The coefficient of variation (CV) for the densities varied from 0.7% to 1.5%. The VOI was separated into a cortical and trabecular region using a threshold-based algorithm. This threshold was set to one third of the cortical vBMD (Ct vBMD). Cortical thickness (Ct.Th) was defined as the mean cortical volume divided by the outer bone surface. Trabecular vBMD (Tb vBMD) in milligrams of hydroxyapatite (HA) per cubic centimeter was computed as the average vBMD within the trabecular VOI. BV fraction (BV/TV, in percent) was derived from Tb vBMD assuming fully mineralized bone to have a mineral density of 1.2 g HA/cm3, that is BV/TV (%) × 100 = [Tb vBMD (mg HA/cm3)/1.2 g HA/cm3]. Trabecular elements were identified by the midaxis transformation method, and the distance between them was assessed three dimensionally by the distance transform method. Trabecular number (Tb.N, per millimeter) was defined as the inverse of the mean spacing of the midaxes. Trabecular thickness (Tb.Th, in millimeters) and separation (Tb.Sp, in millimeters) were derived from BV/TV and Tb.N, that is Tb.Th = (BV/TV)/Tb.N and Tb.Sp = (1 − BV/TV)/Tb.N. Intra-individual distribution of separation (Tb.Sp SD, in millimeters) was quantified by standard deviation of Tb.Sp, a parameter reflecting the heterogeneity of the trabecular network.
The CV values for parameters of the radius and tibia, respectively, were as follows: total vBMD—0.9% and 1.3%, Ct vBMD—0.7% and 0.9%, Ct.Th—1.2% and 0.9%, Tb vBMD—1.0% and 1.5%, Tb.N—3.0% and 3.8%, Tb.Th—3.2% and 4.4%, Tb.Sp—2.8% and 4.3%, and Tb.Sp SD—2.5% and 3.3%. These were determined from three measurements with repositioning among 15 individuals aged 21–47 years [3].
End points
The primary efficacy end point was the BV/TV value at 12 months measured by HR-pQCT at the distal radius. Secondary efficacy end points included cortical thickness; total, cortical, and trabecular vBMD; trabecular number, thickness, and separation measured by HR-pQCT at the distal radius and tibia; areal BMD at the spine, hip, and radius measured by DXA; and the bone turnover markers serum C telopeptide of type I collagen cross-links (CTX) and procollagen type 1 N-terminal propeptide (P1NP). Safety was evaluated by adverse event (AE) reporting and monitoring changes in laboratory values and vital signs.
Biochemical measurements
Fasting serum was collected in the morning and stored at −80°C until assayed. Serum N-terminal extension propeptide of type I collagen (P1NP) and β-isomerized C-terminal telopeptide of type I collagen (CTX) were measured by human-specific two-site immunochemiluminescence assay (ELECSYS; Roche, Indianapolis, IN, USA).
For P1NP, the detection limit is 5 ng/mL. The interassay CV is 4.7% for 41 ng/mL, 4.5% for 74.7 ng/mL, 5.1% for 381.5 ng/mL, and 5.9% for 766.7 ng/mL. For CTX, the detection limit is 0.01 ng/mL. The interassay CV is 3.7% for 0.3 ng/mL, 2.3% for 0.36 ng/mL, 3.8% for 0.69 ng/mL, and 5% for 2.8 ng/mL. The measurements were made at the baseline and 3, 6, 12, 18, and 24 months of follow-up.
Statistics
The sample size calculation was based on the assumption of detecting a difference of 15% in the mean BV/TV (after 12 months of treatment) between the ibandronate and the placebo group (mean BV/TV value for osteopenic women, 0.103) with a common standard deviation of 0.03. On the basis of these assumptions with a two-sided 5% significance level, a sample size of 118 patients randomized (59 per treatment group) provided the study with 90% power to detect a difference between the treatments. The analyses were performed on the intent-to-treat (ITT) population defined as randomized patients who took at least one pill of study treatment. In case of premature withdrawal or missing value on the primary criterion, the following rules were applied:
-
Last observation carried forward method was used if the BV/TV value at 6 months was available and
-
The treatment arm mean value of BV/TV (in which the patient was randomized) replaced if the BV/TV value at 6 months was not available.
Quantitative data were expressed as mean and standard deviation (mean ± SD) or median and range and qualitative data as number and percentage (percentage have been calculated excluding missing values). The comparisons between the two treatment groups at each time point in the bone volumetric and geometric parameters, areal BMD, and bone turnover markers were performed using an analysis of covariance model (ANCOVA), adjusting for baseline values. The correlations between the variation of bone volumetric and geometric parameters and the variation of bone turnover markers were performed using the Pearson correlation coefficients.
Results
Among the 151 patients screened, 149 patients were randomized (72 in ibandronate group and 77 in placebo group). One patient was randomized but did not receive treatment. Thus, the ITT population consists of 148 patients (72 in the ibandronate group and 76 in the placebo group). Patient disposition is displayed in Fig. 1.
The baseline variables did not differ between those women on ibandronate and those on placebo (Table 1). Adherence to treatment was 99% in both groups.
At 12 months, there was no significant difference in BV/TV at the radius (primary end point) between women on ibandronate (10.8 ± 2.5%) and placebo (10.5 ± 2.9%), p = 0.25. Similarly, there was no significant difference in other radius trabecular and cortical microarchitecture parameters at 6, 12, and 24 months (Table 2).
In contrast, at the tibia, total and cortical vBMD in the ibandronate group were significantly greater than in the placebo group as soon as 6 months and also at 12 and 24 months in parallel with better Ct.Th at 6, 12, and 24 months (Table 3). At the radius (Table 2), trabecular area (Tb.Ar) and cortical area (Ct.Ar) did not significantly differ between the ibandronate and placebo groups. At the tibia (Table 3), at the different time points, we observed a significant difference between the two groups, the trabecular area tending to be greater in the placebo group. In parallel, the cortical areas in the ibandronate and placebo groups also significantly differed from 6 to 24 months, tending to be greater in the ibandronate group. At the tibia, the total area (Tt.Ar) did not differ between groups, whereas there was a slight difference at 24 months at the radius. Other tibial microarchitecture trabecular parameters remained unaltered at 6, 12, and 24 months in treated women compared with placebo (Table 3).
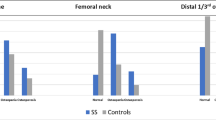
In the ibandronate group, areal BMD was significantly increased at the hip and spine at 12 and 24 months, and significantly superior to placebo only at 24 months at the radius (Fig. 2). After 1 year of treatment, there was a statistically significant difference between patients receiving ibandronate and patients receiving the placebo in BMD measured at the lumbar spine, femoral neck, and total femur (p < 0.001). Mean lumbar and hip BMD was higher in the group receiving ibandronate compared to the group on placebo, with an increase in BMD from screening while change from screening in the placebo group was close to 0 and even negative. These results were confirmed after 2 years of treatment. At the radius site (wrist), there was no difference in mean BMD between the two groups after 1 year of treatment, but mean BMD was significantly higher in the group receiving ibandronate compared to the group receiving the placebo after 2 years of treatment (p = 0.009). We have tested for the interaction between prior estrogen treatment and the variation in BMD in response to therapy. It was not significant, at the femoral neck, spine, and forearm (data not shown).
In the ibandronate group, CTX and P1NP were significantly reduced compared with the placebo group from 3 to 24 months (Fig. 3). The variation of markers was not associated significantly with the variation of microarchitecture parameters at the radius. At the tibia, cortical area was associated moderately with the markers variation at 6 (r = −0.32, p = 0.009 for CTX; r = −0.26, p = 0.03 for P1NP), 12 (r = −0.35, p = 0.003 for CTX; r = −0.3, p = 0.01 for P1NP), and 24 months (r = −0.29, p = 0.02 for CTX; r = −0.28, p = 0.03 for P1NP). This was also the case with cortical thickness at 6 (r = −0.33, p = 0.006 for CTX; r = −0.26, p = 0.03 for P1NP), 12 (r = −0.34, p = 0.004 for CTX; r = −0.27, p = 0.03 for P1NP), and 24 months (r = −0.28, p = 0.03 for CTX; r = −0.26, p = 0.04 for P1NP). Cortical density was also associated with P1NP variation at 12 (r = −0.26, p = 0.03) and 24 months (r = −0.34, p = 0.007).
The percentage of patients with at least one AE or one serious AE was similar in the ibandronate and placebo groups (90% vs. 93% for total AEs and 21% vs. 17% for serious AEs). The most frequent AEs were back pain (16% of the safety population), arthralgia (12%), and bronchitis (15%). More cases of fractures were reported in the placebo group compared to the ibandronate group, and falls were reported only in the placebo group.
For 12 patients [8 in the ibandronate group (11%) and 4 in the placebo group (5%)], AEs were considered related to the study treatment. Most AEs considered related to ibandronate were expected AEs with the use of bisphosphonates, and were completely resolved except for one case of severe rheumatoid arthritis that was persisting. Ten patients (7%) had to discontinue the treatment because of an adverse event: four in the ibandronate group and six in the placebo group. In the placebo group, most treatment discontinuations were due to occurrence of fractures. A total of 28 patients experienced at least one adverse event that was considered serious: 15 in the ibandronate group (21%) and 13 in the placebo group (17%). Only one of them was considered related to the study treatment: visual acuity reduced in the placebo group, and for another one: chest pain in the ibandronate group, the relationship to the treatment was unknown. The study data did not change the known benefit/risk ratio of ibandronate.
Discussion
At the radius, we found that ibandronate increased slightly the cortical and total vBMD at 24 months, but did not modify the trabecular parameters, including the primary end point, the BV/TV at 12 months. At the tibia, cortical vBMD increased progressively in ibandronate, from baseline to 24 months; meanwhile, cortical thickness was maintained on ibandronate, whereas it tended to decline in placebo patients. In parallel, at the tibia also, trabecular parameters were not improved by ibandronate.
We have measured microarchitecture only at two peripheral sites. So our results cannot be inferred to other sites. We found that the tibia was more responsive to treatment than the radius. The cortical bone response was better at this weight-bearing site, which may be more appropriate for treatment monitoring. This observation is consistent with the results of comparable studies [14–16]. It is possible that the mechanical stimulus at the tibia improves response to antiresorptive treatment. Alternatively, the response to bisphosphonates at the radius has always been shown as poorer than at the other sites when examined with the measurement of aBMD. This is also true in three other studies showing that the effect of bisphosphonates at the radius was minimal or undetectable with HR-pQCT [14–16]. An additive effect of exercise and bisphosphonates on bone—which has already been observed in ovariectomized rats [17] and in patients with osteoporosis associated with lung transplantation [18]—could at least in part explain our results, supporting the use of the tibia site as a preferred site for monitoring. It has also been shown that the bone response detectable with HR-pQCT seems to be proportional to the antiresorptive potency, at least at the tibia [14–16]. Thus, if we compare our trial and the three other studies, we can observe that the cortical vBMD and cortical thickness maintenance or improvement were greater with denosumab than with alendronate. With alendronate, this effect seemed greater than with risedronate, but this is an indirect comparison.
There are technical limitations to the longitudinal evaluation of cortical thickness. The analysis we have conducted is limited to geometrical and microarchitectural response to therapy, but does not address the issue of bone material effects. Indeed, mineral changes induced by antiresorptive agents are likely to contribute to the antifracture efficacy [19].
Besides, the common region of interest between baseline and follow-up scans are determined based on cross-sectional area matching, assuming a constant area over time. As a result, the total areas are necessarily the same when measured longitudinally. In addition, it has been shown on cadaver radius that shifting the radius region of interest by ±0.5 mm could lead to a 6% error, whereas this error was only 2% at the tibia [20]. The cross-sectional area is unlikely to have been modified by the bisphosphonate treatment, but the role of a faulty region of interest remains uncertain. The increase in vBMD due to the decrease in bone resorption can influence edge detection and artifactually increase the cortical area. This would result in an artificial increase in cortical thickness. A ±5% change in threshold can produce a 5% difference in cortical area [21]. In fact, the increase in cortical bone density leading to apparent improvement in cortical thickness is likely to be due to filling of cortical porosity—increasing the cortical area—because any real thickening is beyond the resolution of HR-pQCT. This hypothesis is supported by the observed decreased Tb.Ar and increased Ct.Ar on ibandronate compared with placebo at the tibia, while the Tt.Ar remained constant over time, suggesting that the greater Ct.Th is due to refilled endocortical porosity. Increased Ct.Th may improve bone mechanical properties, because Ct.Th measured by this technique has been shown to correlate with mechanical strength [21].
The participants had mild osteopenia. It is uncertain whether women with lower BMD—associated with greater cortical porosity—would have had similar cortical vBMD increase. Their response to treatment may also depend on the time elapsed since menopause because cortical porosity filling by inhibition of bone resorption is probably less efficient in the early phase of rapid bone loss following the menopause. Motion artifacts are common, especially when measuring the radius. The scan quality is not always adequate. In our intent-to-treat analysis, we have not excluded any radius scan, despite the variability in scan quality, to avoid missing values and ensure the validity of the statistical analysis. We recognize that this may bias our results towards the null.
We conclude that 12 months of treatment with ibandronate in women with osteopenia was well tolerated and did not affect trabecular bone microarchitecture, but improved cortical vBMD at the tibia at 12 and 24 months, as well as Ct.Th at the tibia, possibly corresponding to decreased cortical porosity. This might be relevant regarding protection against nonvertebral fracture. The tibia may be a better bone site to evaluate the effects of antiresorptive agents with HR-pQCT.
References
Siris ES, Miller PD, Barrett-Connor E et al (2001) Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: result from the National Osteoporosis Risk Assessment. JAMA 286:2815–2822
Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD (2005) Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res 16:1184–1192
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2006) In vivo assessment of trabecular bone microarchitecture by high resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:6508–6515
Majumdar S, Genant HK, Grampp S et al (1997) Correlation, of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res 12:111–118
Sornay-Rendu E, Cabrera-Bravo JL, Boutroy S, Munoz F, Delmas PD (2009) Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women. J Bone Miner Res 24:737–743
Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD (2007) Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res 22:425–433
Melton LJ III, Riggs BL, Keaveny TM, Achenbach SJ, Kopperdahl D, Camp JJ, Rouleau PA, Amin S, Atkinson EJ, Robb RA, Therneau TM, Khosla S (2010) Relation of vertebral deformities to bone density, structure and strength. J Bone Miner Res 25:1922–1930
Delmas PD, Seeman E (2004) Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with antiresorptive therapy. Bone 34:599–604
Chapurlat RD, Ramsay P, Palermo L, Cummings SR (2005) Risk of fracture among women who lose bone density during treatment with alendronate. The Fracture Intervention Trial. Osteoporos Int 16:842–848
Gordon CL, Lang TF, Augat P, Genant HK (1998) Image-based assessment of spinal trabecular bone structure from high-resolution CT images. Osteoporos Int 8:317–325
Link TM, Vieth V, Langenberg R et al (2003) Structure analysis of high resolution magnetic resonance imaging of the proximal femur: in vitro correlation with biomechanical strength and BMD. Calcif Tissue Int 72:156–165
Chesnut CH 3rd, Rosen CJ (2003) Reconsidering the effects of antiresorptive therapies in reducing osteoporotic fracture. J Bone Miner Res 16:2163–2172
Riggs BL, Melton LJ 3rd (2002) Bone turnover matters: the raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density. J Bone Miner Res 17:11–14
Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S (2010) A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res 25:2558–2571
Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, Kearns A, Thomas T, Boyd SK, Boutroy S, Bogado C, Majumdar S, Fan M, Libanati C, Zanchetta J (2010) Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 25:1886–1894
Seeman E, Chapurlat R, Cheung A, Felsenberg D, Laroche M, Reeve J, Thomas T, Zanchetta J, Bock O, Morris E, Tile L, D’Alo G, Darbie L, Borah B, Rizzoli R (2010) Risedronate reduces microstructural deterioration of cortical bone accompanying menopause. Osteoporos Int 21(Suppl1):S9
Fuchs RK, Shea M, Durski SL, Winters-Stone KM, Widrick J, Snow CM (2007) Individual and combined effects of exercise and alendronate on bone mass and strength in ovariectomized rats. Bone 41:290–296
Braith RW, Conner JA, Fulton MN, Lisor CF, Casey DP, Howe KS, Baz MA (2007) Comparison of alendronate vs alendronate plus mechanical loading as prophylaxis for osteoporosis in lung transplant recipients: a pilot study. J Heart Lung Transplant 26(2):132–137
Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ (2000) Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 27:687–694
Boyd SK (2008) Site-specific variation of bone micro-architecture in the distal radius and tibia. J Clin Densitom 11:424–430
Davis KA, Burghardt AJ, Link TM, Majumdar S (2007) The effects of geometric and threshold definitions on cortical bone metrics assessed by in vivo high-resolution peripheral quantitative computed tomography. Calcif Tissue Int 81:364–371
Conflicts of interest
The study has been designed collaboratively between the investigators and the sponsor (Roche). The study conduct, data collection, statistical analysis, and funding were the responsibility of the sponsor. The manuscript was drafted by R Chapurlat. All other authors participated in collecting data and critical review of drafts and approved the submitted manuscript. Authors had access to all study data. The decision to submit the manuscript was at the discretion of the authors. Roland Chapurlat has received research funding and/or honoraria from Amgen, Servier, Novartis, Roche, Merck, Lilly, Ipsen, Chugai. Thierry Thomas has received research funding and/or honoraria from Amgen, BMS, Chugai, GSK, Ipsen, Lilly, MSD, Novartis, Roche, Servier, Warner-Chilcott. Stéphanie Rouanet is an employee of Roche. Marie-Christine de Vernejoul and Michel Laroche have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pierre D. Delmas is deceased.
Rights and permissions
About this article
Cite this article
Chapurlat, R.D., Laroche, M., Thomas, T. et al. Effect of oral monthly ibandronate on bone microarchitecture in women with osteopenia—a randomized placebo-controlled trial. Osteoporos Int 24, 311–320 (2013). https://doi.org/10.1007/s00198-012-1947-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-1947-4