Abstract
Osteoclastic tartrate-resistant acid phosphatase activity in serum (S-TRACP 5b) was measured in postmenopausal women ( n =59, mean age 56.1 years) with vertebral osteopenia before and during 2-year treatment with an 800-mg daily dose of clodronate, with a non-amino bisphosphonate. Changes in TRACP 5b were compared with those in urinary excretion of type I collagen amino-terminal telopeptide (U-NTX), corrected for creatinine excretion, a well-established marker of bone resorption, and to serum type I procollagen amino-terminal propeptide (S-PINP), a marker of bone formation. Marker changes 1 year after start of treatment were correlated with changes in bone mineral density (BMD). The least significant change (LSC) for each marker and BMD was calculated from values for subjects receiving placebo. Responders to treatment were those exhibiting a change larger than LSC. In response to clodronate treatment S-TRACP 5b (mean change up to −18%) decreased less than did U-NTX (up to −51%) or S-PINP (up to −46%). Marker changes correlated with changes in lumbar spine and trochanter BMD. The most efficient marker for finding responders to treatment was S-PINP, which changed more than the LSC (32%) in 72% of the subjects at the 1-year time point and in 79% at the 2-year time point. S-TRACP 5b change exceeded the LSC (27%) in 40% and 34% of the subjects at each time point, while U-NTX change exceeded the LSC (55%) in 55% and 40%, respectively. We conclude that, in terms of the proportion of subjects exhibiting any change exceeding the LSC, S-TRACP 5b did not appear to be superior to U-NTX and S-PINP in the follow-up of clodronate treatment. The reason may lie in the mechanism of action of clodronate, which rather than reducing the number of TRACP 5b-secreting osteoclasts, reduces the activity of bone proteolytic enzymes and thus the rate of bone organic matrix degradation. This is seen in decreased amounts of type I collagen breakdown products (U-NTX), and through coupling of bone resorption with bone formation, in a decrease in circulating levels of the marker that reflects new collagen formation (S-PINP).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several methods for measuring bone turnover have been developed and, during the last decade, have become commercially available [1–3]. The most widely used resorption markers include various urinary type I collagen degradation products such as free or total pyridinium cross-links pyridinoline (Pyr) or deoxypyridinoline (DPD) and cross-linked amino-terminal and carboxy-terminal telopeptides (NTX, CTX). Markers of bone formation are proteins produced by the osteoblasts. The most widely used are the bone-specific alkaline phosphatase (Bone ALP) and osteocalcin (OC). During type I collagen formation, carboxy-terminal and amino-terminal propeptides of type I procollagen (PICP and PINP) are liberated into the circulation, and their serum concentrations reflect bone formation rate [2]. Through coupling between bone resorption and formation, S-PINP shows promise as a sensitive indicator of the efficacy of antiresorptive therapy [4]. Markers of bone turnover have been correlated with bone mineral density (BMD) and have been evaluated as predictors of future bone loss and fracture [5–8]. Of greatest value for the clinician, bone markers serve to monitor the efficacy of antiresorptive therapy. Significant decreases in markers can be seen in 3–6 months after the start of an efficient treatment, with significant increases in BMD being observed in 1–2 years [9–11].
Several factors may, however, make interpretation of the marker findings difficult. All the type I collagen-derived markers, especially those measured in urine, show great diurnal variation [12–14], which is one contributor to within-subject, day-to-day variability [4]. Collecting urinary samples is laborious, and precise timing is needed when 2-h morning samples are used. Serum assays for the type I collagen telopeptides NTX and CTX have recently been introduced, and these may provide an advantage over the respective urinary assays [15,16]. However, because for serum CTX the amplitude of diurnal variation is aggravated by meals [17], and several markers of bone turnover are lower in morning samples taken after fasting than in samples taken after a light breakfast [18], to reduce within-subject variability, timed sampling is required from fasting individuals. Furthermore, type I collagen is not specific to bone, but is produced by several other tissues as well [19]. Although it is believed that type I collagen-derived markers originate mainly from bone because of high osseous turnover, and bone degradation products contain structures found only in type I collagen of bone, other tissues may contribute in certain situations to their circulating and excreted levels [1–3]. It also has to be kept in mind that renal function has an impact on the serum concentrations of small type I collagen degradation products as well as on their urinary excretion, and liver function has an impact on those markers cleared from the circulation by liver endothelial cells, such as Bone ALP and propeptides of type I procollagen [20–22].
An iron-containing 35 kDa enzyme tartrate-resistant acid phosphatase (TRACP) is expressed in osteoclasts early during their development and has long been used as their biomarker [23,24]. The function of TRACP in osteoclasts is still in part unknown, but it is known to regulate the attachment of osteoclast to bone by dephosphorylating osteopontin and other phosphorylated adhesion proteins in bone organic matrix [25,26]. It also participates in producing reactive oxygen species [27]. In the osteoclasts, vesicles containing TRACP are fused to the transcytotic vesicles, which contain bone matrix degradation products endocytosed by the osteoclasts from the resorption lacunae under them. In these vesicles, reactive oxygen species produced by TRACP further degrade these bone matrix degradation products, which are secreted from the osteoclasts together with active TRACP into the circulation.
Human serum contains two isoforms of TRACP, 5a and 5b, which are products of the same gene and differ from each other in glycosylation, 5a containing sialic acid, 5b not. The isoform 5b has been shown to originate from osteoclasts, and isoform 5a from other sources, most probably from macrophages [28,29]. The amount of secreted active TRACP 5b is believed to correlate with the amount of bone resorbed, making it a potentially useful resorption marker. Several assays have been developed for measuring either TRACP activity or its mass concentration, but thus far they have been disappointing as reflectors of bone resorption. A novel immunoassay measuring specifically TRACP 5b activity has recently been developed [29]. In this assay TRACP isoforms 5a and 5b are both first captured to the solid phase by a TRACP-specific monoclonal antibody, after which the bound TRACP activity is measured at pH 6.1, at which 5b is highly active and 5a almost completely inactive. Serum TRACP 5b activity is practically unaffected by meals, and its diurnal variation is much smaller than that of serum and urinary telopeptides [30]. Thus far, data on the efficacy of this resorption marker in monitoring the effect of antiresorptive treatment have been reported only in healthy postmenopausal women on estrogen replacement therapy [29] and in a small number of postmenopausal women with osteoporosis on alendronate [30].
In the present study, we compared serum TRACP 5b to serum PINP and urinary NTX in postmenopausal women with vertebral osteopenia treated with clodronate for 2 years. Changes in the markers were related to each other and to changes in BMD measured at the lumbar spine, femoral neck, and trochanter. With respect to each marker, the percentage of responders was determined according to the least significant change (LSC) in the marker, calculated from the long-term within-subject variability among the women who had received placebo.
Materials and methods
Subjects
Subjects in the present study were participants in the Probone study [31], a multi-center, double-blind, placebo-controlled dose-finding study consisting of a 3-year primary and a 2-year extension study. A total of 610 women were recruited to the study at six study centers in Finland. The inclusion criteria included: age of 45 years or more, 1–5 years since menopause, good general health, no clinical or laboratory evidence of systemic disease, and a lumbar spine bone mineral density (BMD) at least one standard deviation below the mean for premenopausal women ( T -score ≤−1). Subjects with diseases or medications known to affect bone or calcium metabolism were excluded from the study. The protocol was approved by the ethics committee at each center and at Helsinki University Central Hospital, and all women gave written informed consent. In the primary study the subjects were randomly allocated to five treatment groups (about 120 subjects in each) to receive placebo or different doses of clodronate (BONEFOS, Leiras Oy, Finland) for 3 years; and 509 women completed the primary study. An opportunity to continue in an extension study of 2 years was offered to the women who had been on either placebo or 400 mg or 800 mg of clodronate daily in the primary study. Of a total of 300 women, 187 agreed to participate. Those who had been on placebo ( n =66) were switched to 800 mg of clodronate daily, and those who had been on the two doses of clodronate, were re-randomized to receive either placebo ( n =60) or 800 mg ( n =61) of clodronate daily. One hundred sixty-four completed the extension study.
The population of the present study comprised 59 subjects, aged 48–63 years (mean 56.1 years), who had been on placebo in the primary study and were then switched to 800 mg clodronate for 2 years in the extension study. Samples for bone marker measurements were collected at the beginning of the extension study (=baseline) and after 1 year and 2 years on treatment. BMD of the lumbar spine and at the upper femur was measured at baseline and yearly thereafter. A part of the placebo group of the primary study, those who had had the three markers of bone turnover measured ( n =28), was used to calculate the intra-individual variation (CVi) of the markers from samples taken at entry and at 3 months, 12 months and 24 months, and that of the BMD measurements at entry and at 12 months and 24 months. The control group of the extension study could not be used for this purpose, since all the subjects in this group were treated with clodronate during the preceding 3 years.
DXA measurements
Three different brands of bone densitometers were used; Lunar (Lunar DPX, Madison, WI, USA), Hologic (Hologic QDR-1000, Waltham, MA, USA) and Norland (Norland XR 26, Medical System, Fort Atkins, WI, USA) to measure the BMDs at the lumbar spine, the femoral neck, and the trochanter. Percentage changes from baseline in the treated group were calculated. The placebo group of the primary study was used to calculate the LSCs for the BMD measurements.
Bone turnover measurements
Blood and urine samples were taken in the morning after an overnight fast of 10 h. Two-hour second morning void urine samples were used for urine analyses. Samples were kept frozen at −70°C until the assay.
Urinary NTX was measured by an enzyme-linked immunosorbent assay (ELISA) with commercial kits (Osteomark) from Ostex International, Seattle, WA. Analytical sensitivity of the assay was 30 nmol of bone collagen equivalents (BCE)/l, and the intra-assay and inter-assay CVs were 11% or less. The values were corrected for creatinine excretion, measured by a kinetic Jaffe method on a Hitachi 911 analyzer. Serum-intact PINP was determined by a competitive radioimmunoassay with commercial kits (Intact PINP RIA) from Orion Diagnostica, Oulunsalo, Finland. Analytical sensitivity of this assay was 2 μg/l, and the intra-assay and inter-assay CVs for this assay ranged 2–6%. Serum TRACP 5b activity was measured by an immuno-extraction method with kits (BoneTRAP) from Suomen Bioanalytiikka Oy, Oulu, Finland. The analytical sensitivity of this assay was 0.1 U/l, and the intra-assay and inter-assay CVs were 6% or less.
Calculations
For each subject in the placebo group of the primary study ( n =28), the long-term intra-individual variability of bone markers (CVi = SDi/meani) was calculated from four measurements at 0, 3 months, 12 months, and 24 months. Similarly, the CVi of BMD measurements at 0, 12 months and 24 months was calculated. The least significant change (LSC) for each bone marker and BMD at measured sites was calculated from these CVis by the formula LSC=1.96×sqr 2 × (ΣCVi2)/ n, which gives the LSC with 95% confidence interval. Responders with respect to changes in bone markers or BMD were those who exhibited a change greater than or equal to the LSC.
Statistics
Bone metabolism markers were analyzed with analysis of variance for repeated measures with mixed modeling, including treatment group, time point and their interaction as factors in the model. The analysis checks for skewness of the data. The percentage changes from baseline were assessed with one-way analysis of variance. Pearson correlation coefficients were calculated to measure the strength of the linear relationship between different marker changes and also between the marker and BMD changes. A p value of equal to or less than 0.05 was considered significant.
Results
Over-time marker values and percent changes in the markers
At baseline, the median value for S-TRACP 5b and S-PINP fell within reference ranges for premenopausal women, whereas the median U-NTX was above the reference range (Table 1). S-TRACP 5b was above the reference range for 42% (25) of the subjects, U-NTX was elevated in 70% (41), whereas S-PINP was elevated in only 6.9% (4) of these subjects. All the marker values decreased significantly during the first year of clodronate, stabilizing thereafter. At the end of the study 8 (14%), 7 (13%) and 0 subjects had values exceeding the upper reference range for premenopausal women for S-TRACP 5b, U-NTX, and S-PINP, respectively (Table 1). At 1-year time point 7 patients (12%) and at 2-year time point 2 patients (4%) had their S-PINP lower than the lower premenopausal reference range of the marker. The respective percentages were 19% and 10% for S-TRAPC 5b and 3% and 4% for U-NTX.
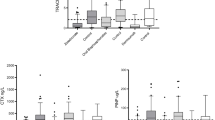
The percentage changes in all measured markers from baseline to the 1-year and 2-year time points were significant ( p <0.0001). The mean decrease in S-TRACP was 20% (SD 17%) at the 1-year time point and 18% (SD 17%) at the 2-year time point, the mean decrements in U-NTX being 51% (SD 27%) and 47% (SD 33%), and in S-PINP 46% (SD 33%) and 42% (SD 41%) at the two time points (Fig. 1).
Correlation of marker percentage changes
Significant correlations existed between all the marker changes from baseline to the two other time points (1 year and 2 years), with correlation coefficients ranging from 0.34 ( p =0.009) between S-TRACP 5b and S-PINP at 2 years to 0.48 ( p =0.0002) between TRACP 5b and U-NTX at 2 years.
Effects of clodronate on BMD
Clodronate treatment for 2 years resulted in a mean change in the lumbar spine BMD of +2.1% (95% confidence interval, +1.1 to +3.1, p =0.0002), and a mean change in the trochanter BMD of +2.6% (95% confidence interval, +1.3 to +3.8, p =0.0001). It did not change the femoral neck BMD significantly.
Correlations of marker percentage changes with BMD percentage changes
Correlations between percentage changes in the bone turnover markers and BMD from baseline to each time point are presented in Table 2. The percentage change in S-TRACP 5b from baseline to 1 year correlated with the percentage change in lumbar spine and trochanter BMD at the 1-year and 2-year time points. The percentage change in U-NTX from baseline to 1 year correlated with the percentage change in trochanter and lumbar spine BMD at the 2-year time point. The percentage change in S-PINP from baseline to 1 year correlated with the percentage changes in lumbar spine BMD at the 1-year and 2-year time points, and also with the percentage changes in trochanter BMD at the 2-year time point.
Responders
The LSCs for the biochemical markers were 26.6% for S-TRACP 5b, 55.1% for U-NTX, and 32.4% for S-PINP. For the BMDs they were 6.7% at the lumbar spine, 8.2% at the trochanter, and 7.7% at the femoral neck.
The S-TRACP 5b change showed 39.7% of the subjects (23/58) at the 1-year time point and 34.4% of them (20/58) at the 2-year time point to be responders. The respective percentages for U-NTX were 55.2 (32/58) at 1 year and 40.0 (22/55) at 2 years. In terms of the S-PINP change, the majority of the subjects were responders, 72.4% (42/58) at the 1-year time point, and 79.3% (46/58) at the 2-year time point. The mean percentage change of the whole study group exceeded the LSC only for S-PINP. The individual percentage changes in the markers as response to clodronate treatment at the two time points are shown in Fig. 2.
Individual percentage changes of the markers from baseline to 1-year and 2-year time points, A for S-TRACP 5b, B for U-NTX and C for S-PINP. The horizontal line represents the LSC of the marker. The percentage of responders identified by TRACP 5b change was 39.7% at the 1-year time point and 34.4% at the 2-year time point, by NTX change 55.2% and 40.0%, and by PINP change 72.4% and 79.3% at 1-year and 2-year time points, respectively
In terms of the BMD changes at the lumbar spine and the trochanter, two and three subjects were responders at the 1-year time point and five at both sites at the 2-year time point. None was a responder with respect to the BMD change at the femoral neck. One subject was a responder in terms of changes in BMD at the 2-year time point but not of those in the markers.
Discussion
In comparison to the reference range for premenopausal women, serum TRACP 5b concentration was elevated in 42% of the subjects, and the other marker of bone resorption, U-NTX, was elevated in 70% of the subjects, whereas the marker of bone formation, S-PINP, rose above the reference range in only 7% of the subjects. In addition, the median value of U-NTX was above the upper reference limit for premenopausal women, but the median of S-TRACP 5b fell within the reference range. In this group of postmenopausal women with vertebral osteopenia, such findings may indicate insufficient coupling of bone formation and resorption. Moreover, urinary excretion of NTX seemed to detect osteopenic subjects more efficiently than did S-TRACP 5b. In several studies the mean values of U-NTX and S-PINP in healthy postmenopausal women have been shown to be somewhat higher than in premenopausal women, whereas in osteoporotic postmenopausal women the marker means are significantly higher [32–34]. In one study S-TRACP 5b was shown to be elevated in 48% of 29 osteopenic women, a result consistent with the result of the present study [35].
The percentage changes in TRACP 5b were less than half the percentage changes in U-NTX. This may be explained by clodronate action. Based on recent studies in vitro, bisphosphonates are known to affect bone resorption at several levels [36]. They inhibit the adhesion of osteoclasts to bone matrix, preventing the formation of actively resorbing cells. They also cause osteoclast apoptosis: the bisphosphonates containing amino groups do so by preventing protein prenylation, and the non-amino bisphosphonates by forming complexes with adenosine 5’-triphosphate (ATP) [37,38]. Bisphosphonates also inhibit the activity of proteolytic enzymes secreted into the resorption lacunae by the osteoclasts [39]. Depending on which bisphosphonate, different mechanisms may predominate in vivo [40]. The finding that TRACP 5b was reduced to a lesser extent than U-NTX could be explained as follows: First, clodronate acts mainly by causing osteoclast apoptosis, but does not affect the formation of early osteoclasts, which would still secrete TRACP 5b into the circulation. Second, clodronate inhibits the activity of bone resorbing enzymes, leading to decreased amounts of type I collagen breakdown products. In one study on men with Klinefelter’s syndrome and osteoporosis, treated with an aminobisphosphonate ibandronate, serum TRACP 5b was reduced to only about half of the extent of the reduction in serum CTXβ [41], an observation that supports our results owing to similarities in the action of clodronate and ibandronate.
The TRACP 5b changes correlated with BMD changes no better than did the type I collagen-derived markers, and the marker changes correlated with changes in BMD at the lumbar spine and trochanter only. These results are in agreement with those of several other studies showing early marker changes in a response to HRT or bisphosphonates correlating with later BMD changes only slightly [9–11, 16,17]. The markers used in those trials have covered all the markers measurable with commercially available reagents. Urinary NTX, CTX, DPD, and S-CTXβFootnote 1 changes from baseline to 3–6 months have been found to be significant predictors of spinal BMD changes only, as have been the changes in serum formation markers Bone ALP or OC. In some studies, marker changes have also been predictive for BMD changes at other skeletal sites, especially in trials involving elderly populations [10], whereas some studies have found no correlations at all between early marker changes and later BMD changes [7,8].
The LSCs of the markers, calculated from long-term intra-individual variation in early postmenopausal subjects in the placebo group of the primary study, were consistent with those reported previously [4,16], although somewhat lower LSCs have been obtained in premenopausal women [30]. Our LSC for S-TRACP 5b, 26.6%, was much smaller than that for U-NTX (55.1%). However, TRACP 5b changes identified somewhat fewer responders to treatment with clodronate than did NTX changes, and markedly fewer than did PINP changes. These results are not in agreement with results for TRACP 5b changes as a response to HRT [29]. In that small study of healthy postmenopausal women, a highly significant mean decrease of 48% in S-TRACP 5b was apparent after 6 months on HRT, and a decrease larger than an LSC of 26.2% occurred in 13 of 15 subjects on HRT. The difference between the results of these two studies may be in part explained by the differing mechanisms of the interventions. Estrogen replacement directly affects the number of osteoclasts by inhibiting cytokines essential for osteoclast formation [42,43], whereas clodronate treatment influences at a later stage of osteoclast development and bone resorption [40]. This difference in response of S-TRACP 5b to treatment with estrogen vs bisphosphonate could indicate that TRACP 5b activity reflects the number of osteoclasts rather than their activity.
The marker of the greatest value in finding the responders to clodronate treatment was S-PINP. Although published data on S-PINP as a marker of the efficacy of antiresorptive treatment are scanty, some studies support the present finding. In a study of subjects with surgical menopause [34], among several markers of bone formation (Bone ALP, PINP, PICP, OC), S-PINP exhibited the highest response to HRT. Of the resorption markers measured (U-CTXβ, U-CTXαFootnote 2, U-NTX, S-CTX-MMP (S-ICTP)Footnote 3, S-CTXβ, S-TRACP) S-CTXβ was the most efficient, showing as high a response as S-PINP. S-PINP was also shown to respond similarly to HRT use in natural menopause [4]. Also, in a small study of the effects of alendronate on several markers of bone turnover, S-PINP showed a similar response to the resorption markers S-CTXβ and U-NTX [44], consistent with the response to clodronate in our study. The reason for S-PINP being superior to U-NTX in detecting the responders to clodronate treatment is mainly the larger intra-individual variability in U-NTX; the mean percentage decreases in these markers were of the same magnitude.
The findings of the present study do not necessarily mean that S-TRACP 5b activity is an inefficient marker of bone resorption, although it appeared inefficient for the follow-up of clodronate treatment. The markers seem to behave differently in different situations, and their responses to antiresorptive therapies may depend on the mode of action of the therapy used. This was shown in one study for free and peptide-bound cross-link excretion as a response to bisphosphonate treatment and estrogen replacement therapy [45]. Bisphosphonates had no effect on free cross-link excretion, whereas HRT reduced it significantly.
Our study was aimed at comparing different bone markers in the follow-up of treatment with clodronate, not at predicting forthcoming BMD changes by early marker changes. However, in determining responders by the LSC, the markers appeared to be indeed superior to the BMD. Theoretically, the best bone marker is the one that best predicts the prevention of bone fractures. For this purpose, bone markers appear to be of more significance than BMD, even when similar 1-year changes used in the present study have been employed [46,47]. As a response to raloxifene therapy, reduced fracture risk was related to the change in bone turnover but not at all to the change in BMD [46]. Interestingly, the bone formation markers S-Bone ALP and S-OC were more advantageous than urinary CTX. The decrease in bone resorption markers at 3–6 months with risedronate therapy predicted up to two-thirds of the reduction in vertebral fracture risk [48], while the change in BMD explained only 28% of the reduced risk [49]. Similar calculations are not available for clodronate therapy, although it reduces the risk of vertebral fractures [50]. Various antiresorptive therapies may reduce fracture risk by differing mechanisms, which alter different markers of bone turnover to varying magnitudes. Thus, the best marker to predict reduced fracture risk during one therapy is not necessarily the best during another treatment.
Limitations in our study were the rather small number of patients and the lack of a control group. Since in the extension of the Probone study all the subjects in the placebo group had been on clodronate during preceding years, we had to produce the LSC values from the control group of the primary part of the study. This may have increased the LSC values due to a greater intra-individual variation in women who had approximately 3 fewer years since menopause than did the present subjects. Furthermore, TRACP 5b may be relatively unstable on storage, at least when stored at −4°C for days and during prolonged storage at −20°C [29], a fact that diminishes its value as a resorption marker in clinical practice. However, the loss of activity reaches a plateau when stored at −70°C or colder. Therefore, the samples in this study, stored 2–7 years at −70°C, were considered adequately well preserved.
In conclusion, as a response to clodronate treatment in osteopenic postmenopausal women, the change in serum TRACP 5b activity was smaller than were changes in the two type I collagen-derived markers, urinary excretion of NTX, and serum concentration of PINP. Serum PINP seemed to be the most efficient marker in finding the responders to clodronate treatment.
Notes
Beta-isomerized form of the cross-link region peptide from the type I collagen carboxy-terminal telopeptide
The alpha-isomerized form of CTX
Cross-linked carboxy-terminal telopeptide of type I collagen produced by matrix metalloproteinases
References
Risteli L, Risteli J (1993) Biochemical markers of bone metabolism. Ann Med 25:385–393
Blumsohn A, Eastell R (1997) The performance and utility of biochemical markers of bone turnover: do we know enough to use them in clinical practice? Ann Clin Biochem 34:449–459
Seibel MJ (2000) Molecular markers of bone turnover: Biochemical, technical and analytical aspects. Osteoporos Int 10 [Suppl 6]:S18–29
Hannon R, Blumsohn A, Naylor K, Eastell R (1998) Response of biochemical markers of bone turnover to hormone replacement therapy: impact of biological variability. J Bone Miner Res 13:1124–1133
Melton LJ 3rd, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL (1997) Relationship of bone turnover to bone density and fractures. J Bone Miner Res 12:1083–1091
Garnero P, Dargent-Molina P, Hans D, Schott AM, Bréart G, Meunier PJ, Delmas PD (1998) Do markers of bone resorption add to bone mineral density and ultrasonographic heel measurement for the prediction of hip fracture in elderly women? The EPIDOS prospective study. Osteoporos Int 8:563–569
Bauer DC, Sklarin PM, Stone KL, Black DM, Nevitt MC, Ensrud KE, Arnaud CD, Genant HK, Garnero P, Delmas PD, Lawaetz H, Cummings SR for the study of osteoporotic fractures research group (1999) Biochemical markers of bone turnover and prediction of hip bone loss in older women: the study of osteoporotic fractures. J Bone Miner Res 14:1404–1410
Marcus R, Holloway L, Wells B, Greendale G, James MK, Wasilauskas C, Kelaghan J (1999) The relationship of biochemical markers of bone turnover to bone density changes in postmenopausal women: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. J Bone Miner Res 14:1583–1595
Rosen CJ, Chesnut CH 3rd, Mallinak NJS (1997) The predictive value of biochemical markers of bone turnover for bone mineral density in early postmenopausal women treated with hormone replacement or calcium supplementation. J Clin Endocrinol Metab 82:1904–1910
Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsay L, Karpf DB (1998) Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res 13:1431–1438
Ravn P, Hosking D, Thompson D, Cizza G, Wasnich RD, McClung M et al (1999) Monitoring of alendronate treatment and prediction of effect on bone mass by biochemical markers in the early postmenopausal intervention cohort study. J Clin Endocrinol Metab 84:2363–2368
Hassager C, Risteli J, Risteli L, Jensen SB, Christiansen (1992) Diurnal variation in serum markers of type I collagen synthesis and degradation in healthy premenopausal women. J Bone Miner Res 7:1307–1311
Blumsohn A, Herrington K, Hannon RA, Shao P, Eyre DR, Eastell R (1994) The effect of calcium supplementation on the circadian rhythm of bone resorption. J Clin Endocrinol Metab 79:730–735
Panthegini M, Pagani F (1996) Biological variation in urinary excretion of pyridinium crosslinks: recommendations for the optimum specimen. Ann Clin Biochem 33:36–42
Woitge HW, Pecherstorfer M, Li Y, Keck A-V, Horn E, Ziegler R, Seibel M (1999) Novel serum markers of bone resorption: clinical assessment and comparison with established urinary indices. J Bone Miner Res 14:792–801
Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, Greenspan SL (2000) Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int 66:100–103
Christgau S, Bitsch-Jensen O, Hanover Bjarnason N, Gamwell Henriksen E, Qvist P, Alexandersen P, Bang Henriksen D (2000) Serum CrossLaps for monitoring the response in individuals undergoing antiresorptive therapy. Bone 26:505–511
Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R (2002) Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone 30:886–890
Prockop DJ, Kivirikko KI, Tuderman L, Guzman RA (1979) The biosynthesis of collagen and its disorders. N Engl J Med 301:13–23, 77–85
Smedsrod B, Melkko J, Risteli L, Risteli J (1990) Circulating C-terminal propeptide of type I procollagen is cleared mainly via the mannose receptor in liver endothelial cells. Biochem J 271:345–350
Melkko J, Hellevik T, Risteli L, Risteli J, Smedsrod B (1994) Clearance of the amino-terminal propeptides of types I and III procollagen is a physiological function of the scavenger receptor in liver endothelial cells. J Exp Med 179:405–412
Blom E, Ali MM, Mortensen B, Huseby NE (1998) Elimination of alkaline phosphatases from circulation by the galactose receptor. Different isoforms are cleared at various rates. Clin Chim Acta 270:125–136
Minkin C (1982) Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int 34:285–290
Clark SA, Ambrose WW, Anderson TR, Terrell RS, Toverud SU (1989) Ultrastructural localization of tartrate-resistant, purple acid phosphatase in rat osteoclasts by histochemistry and immunocytochemistry. J Bone Miner Res 5:399–405
Andersson G, Ek-Rylander B (1995) The tartrate-resistant purple acid phosphatase of bone osteoclasts—a protein phosphatase with multivalent substrate specificity and regulation. Acta Orthop Scand 66 [Suppl 266]:189–194
Halleen JM, Kaija H, Stepan JJ, Vihko P, Väänänen HK (1998) Studies on the protein tyrosine phosphatase activity of tartrate-resistant acid phosphatase. Arch Biochem Biophys 352:97–102
Halleen JM, Räisänen S, Salo JJ, Reddy SV, Roodman GD, Hentunen TA et al (1999) Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartrate-resistant acid phosphatase. J Biol Chem 274:22907–22910
Lam KW, Eastlund DT, Li CY, Yam LT (1978) Biochemical properties of tartrate-resistant acid phosphatase in serum of adults and children. Clin Chem 24:1105–1108
Halleen JM, Alatalo SA, Suominen H, Cheng S, Janckila AJ, Väänänen HK (2000) Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res 15:1337–1345
Hannon RA, Clowes JA, Eagleton AC, Al Hadari A, Eastell R, Blumsohn A (2004) Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone 34:187–194
Välimäki MJ, Laitinen K, Patronen A, Puolijoki H, Seppänen J, Pylkkänen L et al for the Probone Study Group (2002) Prevention of bone loss by clodronate in early postmenopausal women with vertebral osteopenia: a dose-finding study. Osteoporos Int 13:937–947
Garnero P, Hih WJ, Gineyts E, Karpf DB, Delmas PD (1994) Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab 79:1693–1700
Dominguez Cabrera C, Sosa Henriques M, Traba ML, Alvarez Villafane E, de la Piedra C (1998) Biochemical markers of bone formation in the study of postmenopausal osteoporosis. Osteoporos Int 8:147–151
Peris P, Alvarez L, Monegal A, Guanabens N, Duran M, Pons F et al (1999) Biochemical markers of bone turnover after surgical menopause and hormone replacement therapy. Bone 25:349–353
Halleen JM, Alatalo SA, Janckila AJ, Woitge HW, Seibel MJ, Väänänen HK (2001) Serum tartrate-resistant acid phosphatase 5b is a specific and sensitive marker of bone resorption. Clin Chem 47:597–600
Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Mönkkönen J, Frith JC (2000) Cellular and molecular mechanisms of action of bisphosphonates. Cancer 88:2961–2978
Selander KS, Mönkkönen J, Karhukorpi EK, Härkönen P, Hannuniemi R, Väänänen HK (1996) Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages. Mol Pharmacol 50:1127–1138
Lehenkari PP, Kellinsalmi M, Napankangas JP, Ylitalo KV, Mönkkönen J, Rogers MJ et al (2002) Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol 61:1255–1262
Teronen O, Heikkilä P, Konttinen YT, Laitinen M, Salo T, Hanemaaijer R et al (1999) MMP inhibition and downregulation by bisphosphonates. Ann N Y Acad Sci 878:453–465
Halasy-Nagy JM, Rodan GA, Reszka AA (2001) Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone 29:553–559
Stepan JJ, Burckhardt P (2002) Serum activity of type 5b ACP and biochemical markers of type I collagen degradation in osteoporotic men with Klinefelter’s syndrome treated with intravenous ibandronate. Calcif Tissue Int 70:279, P78
Hofbauer LC, Khosla S, Dunstan CR, Lacey D, Boyle WJ, Riggs BL (2000) The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15:2–12
Shevde NK, Bendixen AC, Dienger KM, Pike JW (2000) Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci U S A 97:7829–7834
Stepan JJ, Vokrouhlicka J (1999) Comparison of biochemical markers of bone remodelling in the assessment of the effects of alendronate on bone in postmenopausal osteoporosis. Clin Chim Acta 288:121–135
Garnero P, Gineyts E, Arbault P, Christiansen C, Delmas PD (1995) Different effects of bisphosphonate and estrogen therapy on free and peptide-bound bone cross-links excretion. J Bone Miner Res 10:641–649
Bjarnason NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C (2001) Six and 12-month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int 12:922–930
Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, Thompson DE, Ewing SK, Delmas PD, The Fracture Intervention Trial Study Group (2004) Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: The Fracture Intervention Trial. J Bone Miner Res 19:1250–1258
Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18:1051–1056
Li Z, Meredith MP, Hoseyni MS (2002) A method to assess the proportion of treatment effect explained by a surrogate endpoint. Stat Med 20:3175–3188
McCloskey E, Selby P, Davies M, Robinson J, Francis RM, Adams J, Kayan K, Beneton M, Jalava T, Pylkkanen L, Kenraali J, Aropuu S, Kanis JA (2004) Clodronate reduces vertebral fracture risk in women with postmenopausal or secondary osteoporosis: results of a double-blind, placebo-controlled 3-year study. J Bone Miner Res 19:728–736
Acknowledgment
We thank Suomen Bioanalytiikka Oy for providing us with reagents for TRACP 5b measurement
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tähtelä, R., Seppänen, J., Laitinen, K. et al. Serum tartrate-resistant acid phosphatase 5b in monitoring bisphosphonate treatment with clodronate: a comparison with urinary N-terminal telopeptide of type I collagen and serum type I procollagen amino-terminal propeptide. Osteoporos Int 16, 1109–1116 (2005). https://doi.org/10.1007/s00198-004-1819-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-004-1819-7