Abstract
Introduction
Bone metabolism disturbances following renal transplantation (RT) are complex and multifactorial in origin. Abnormalities in 1,25-dihydroxyvitamin D levels in RT patients under treatment at our Bone Center prompted this retrospective study.
Methods
Parameters of vitamin D metabolism were compared in RT patients and a cohort of patients with primary hyperparathyroidism (PHTP) who mimicked the hyperparathyroid state of the RT patients. Thirty-one RT recipients (from 300 reviewed) matched our inclusion criteria with a stable graft function for more than 1 year and a glomerular filtration rate (GFR) >50 mL/min per 1.73 m2 (Group A); these were compared with 42 consecutive patients with PHTP who had been referred to the same Bone Center for treatment for over 1 month (Group B). Statistical analysis included the chi-square or Fisher’s exact tests for categorical data and the Wilcoxon rank sum test for quantitative measures.
Results
The mean (±SD) 1,25-dihydroxyvitamin D level was significantly lower (p < 0.001) in Group A patients (29.8 ± 16.2) than in Group B patients (70.2 ± 25.9) despite non-significant differences in the levels of parathyroid hormone (PTH) (mean: 184.0 vs.101.1;p < 0.29), phosphorus (mean: 3.2 vs. 3.1; p < 0.3) and 1,25-vitamin D (mean: 19.5 vs. 25.2; p < 0.06). Group A patients had lower levels (p < 0.05) of mean serum calcium and calculated GFR (9.3 mg/dL, 65.7 mL/min) than Group B patients (10.6 mg/dL, 97.6 mL/min). 1,25-Dihydroxyvitamin D significantly correlated with calcium (p < 0.001), 25-vitamin D (p < 0.005) and GFR (p < 0.001) in both groups, but there was a notable lack of association between 1,25-dihydroxyvitamin D and PTH (p < 0.64) or phosphorus (p < 0.26) in Group A patients. In this group, 1,25-dihydroxyvitamin D was not influenced by the type of immunosuppresion regimen (p < 0.06), use of biphosphonates (p < 0.73), presence of diabetes (p < 0.59), menopause in women (p < 0.08), season (p < 0.43) or race (p < 0.31). Our data indicate that 1,25-dihydroxyvitamin D metabolism remains disturbed for a considerable time after successful RT, with the result that the level of 1,25-dihydroxyvitamin D in RT patients is lower despite physiological signals that should stimulate its production. Our analysis of many clinical variables was unable to elucidate the underlying mechanism(s) for this disturbance.
Conclusion
Successful RT may not produce appropriate levels of 1,25-dihydroxyvitamin D commensurate to the elevated levels of PTH. This abnormality along with sustained hyperparathyroidism may contribute to bone loss following transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compared to other solid organ transplantation, renal transplantation (RT) is characterized by renal osteodystrophy, a unique pre-transplantation bone disease that includes osteitis fibrosa, osteomalacia, osteoporosis and, less often, adynamic bone disease. Post-transplantation bone disease is an extension of this state coupled with the effects of anti-rejection drugs. In successful RT, the degree of renal function recovery is usually incomplete. While the definition of optimal graft function is arbitrary, a creatinine level of more than 1.5 mg/dL is considered to indicate renal dysfunction [1]. Information is scarce about the recovery of endocrine function, with some data showing a reversal of hyperparathyroidism in 45% of the cases at 2 years [2] or in just 46% of the cases even after 8 years [3].
Several other studies have shown that the levels of parathyroid hormone (PTH) and osteocalcin generally decrease after transplantation [3–7], while those of alkaline phosphatase [8, 9] and urinary NTX [3] are unpredictable. The levels of 1,25-dihydroxyvitamin D in patients following RT have been found to be variable, with various studies reporting increased [5], decreased [6] or normal [8, 9] levels.
How much of the exaggerated bone loss after the transplant is due to 1,25-dihydroxyvitamin D is not completely understood. Abnormalities in 1,25-dihydroxyvitamin D levels in some RT patients seen at our Bone Center prompted this study. We present our retrospective cross-sectional analysis of 1,25-dihydroxyvitamin D levels in two groups of patients – RT patients and patients with primary hyperparathyroidism (PHTP) – and present an analysis of several variables to try to explain the discrepancy.
Methods
We retrospectively reviewed data from 300 patients who received a renal transplant, ultimately choosing 31 patients (Group A) that matched our inclusion criteria. These patients had a serum creatinine < 1.5 mg/dL, a GFR >50 mL/min per 1.73 m2 and a stable graft function for more than 1 year; the mean (±SD) length of time since the RT was 1.9 ± 0.8 years. This group of patients was compared with 42 patients with primary hyperparathyroidism (Group B).
GFR was calculated using abbreviated the MDRD (Modification of Diet in Renal Disease) equation: GFR (in mL/min per 1.73 m2) = 186.3 × PCr [exp(−1.154)] × Age [exp(−0.203)] × (0.742 if female) × (1.21 if black) where exp is the exponential [10]. The study was approved by the Institutional Board of the Bone Center.
Serum calcium, creatinine and urinary calcium were measured by autoanalyzer, PTH was measured by chemiluminescence immunoassay (CLIA), 25-OH vitamin D by chemiluminescence (CL), 1,25-dihydroxyvitamin D by radioimmunoassay (RIA) and urinary NTx ((cross-linked N-telopeptides of type I collagen) by enzyme immunoassay (EIA). The PTH assay had a 6% inter-assay and 2% intra-assay coefficient of variability (CV). The CV for 1,25-dihydroxyvitamin D was 14.7–15.3% (intra-assay) and 9.5–11.0% ( inter-assay). Bone mineral density (BMD) was measured by dual-energy X-ray absorptiometry (DXA) at the lumbar spine and femoral neck with a Lunar Prodigy scanner DF 1003 (Lunar Corp; General Electric, Madison, Wis.; CV: 3–4%).
Immunosupression therapy in Group A patients: four different regimens
The Group A patients received one of four different immunosupression therapy regimen: cyclosporine, tacrolimus and sirolimus (cycl,t,s); prednisone, mycophenolate and sirolimus (p,m,s); prednisone, mycophenolate and tacrolimus (p,m,t) and prednisone, cyclosporine and tacrolimus (p,cycl,t), respectively. None of the patients were treated with calcitriol.
Statistical analysis
1,25-Dihydroxyvitamin D was studied for any relationship to categorical or quantitative variables. Categorical variables were analyzed by the Kruskal-Wallis test and quantitative variables by the Spearman correlation.
Results
Table 1 presents the clinical and socio-demographic characteristics of the patients participating in this study. The study was carried out at a mean 1.9 ± 0.8 years after the RT, with Group A patients being on dialysis prior to the RT for a mean of 8.3 ± 7.3 years. The clinical parameters of Group A patients are shown in Table 2.
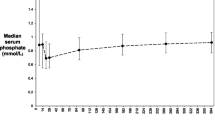
The mean (±SD) 1,25-dihydroxyvitamin D level was significantly lower (p < 0.001) in Group A (29.8 ± 16.2) than in Group B patients (70.2 ± 25.9) despite non-significant differences in the mean levels of PTH (184.0 vs. 101.1;p < 0.29), phosphorus (3.2 vs. 3.1; p < 0.3) and 25 hydroxyvitamin D (19.5 vs. 25.2; p < 0.06). Group A patients had lower levels of (p < 0.05) mean serum Ca and calculated GFR (9.4 mg/dL; 65.7 mL/min) than Group B patients (10.6 mg/dL, 97.6 mL/min) (Tables 1 and 3).
In Group A patients, the 1, 25-dihydroxyvitamin D level was significantly correlated with the levels of calcium (p < 0.001), 25-hydroxyvitamin D (p < 0.005) and GFR (p < 0.001), but not with PTH (p < 0.640) or phosphorus (p < 0.26) (Table 4).
The level of 1,25-dihydroxyvitamin D in Group A patients was not influenced by the type of immunosuppression regimen (p < 0.06), use of bisphosphonates (p < 0.73), presence of diabetes (p < 0.59), menopause in women (p < 0.08), season (p < 0.43) or race (p < 0.31). Turnover markers of bone resorption as NTx (mean: 71.2 vs. 58.9; p < 0.98), lumbar T scores (mean: −1.4 vs. −1.2; p < 0.95) and hip T-scores (mean: −1.7 vs. −1.4; p < 0.23) were not significantly different in the two groups. Bisphosphonates were used in both groups.
Discussion
Several factors that are known to limit the chance of complete osseous recovery in renal allograft recipients are incomplete restoration of renal function or the development of graft failure, continued hyperparathyroidism and chronic administration of drugs that interact negatively with bone metabolism. Our study suggests that vitamin D abnormalities may also be a factor.
Even in the theoretical setting of a perfectly functioning renal graft donated between monozygotic twins, the reversibility of renal osteodystrophy is only partial [11]. A recent study reports that bone histomorphometric dynamic parameters became normal in less than 50% of any one group of patients at 12 months post-RT [8].
Increased bone resorption driven by continued hyperparathyroidism limits recovery, but abnormalities in the vitamin D level may play a heretofore unrecognized effect independent of PTH. In chronic renal failure, the impairment of renal 1,25-dihydroxyvitamin D production is an important player in the initial sequence of events leading to secondary hyperparathyroidism (absence of negative feedback loop) [12, 13]. This failure to produce enough biologically active vitamin D following RT could prevent the resolution of the hyperparathyroidism [6]. Several hypotheses have been proposed to explain post-RT dysfunctional vitamin D metabolism. These include an increased level of circulating fibroblastic growth factor-23 (FGF-23), which has been found to have an impact on the renal synthesis of 1,25-dihydroxyvitamin D with the progression to chronic renal failure [14]. 1,25-Dihydroxyvitamin D levels were lower in RT patients despite the presence of high circulating levels of its physiological stimulator PTH, which were comparable to the levels seen in PHPT patients (p < 0.001). The statistical significance persisted even all other factors had been adjusted.
We know that there are discrepancies between the measurements of PTH using a bio-intact assay and the usual common intact PTH assay as the latter will pick up not only 1-84 PTH, but also 7-84 fragments [15]. Diagnostic information on parathyroid activity provided by common PTH or bio-intact PTH may differ in individual patients. It is conceivable that in using bio-intact PTH as an indicator we would have marginally lower levels of PTH in the transplant group but a higher common intact assay (more fragment 7-84 due to lower GFR). However, it has to be recognized that a bio-intact PTH will produce lower values in the HPT group, so that proportionately, we may see the same results.
The data on 1,25-dihydroxyvitamin D levels in patients following RT are contradictory, with published values ranging from very low (0.01 pg/mL) to very high (105 pg/mL) [16–20]. Some authors have suggested that the 1,25-dihydroxyvitamin D level is normal several weeks after transplantation [9, 19], while others have found low levels of 1,25-dihydroxyvitamin D in 78% of the patients at 30 days post-RT [16, 17]. Tubular necrosis and high doses of cytotoxic drugs were implicated in low calcitriol levels the first several weeks after RT [6, 17]. This hypothesis does not fit our case, where the median interval after RT was 2 years.
Researchers carrying out a long-term study reported that successful RT restored the capacity to synthesize active vitamin D after 1–131 months (mean ± SD: 23 ± 23 months) albeit to lower levels than those found in normal healthy subjects [20]. These values were highly correlated (p value < 0.001) with GFR, as also found in our patients. On the other hand, a prospective study demonstrated that 1,25-dihydroxyvitamin D levels remained below the normal range in one half of the patients, even at 6 months post-RT, and did not correlate with PTH but did significantly correlate with GFR, as in our patients [6].
It has been suggested that lower levels of 1,25-dihydroxyvitamin D could be related to the steroid dose. This was not confirmed in our study because all of the participants received the same cumulative steroid dose per protocol. However, there was no significant relationship between 1,25-dihydroxyvitamin D and any one of the immunosupression regimens. Interestingly, the one patient in our study on a steroid-free regimen had a 1,25-dihydroxyvitamin D level that fell in the lower tertile.
One could assume that the lower 1,25-dihydroxyvitamin D level could be due to low levels of precursor or enzymatic deficiency. In our data, serum 25-hydroxyvitamin D levels tended to be lower( p < 0.06) in RT patients than in the controls (PHPT), which is in agreement with their results from other studies [18], but the statistical difference in 1,25- dihydroxyvitamin D levels in the two groups persisted even after we had adjusted for 25-hydroxyvitamin D levels.
A deficit in 1 alpha hydroxylase (reduction in synthesis, activity or relative resistance to PTH) could cause low levels of 1,25-dihydroxyvitamin D (effects of steroids, ischemic damage or suboptimal graft function) [6]. Interestingly, three patients using cyclosporine, a known stimulator of this enzyme [21], had levels of 1,25-dihydroxyvitamin D that fell in the lower tertile.
Finally, the lower levels of 1,25-dihydroxyvitamin D could be due to its increased catabolism. Further studies will be required to address these possibilities.
In our patient cohort, menopause appeared to be a possible factor in the decline of 1,25-dihydroxyvitamin D levels (the relationship almost reached statistical significance). Although the reason for this is unclear, animal models (chicks) have shown that acute administration of estrogens increases renal 1 alpha hydroxylase activity and vitamin levels up to 25-fold, perhaps through an indirect effect of estradiol [22]. Moreover, serum 1,25-dihydroxyvitamin D levels were found to increase in postmenopausal women after treatment with estrogen [23], hence our findings. Interestingly enough, estrogen replacement may not protect the patient from bone loss after RT [24]. It is unclear how beneficial vitamin D may be for these patients. The latest Cochrane Database Systematic Review (2005) of trials reporting the use of bisphosphonates, vitamin D analogues, calcitonin and hormone replacement therapy to treat bone disease following renal engraftment did not find any benefit from any of these interventions in reducing the fracture risk [25].
Accelerated bone loss is a well-recognized complication after cardiac transplantation. In clinical studies, the administration of low-dose calcitriol has not been shown to have any significant extra benefit with respect to bone mineral density (BMD) and fracture rate in the long-term period after heart transplant [26].
Overall, a review of the literature on the benefits of vitamin D supplementation on bone after RT reveals contradictory results. The treatment of 111 patients with a low dose (0.25 μg) of calcitriol and 1000 mg elemental calcium was reported to partially prevent bone loss at the lumbar spine and proximal femur during the first 6 months after RT, and protection at the proximal femur was seen with use of calcium supplements for 1 year plus intermittent calcitriol for the first 3 months [27]. Conversely, a controlled study using calcium and a monthly dose of 25,000 IU of vitamin D3 for 12 months versus just calcium showed that the addition of vitamin D to the treatment regime normalized PTH but did not prevent bone loss in the lumbar spine in RT patients on low-dose steroids [28]. In addition, the prophylactic administration of vitamin D and calcium was not sufficient to prevent the progression of osteopathy after RT, and changes in bone density were more affected by graft function [29].
One explanation for these different results in BMD response to calcium or vitamin D in RT patients could be related to the vitamin D receptor (VDR) genotype. The VDR genotype polymorphism affects the bone density of renal transplant recipients via its effects on the severity of SHPT (secondary hyperparathyroidism). The prevalence of the VDR polymorphism is higher in patients with sporadic PHPT [30], with SHPT as a result of chronic kidney disease and in RT patients.
In a study on 75 RT patients, mean PTH levels were higher in these patients than in the normal population, [3] but they were elevated only in the presence of the bb VDR polymorphism. The BB VDR genotype, on the contrary, was associated with almost normal PTH and the lowest proportion of persisting hyperparathyroidism [4, 31–33]. It has been suggested that a decreased transcriptional activity or stability of the VDR mRNA in patients with the bb aplotype could explain the decreased effects of calcitriol on the parathyroid gland [34]. A better understanding of this mechanism would help identify patients in whom calcitriol treatment may have a protective role on bone density. Moreover, calcitriol therapy may have a beneficial effect on graft survival in addition to its skeletal role [35, 36].
Conclusion
Abnormalities of bone metabolism are complex and multifactorial in patients who have had a RT. The successful RT does not produce appropriate levels of 1,25-dihydroxyvitamin D. Patients with long-term RT and normal renal function frequently present with increases in PTH but low levels of 1,25-dihydroxyvitamin D. The levels of 1,25-dihydroxyvitamin D in RT patients are lower despite the physiological signals that should stimulate its production. Although many clinical variables have been analyzed, the underlying mechanism of this shortfall in 1,25-dihydroxyvitamin level remains unclear. This abnormality along with sustained hyperparathyroidism may contribute to bone loss following transplantation.
Further studies will be required to address these abnormalities, provide novel information on their pathophysiology and devise ‘the best’ therapy to prevent bone disease in patients following a kidney transplant.
References
Siddiqi N, McBride M, Hariharan S (2004) Similar risk profiles for post-transplant renal dysfunction and long-term graft failure: UNOS/OPTN database analysis. Kidney Int 65(5):1906–1913
Lobo R, Cortez MS, Stevenson WC, Pruett TL (1995) Normocalcemic hyperparathyroidism associated with relatively low 1:25 vitamin D levels post-renal transplant can be successfully treated with oral calcitriol. Clin Transplant 9(4):277–281
Rubello D et al (2005) Secondary hyperparathyroidism is associated with vitamin D receptor polymorphism and bone density after renal transplantation. Biomed Pharmacother 59(7):402–407
Messa P et al (1998) Persistent secondary hyperparathyroidism after renal transplantation. Kidney Int 54(5):1704–1713
Mikuls T et al (2003) Bone mineral density changes within six months of renal transplantation. Transplantation 75(1):49–54
de Sevaux R et al (2003) Abnormal vitamin D metabolism and loss of bone mass after renal transplantation. Nephron Physiol 93(1):C21–C28
Kim H et al (1998) Bone mineral density after renal transplantation. Transplant Proc 30(7):3029–3030
Abdallah K et al (2006) Improvement of adynamic bone disease after renal transplantation. Braz J Med Biol Res 39(1):31–41
Julian et al (1991) Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med 325(8):544–550
Levey A et al (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130(6):461–470
Brandenburg V, Westenfeld R, Ketteler M (2004) The fate of bone after renal transplantation. J Nephrol 17(2):190–204
Silver J, Levi R (2005) Regulation of PTH synthesis and secretion relevant to the management of secondary hyperparathyroidism in chronic kidney disease. Kidney Int Suppl 95:S8–S12
Levi R, Silver J (2005) Pathogenesis of parathyroid dysfunction in end-stage kidney disease. Pediatr Nephrol 20(3):342–345
Gutierrez O et al (2005) Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16(7):2205–2215
Reichel H et al (2003) Influence of PTH assay methodology on differential diagnosis of renal bone disease. Nephrol Dial Transplant 18(4):759–768
Bonnin M et al (1990) 1,25-Dihydroxycholecalciferol as measured by a radioreceptor assay in normal subjects and patients after kidney transplantation. Clin Chem 36(2):389–390
Bonnin M et al (1992) Serum calcitriol concentrations in the early follow-up after renal transplantation. Transplant Proc 24(1):103–104
Querings K et al (2006) 25-hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab 91(2):526–529
Reinhardt W et al (1998) Sequential changes of biochemical bone parameters after kidney transplantation. Nephrol Dial Transplant 13(2):436–442
Riancho J et al (1988) Serum levels of 1,25-dihydroxyvitamin D after renal transplantation. Miner Electrolyte Metab 14(6):332–337
Stein B et al (1991) Cyclosporin-A increases synthesis of 1,25-dihydroxyvitamin D3 in the rat and mouse. Endocrinology 128(3):1369–1373
Henry H (1981) 25(OH)D3 metabolism in kidney cell cultures: lack of a direct effect of estradiol. Am J Physiol 240(2):E119–E124
Gallagher J, B Riggs, DeLuca H (1980) Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab 51(6):1359–1364
Heaf J et al (2003) Hyperparathyroidism and long-term bone loss after renal transplantation. Clin Transplant 17(3):268–274
Palmer S, McGregor D, Strippoli G (2005) Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst Rev 2:CD005015
Stempfle H et al (1999) Prevention of osteoporosis after cardiac transplantation: a prospective, longitudinal, randomized, double-blind trial with calcitriol. Transplantation (Baltimore) 68(4):523–530
Torres A et al (2004) Treatment with intermittent calcitriol and calcium reduces bone loss after renal transplantation. Kidney Int 65(2):705–712
Wissing K et al (2005) A controlled study of vitamin D3 to prevent bone loss in renal-transplant patients receiving low doses of steroids. Transplantation (Baltimore) 79(1):108–115
Al-Gabri S et al (2005) Changes in bone mineral density and selected metabolic parameters over 24 months following renal transplantation. Transplant Proc 37(2):1014–1019
Carling T et al (1997) Vitamin D receptor polymorphisms correlate to parathyroid cell function in primary hyperparathyroidism. J Clin Endocrinol Metab 82(6):1772–1775
Giannini S et al (2002) The effects of vitamin D receptor polymorphism on secondary hyperparathyroidism and bone density after renal transplantation. J Bone Miner Res 17(10):1768–1773
Toro J, Gentil MA, Garcia R, Perez-Valdivia MA et al (2005) Alendronate in kidney transplant patients: a single-center experience. Transplant Proc 37(3):1471–1472
Falkiewicz K, BidziDska B, Demissie M, BoratyDska M, Zmonarski SC, Tworowska K et al (2005) Influence of vitamin D receptor gene polymorphisms on secondary hyperparathyroidism and bone density after kidney transplantation. Transplant Proc 37(2):1023–1025
Bover J, Bosch R (1999) Vitamin D receptor polymorphisms as a determinant of bone mass and PTH secretion: from facts to controversies. Nephrol Dial Transplant 14(5):1066–1068
Hullett D, Laeseke PF, Malin G, Nessel R et al (2005) Prevention of chronic allograft nephropathy with vitamin D. Transpl Int 18(10):1175–1186
Sezer S, Uyar M, Arat Z, Ozdemir FN et al (2005) Potential effects of 1,25-dihydroxyvitamin D3 in renal transplant recipients. Transplant Proc 37(7):3109–3111
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fleseriu, M., Licata, A.A. Failure of successful renal transplant to produce appropriate levels of 1,25-dihydroxyvitamin D. Osteoporos Int 18, 363–368 (2007). https://doi.org/10.1007/s00198-006-0238-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0238-3