Abstract
Introduction and hypothesis
An animal model of vaginal distention (VD) was developed to reproduce the acute urethral injury and deficiency underlying stress urinary incontinence (SUI). Data on the chronic effects of urethral trauma and the recovery process are still scarce. We investigated acute, short- and long-term histomorphological and molecular changes in the urethra of rats post 12-h intermittent VD.
Methods
We evaluated the urethra of four groups of female rats (n = 72): control without trauma, 1 h, 7 days and 30 days post VD. We compared the gene and protein expression of the VEGF and NGF growth factors, collagens (COL1a1 and COL3a1), desmin, smooth muscle myosin (MYH11), skeletal muscle myosins (MYH1, MYH2 and MYH3) and cell proliferation marker MKi67. We used real-time RT-qPCR, and immunohistochemistry.
Results
Histology showed urethral damage after VD mainly involving the muscular layers. VEGF, NGF, desmin and MKi67 mRNA were significantly upregulated in the urethras of rats 1-h post VD compared with controls (P < 0.05 for all). By 7 days post trauma, COL1a1, MYH11 and MYH3 genes were overexpressed compared with controls (p < 0.05 for all). The COL3a1 protein level was increased by 2.6 times by day 7, while MYH2 protein was significantly decreased (around two times) from 7 to 30 days post VD compared with controls (p < 0.05 for both).
Conclusions
The 12-h intermittent VD causes chronic alterations in the urethra represented by increased COL3a1 and decreased MYH2 protein levels in the long term. The model can potentially be used to study the mechanisms of urethral injury and recovery as well as the physiopathology of SUI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) is the involuntary loss of urine on effort or physical exertion [1]. It has been demonstrated that urethral sphincter deficiency and, consequently, a decrease in the intra-urethral pressure underlie the dysfunction [2]. To better understand the pathophysiological mechanisms of SUI, experimental models that reproduce lesions of the urethral tissue have been developed [3, 4]. Among them is vaginal distention (VD) in which an insufflated Foley catheter is placed in the vagina of rats for ≤ 8 h [4, 5].
Based upon histomorphological, structural-anatomical and biochemical analysis, several authors have described that VD causes damage to nerves [6], skeletal muscle [3, 4, 7], smooth muscle [8, 9] and extracellular matrix content [10] of the urethra of female rats. Functional studies have also demonstrated that VD reduces the urethral outlet resistance and leak point pressure [3, 6, 11]. However, the majority of previous studies have only investigated the immediate and short-term effects of VD, confirming the causality between vaginal and urethral traumas and potentially ensuing SUI. Furthermore, injuries caused by the previous models of VD alone are potentially recoverable [11] and therefore do not serve as ideal models of chronic urethral injury or SUI.
The long-term effects of trauma by VD on the urethra have been poorly investigated. It is known that the longer the duration of VD is, the greater the urethral damage and the longer its recovery time [4, 11]. Thus, we developed an animal model of prolonged trauma that would provoke acute and chronic alterations in the urethra that may impair urethral function associated with SUI. Based on this, we tested the hypothesis that 12-h intermittent VD causes structural, histological and molecular changes in the urethra of rats immediately and in the short- and long-term following trauma. We intended to assess the histomorphology and gene and protein expression profiles of the urethra of female rats after prolonged VD at different time points: 1 h, 7 days and 30 days after trauma. We investigated several molecules involved in the metabolism of urethral components: the vessels, nerves, skeletal and smooth muscles and extracellular matrix. The time period following induced trauma with the corresponding data of various markers would also allow for an understanding of the sequence of molecular events following urethral injury and the regeneration process.
Methods
Study groups
Animal care and experimental procedures are in accordance with the ethical principles for animal research as adopted by the Brazilian College of Animal Experimentation. The protocol was approved by the Local Institutional Ethics Review Board (CEP1607/11) from the Federal University of São Paulo, Brazil.
We studied 72 adult female Wistar rats, approximately 120 days old, retired breeders, weighing approximately 250 g, housed at 22 ± 1 °C under a 12-h light-dark cycle. The rats were distributed in 4 groups of 18 animals each: the control group: rats without VD trauma; immediate trauma group: rats that had acute trauma (1 h post VD); recent trauma group: rats that had short-term trauma (7 days post VD); late trauma group: rats that had long-term induced trauma (30 days post VD). The time points were decided according to the phases of the urethral healing (inflammation, proliferation, maturation and remodeling) described in male rats in a surgical model of urethroplasty [12] and the molecular markers of interest for our study.
The inflammatory phase is characterized by high levels of growth factors and the infiltrate of polymorphonuclear neutrophils and macrophages (peaked at day 2 post injury and decreased progressively afterwards) together with increased cytokine levels. The decrease in inflammatory cells coincided with an increase in myofibroblasts responsible for the provisional extracellular matrix (ECM) and collagen (COL) III synthesis (peaked from day 6–8), and cells expressing MKi76 increased (peaked on day 6), representing the proliferative phase of the urethral healing (extended until day 10). The peak of vessel formation was on days 6–8. The collagen III level was higher on day 8, which increased the COL III/I ratio. The maturation or remodeling phase started at day 12 and was determined by the decrease in the ratio of preliminary collagen III to mature collagen I and rearrangement of the ECM [12].
The 1 h following trauma represented the immediate acute inflammatory phase of healing, and 7 days represented the proliferative phase of healing. We waited up to 30 days to study the urethras (matured and consolidated phase of urethral healing) to certify that no additional regeneration would happen and that it would represent chronic effects (equivalent to 3 human years) [13].
Vaginal distension model
The trauma model consisted of a 12-h intermittent VD. Animals were anesthetized by intraperitoneal administration of a 15-mg/kg dose of xylazine (Rompun; Bayer, Leverkusen, Germany) and 80-mg/kg dose of ketamine (Narketan 10; Vetoquinol, Bern, Switzerland). A 10-Fr Foley catheter was inserted into the vagina and inflated with 3 ml of water. The inflated balloon had a final diameter of 0.9 cm, which corresponded to two times the diameter of the cephalic pole of the newborn rat.
Two stitches were made using 3–0 nylon sutures to fix the balloon, and the animals were placed on the table in a fixed prone position with their lower limbs flexed next to the abdomen and the pubic symphysis toward the edge of the table. The opposite end of the catheter was connected to a fixed 100-g weight (pulling it down). During this period, if the catheter came out, it was deflated and replaced in its original position. Vaginal distention was intermittently performed. The balloon was left inflated for 4 h and then deflated for 30 min. The balloon was then re-inflated and remained in the vagina for 4 additional h and was then removed, performing 8-h trauma on the first day. The trauma was then completed after 15 h (day 2) with the remaining 4 h of VD. The animals were kept anesthetized throughout the procedure while they had the catheter in place.
Rats were killed with intravenous thiopental (60 mg/kg) and the specimens obtained by a longitudinal abdominal incision exposing the abdominal cavity 1 h, 7 or 30 days after trauma. The bladder, urethra and proximal vagina were extracted en bloc to facilitate their handling.
Histological analyses
Immediately after removal, the urethras were washed three times in PBS 10X, immersed and fixed in 10% formalin for 12 h and then embedded in paraffin for further evaluation of proteins related to vessels, nerves and ECM: vascular endothelial growth factor (VEGF), nerve growth factor (NGF) and collagen types I and III (COL1a1 and COL3a1) (n = 6 per group).
To evaluate muscle components, we studied the proteins: myosin heavy chain 11 of smooth muscle (MYH11), myosin heavy chain 1 of adult skeletal muscle (MYH1) and myosin heavy chain 2 of adult skeletal muscle (MYH2). For this, urethras were fixed to a piece of cork using Tissue-Tek (Sakura, The Netherlands). Then, the segment was snap-frozen in liquid nitrogen and stored at −80 °C (n = 6 per group).
Urethral sections of 5 μm thickness were made transversely to the longest axis of the organ, and slides were prepared according to standard procedures for histological examination. Slides were stained with hematoxylin and eosin (for frozen segments) and Masson’s trichrome (for segments in paraffin) and qualitatively compared in a blinded fashion by visual analysis with a light microscope (Olympus BX 10 l) coupled to a video camera (Q Color 3 Olympus).
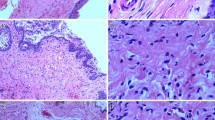
The content and morphology of the rat urethra vary along its length [14]. The maximum amount of muscle occurs at about the midpoint along the length of the urethra, where striated muscle predominates and completely encircles the lamina propria, forming a structure that is circular in transverse section (Fig. 1).
Histological analyses of urethral cross-sections of female rats. Immediate trauma group: note the presence of edema characterized by areas of light blue and spaces separating the urethra from the vaginal mucosa anteriorly and separating the urethral layers (black arrows). Note the overall tissue disorganization with urethral layers not well defined. Note the extensive disruption of the striated muscle layer with stretched, fragmented and waved morphological fibers (red arrows). Smooth muscle bundles were distorted and lost their alignment. Recent trauma group: note the improvement in the tissue organization in layers, with newly formed smooth muscle and striated muscle fibers. Note the increased number of blood vessels (green arrows). Note areas of interruption within the striated muscle fibers (blue arrow). Late trauma group: note the structural reorganization of the urethra, with smooth muscle bundles formed and aligned. Note the striated muscle layer alignment and the circumferential aspect of fibers, but irregular and marked thinning of the EUS, with regions of smaller width. V: vagina; M: urethral mucosa; EUS: external urethral sphincter; SM: smooth muscle layer; Masson’s trichome stain (line 1); desmin immunostaining is indicated by a dark brown deposition (lines 2 and 3). Magnification: 5× and 30×
For histological and immunohistochemical analyses, we used only the mid-urethra where the external urethral sphincter is located. To avoid biases in the analyses by selecting sections from different portions of the organ, we standardized our methodology. We performed serial transverse sections in the whole urethra and separated its middle portion characterized by the lumen in a horseshoe or “H” format according to the urethral morphology described by Phillips et al. [15] (Fig. 1).
Immunohistochemistry
We performed immunohistochemical analyses for quantification of the proteins VEGF, NGF, COL1a1, COL3a1, MYH2 and MYH11. We did not consider these analyses for the immediate trauma group, but for the control, recent and late trauma groups (n = 5 each) since we did not expect protein levels to be affected immediately right after injury (n = 5 each).
The slides from paraffin blocks were deparaffinized in xylene, rehydrated through a graded series of ethanol and washed with distilled water. Antigen was retrieved by incubating the slides in citrate buffer (pH 6.0) in a water bath at 95–100 °C for 20 min. The slides were cooled for 15 min at room temperature. After that, they were immersed in endogenous peroxidase blocker (Spring, CA, USA) for 20 min. After rinsing in TBST, the slides were incubated with primary antibodies directed against VEGF (ab1316; Abcam, Cambridge, MA) 1:50, NGF (ab52918; Abcam, Cambridge, MA) 1:100, COL1 (20,111 Novotec, Lyon, France) 1:100, for 1 h at room temperature or COL3 (ab6310; Abcam, Cambridge, MA) 1:50 for 2 h at room temperature.
The slides were washed in TBST (3 washes of 5 min each) and incubated with secondary antibody (N-Histofine® Simple Stain MAX PO, Nichirei, Tokyo, Japan Code 414191 - F) for 30 min in a wet box. After washing in TBST, color was developed using DAB–diaminobenzidine 3,3′ (Spring, CA, USA) for 5 min at room temperature. The slides were washed in running water, counterstained with hematoxylin and sequentially washed and dehydrated.
The slides obtained from frozen tissue were incubated with blocking buffer containing bovine serum albumin (BSA) in PBS-Tween. They were incubated in a sequence with primary antibodies directed against MYH11 (ab681; Abcam, Cambridge, MA) 1:100 and MYH2 (ab51263; Abcam, Cambridge, MA) 1:100 for 1 h at room temperature. The slides were washed in PBS-Tween (4 washes of 10 min each) and incubated with secondary antibody for 40 min at room temperature. The slides were again washed in PBS-Tween (4 washes of 10 min each). The slides were incubated in H2O2 for 30 min. After washing in PBS-Tween, color was developed using DAB (Spring, CA, USA) for 5 min at room temperature. The slides were then washed in running water, counterstained with hematoxylin, rewashed and dehydrated. A negative control slide without primary antibody incubation was used in all experiments.
The slides were analyzed after whole-slide digitalization (Panoramic Viewer, 3DHISTECH, Budapest, Hungary). The HistoQuant (3DHISTECH) image analysis software was used to selected immunostained areas. VEGF, NGF, COL1 and COL3 proteins were quantified in the total area of the urethral section. For muscle proteins MYH11 and MYH2, quantification was performed only in the muscular area of the urethra. The slides were analyzed in a blinded fashion. The quantification value was obtained by the mean of two slides for each sample. The quantification intra-groups were obtained by the mean of five samples.
Real-time polymerase chain reaction
For gene expression analyses, total urethras were crushed frozen in liquid nitrogen (n = 6 per group). Tissues were homogenized using Trizol (Life Technologies, São Paulo, Brazil) and Polytron (Kynematica, NY, USA). Total mRNA was extracted using Purelink RNA Mini Kit (Life Technologies, São Paulo, Brazil) according to the manufacturer’s instructions. The mRNA concentration and purification were checked using the NanoDrop 2000 spectrophotometer (Thermo Scientific, São Paulo, Brazil), and its integrity was assessed by agarose gel. Reverse transcription was performed in a 20-ul final volume with 2.5 μg of RNA using Super Script Vilo Master Mix (Life Technologies, São Paulo, Brazil) in the Verity Thermal Cycler (Applied Biosystems, São Paulo, Brazil). Quantitative RT-PCR was performed using the Step-One Plus Real Time PCR System (Applied Biosystems, São Paulo, Brazil). The reaction mixture contained Taqman® Universal PCR Master Mix (Applied Biosystem, São Paulo, Brazil), 50 ng cDNA, Taqman Gene Expression Assay (Applied Biosystem, São Paulo, Brazil) and ultrapure water (Table 1). Relative mRNA levels were determined by normalization to the GAPDH internal control by the ∆∆ Ct method and presented in fold change for comparison between groups.
Data analyses
We used the ANOVA test for gene and protein expression comparison among the four study groups. If significance was observed, we performed additional Tukey’s multiple comparisons post-tests to evaluate groups that were significantly different from each other. Histological analyses were performed qualitatively. P ≤ 0.05 was considered statistically significant.
Results
Histological analyses
Control
Control samples showed well-defined vaginal mucosa and the urethra with distinguishable and organized histological layers. The mid-urethra consists of the mucosa surrounded by a layer of smooth muscle mainly comprised of longitudinal smooth muscle fibers arranged in dense and aligned bundles, displayed in transverse sections in a skein format and separated from each other by connective tissue. We also observed the external urethral sphincter (EUS), which is formed by circumferential striated muscle fibers arranged in parallel, contiguous and evenly spaced with surrounding connective tissue (Fig. 1).
Immediate trauma
One hour after vaginal distention release, histological evaluation by Masson’s trichrome stain demonstrated the presence of intense tissue edema characterized by areas of light blue staining and spaces that separated both the urethra from the vaginal mucosa anteriorly and also the urethral layers, which lost their pattern of continuity. Tissue disorganization was marked overall by poorly defined urethral layers. The muscle layer morphology was well visualized using desmin immunostaining. We noticed that both smooth and striated muscles were dramatically injured after trauma. There was an extensive disruption of the morphology of the striated muscle layer with stretched, fragmented and waved fibers. Smooth muscle bundles were distorted and lost their alignment (Fig. 1).
Recent trauma
On the 7th day following trauma, we observed a significant structural organization of the urethra. There was no edema, and the borders of the vaginal mucosa were better defined, as were the urethral layers. The vaginal epithelium recovered its undulating wave morphology, which was not immediately observed after trauma. Intense desmin stains were noted, suggesting muscle regeneration. More dense muscle layers with newly formed smooth muscle and striated muscle fibers but not completely organized in bundles were observed. Also an increased number of blood vessels was detected, suggesting neovascularization (Fig. 1).
Late trauma
The urethra was better organized on the 30th day after VD mainly with respect to muscle layers. Smooth muscle bundles were better formed and aligned with a structure very similar to the control urethras. We noticed the striated muscle layer alignment and the circumferential aspect of fibers, but marked thinning of the EUS in comparison with controls. In addition, the EUS thickness seems heterogeneous and irregular with regions of smaller width (Fig. 1).
Gene and protein expression profile
Growth factors
-
VEGF
Analysis of variance showed a difference between the groups regarding VEGF gene expression (p < 0.0001). Post-tests showed a three-fold increased expression of the gene immediately post trauma compared with control rats. Afterwards, we observed continuous reduction of mRNA with time, reaching a level comparable to the control group at the 30th day post VD (Fig. 2).
We did not detect statistical differences in protein expression between the groups (p = 0.09) (Fig. 2). VEGF protein detection in the urethral tissue of the groups is presented in Fig. 3.
-
NGF
Representation of immunohistochemistry of the urethra of female rats in the study groups: COL1a1, VEGF, NGF and MYH11. V: vagina; M: urethral mucosa; EUS: external urethral sphincter; SM: smooth muscle layer. Positive immunostaining is indicated by a dark brown deposition. Magnification: 10× and 40×
Analysis of variance showed differences between the groups considering NGF gene expression (p < 0.0001). Following the same pattern of VEGF, post-test analyses detected overexpression of the NGF gene 1 h immediately after trauma compared with controls by a 3.3-fold change. Afterwards, we observed continuous reduction of mRNA throughout the time, almost back to the level established in the control group at the 30th day post injury (Fig. 2).
We did not detect statistical differences in protein expression between the groups (p = 0.42) (Fig. 2). NGF protein detection in the urethral tissue of the groups is presented in Fig. 3.
Extracellular matrix markers
-
COL1a1
We detected different expressions of COL1a1 mRNA between the groups (p < 0.0024). A 6.2-fold increased expression was shown in the recent trauma group compared with controls. We noticed downregulation of the COL1a1 gene in the late trauma group, being decreased 5.7-fold compared with the recent trauma group (Fig. 4).
We did not detect a statistical difference in protein expression between the groups (p = 0.44) (Fig. 4). COL1a1 protein detection in the urethral tissue of the groups is presented in Fig. 3.
-
COL3a1
Analysis of variance did not show differences between the groups considering COL3a1 mRNA expression (p = 0.14) (Fig. 4). However, we did observe significant changes in the COL3a1 protein level by analyzing the groups together (p = 0.018). The post-test detected 2.6-fold upregulation in the recent trauma compared with the control group (Fig. 4). COL3a1 protein detection in the urethral tissue of the groups is presented in Fig. 5.
-
Muscle markers
Representation of immunohistochemistry of the urethra of female rats in the study groups: COL3a1 and MYH2. Recent trauma group: note the more intense collagen deposition surrounding the smooth muscle bundles and the striated muscle layer; note the disorganized and disrupted striated muscle fibers. Late trauma group: note the partial muscle layer recovery marked by thinner fibers of the EUS interrupted by connective tissue. V: vagina; M: urethral mucosa; EUS: external urethral sphincter; SM: smooth muscle layer. A positive immunostaining is indicated by a dark brown deposition. Magnification: 10× and 40×
Six markers were evaluated to study genes related to the muscle components: desmin, Mki67, MYH1, MYH2, MYH3 and MYH11. Considering the relevance, we selected desmin, MYH2 and MYH11 to analyze the protein levels.
-
Desmin
We observed significant changes in the desmin gene expression by analyzing the four groups together (p < 0.014). The post-test detected 2.7-fold upregulation in the immediate trauma compared with the control group. mRNA expression dropped to a level comparable to controls in the long term (Fig. 7).
We observed significant changes in the desmin protein level by analyzing the groups together (p = 0.03). The post-test detected a 1.4 times decreased level in the recent trauma group compared with the controls, returning to a normal level in the long term (Fig. 7).
-
Mki67
Analysis of variance showed the difference between the groups considering Mki67 mRNA expression (p < 0.0001). Similar to the growth factor pattern, post-tests showed 5.5-fold increased expression of the gene immediately post trauma compared with the control group. Also, the Mki67 gene underexpression in the recent and late trauma groups compared with the immediate trauma group showed 4.2- and 6-fold changes, respectively (Fig. 6).
-
MYH1
We detected different expressions of the MYH1 gene between the study groups (p < 0.0092). Increased mRNA expression of 10.7 fold in the recent trauma group compared with the control and of 9.7 fold compared with the immediate trauma group was observed (Fig. 6).
-
MYH2
Analysis of variance showed a difference between the groups considering MYH2 gene expression (p < 0.042). The MYH2 gene was upregulated by 3.8 fold in the late trauma compared with the immediate trauma group (Fig. 7).
The protein level varied significantly between the groups (p = 0.0008). Importantly, we detected a reduction in MYH2 protein in the recent and late trauma groups compared with the control group with 2.2- and 1.8-fold changes, respectively (Fig. 7). MYH2 protein detection in the urethral tissue of the groups is presented in Fig. 5.
-
MYH3
We detected different expressions of MYH3 mRNA between the groups (p < 0.007). The MYH3 gene was overexpressed in the recent trauma group compared with the control group with a 48-fold change and compared with the immediate trauma group with a 23.6-fold change, but decreased afterwards, reaching levels similar to the control group in the long term (Fig. 6).
-
MYH11
Analysis of variance showed differences between the groups considering MYH11 gene expression (p < 0.003). Post-test analyses detected overexpression of the MYH11 gene in the recent and late trauma groups compared with the control group with 3.4- and 3.1-fold changes, respectively (Fig. 7).
We did not detect statistical differences in protein expression between the groups (p = 0.09) (Fig. 7). MYH11 protein detection in the urethral tissue of the groups is presented in Fig. 3.
Gene and protein expression profile according to the time period post trauma
Immediate trauma
This time period of 1 h post trauma was marked by changes in mRNA expression of VEGF, NGF, sesmin and Mki67, all being upregulated (Figs. 2 and 7).
Recent trauma
The short-term effects of trauma in the urethra were marked by high expression of the COL1a1, MYH1, MYH3 and MYH11 genes, peaking 7 days post VD (Figs. 4 and 6). At this time point, upregulation of desmin and COL3a1 proteins was identified, while the MYH2 protein was still underexpressed (Figs. 4 and 7).
Late trauma
The long-term effects of trauma in the urethra were marked by overexpression of the MYH2 and MYH11 genes (Figs. 6 and 7). While the MYH11 protein level did not alter statistically during the post-trauma phases, the MYH2 protein level consistently showed a reduction up to 30 days post VD (Fig. 7).
Discussion
Clinical and basic science research suggests a complex and multifactorial etiology of SUI in which repetitive trauma, insufficient regeneration and biochemical changes with age contribute to intrinsic urethral sphincter deficiency [7]. Previous experimental models have linked vaginal distention, urethral injury and the pathophysiology of SUI [3, 16, 17]. However, those models provoked short-term urethral damage with evidence of urethral regeneration [14]. By prolonging the duration of VD into an intermittent 12-h period divided into 2 days, we created a VD model that caused persistent and long-term histomorphological and molecular alterations in the urethral tissues of female rats. Furthermore, the use of a clear time frame from immediate to long-term trauma with corresponding acquired data concerning molecular changes incurred throughout allowed for a greater understanding of the sequence of molecular events following VD leading to urethral regeneration and fibrosis.
Similar to other studies, our results showed extensive damage mainly in the muscle layers post trauma, confirming that VD is primarily a model of urethral myogenic damage [3, 14]. The urethral structure and tissue organization progressively and spontaneously regenerate throughout time, suggesting that the urethras of rats have an important self-recovery ability, although it seems to be only partial in our model.
We evaluated the VEGF and NGF growth factors and the MKi67 levels, which stimulate neoangiogenesis, facilitate neural repair and indicate cell proliferation, respectively, in response to injury and inflammation [18,19,20]. We observed that the mRNA expression of those markers is upregulated 1 h after trauma, but with no change in the respective protein levels. This is in accordance with previous studies that also detect overexpression of VEGF genes immediately and 24 h after VD [16, 17, 21]. Also, Pan et al. did not notice changes in NGF protein expression 1 day after VD [7].
At 7 days post trauma, we observed no changes in the gene and protein expression of both growth factors. Taken together, these findings may suggest that acute trauma initially stimulates their mRNA increase and the levels return to normal in less than a week post VD. Perhaps the 1-h and 7-day analyses failed to detect a response in the proteins level, which might have occurred in between. It seems that growth factors are markers of acute injury and of the inflammatory phase of the healing process.
It is known that active muscle degeneration and inflammation occur in the first few days following injury, whereas muscle regeneration usually occurs 7 to 10 days later [22]. Desmin plays a critical role in the maintenance of the structural and mechanical integrity of the contractile apparatus in smooth and striated muscle tissues. We observed that desmin mRNA expression is upregulated 1 h after acute trauma but progressively decreases with time.
The desmin protein level is very low in necrotic fibers, and it is further found in newly formed muscular structures, being a marker of muscle regeneration [23]. Our study showed a progressive increase in desmin protein levels throughout time similar to control levels in the long term. Both the desmin gene and protein expression profiles potentially reflect the response of smooth and striated muscles to injury and regeneration. Other major changes in muscle content initiated 7 days post VD were observed, such as the increased gene expression of smooth muscle MYH11 and skeletal muscle MYH1 and MYH3.
The MYH11 gene was overexpressed up to the 30th day following trauma. However, the MYH11 protein level did not change at any time point of our evaluation. MYH11 protein is expressed in the final stage of vascular development and is considered the definitive marker of mature smooth muscle cells [24]. Those molecular findings together with the histomorphological analyses among the groups suggest that urethral smooth muscle cells suffer from VD injury but self-regenerate efficiently.
MYH3 is the embrionic form of myosin used as a marker of regeneration in mature striated muscle [25]. In our study, MYH3 gene expression was very low on the 30th day following trauma, meaning that there was little or no active regeneration in the EUS of rats at this time point. These results suggest that the remodeling process is consolidated, and the urethral changes observed in our study may be persistent.
MYH2 is one of the most important myosins of mature striated muscle, a major contractile protein that converts chemical energy into mechanical energy through the hydrolysis of ATP. Even though MYH2 protein expression increased from the 7th to 30th days following trauma, the levels still were decreased compared with controls, suggesting the incomplete regeneration of striated muscle fibers. There is a lack of studies investigating the expression of muscle structural proteins in the long term after VD, making it difficult to compare with other results.
Similar to muscles, collagen expression alterations were more pronounced in the recent 7 days post trauma with increased COL1a1 mRNA and COL3a1 protein expressions. This was expected as the proliferation phase of healing is characterized by the formation of a provisional ECM with more pronounced collagen III deposition. We could not find significant changes in COL1a1 protein expression between the groups at different time points. However, the progressive decrease in the ratio of newly formed COL3a1 over COL1a1 throughout the period is in agreement with the remodeling phase of healing [12], marked by the rearrangement of the ECM with partial replacement of the immature COL3 by mature COL1. The relative proportion of collagen I and collagen III fibers is an important index for tensile strength [26]. Whether our biochemical findings are in accordance with ECM physiology still requires further investigation.
An ideal regeneration process would involve the replacement of the injured cells by cells of the same type, leaving no lasting evidence of damage. Fibrosis is a process that results in substantial deposition of ECM components in which normal tissue is replaced by permanent scar [27]. We observed higher COL3a1 and lower MYH2 protein levels after injury in the long term compared with controls. These findings suggest some degree of fibrosis caused by this experimental model. While connective tissue is formed, the complete recovery of muscular tissue is not achieved [22]. The development of possible therapeutic interventions that limit the progression of fibrosis without adversely affecting the overall repair process or accelerating the healing process would be beneficial.
The literature shows a wide range of VD models [3, 7, 14, 17]. However, no previous study has evaluated the chronic effects of VD on the urethra of female rats by examining a broad array of factors through mRNA analysis and protein synthesis involved in the mechanisms of post-traumatic urethral injury and recovery.
In conclusion, we developed an animal model of trauma 12-h intermittent VD that provoked chronic morphological and molecular alterations in the urethra of rats characterized mainly by the increased COL3a1 and decreased MYH2 protein levels in the long term. These findings suggest fibrosis formation together with incomplete muscle regeneration. This may translate into impaired urethral function establishing the basis of SUI development. However, further investigation is needed to understand the impact of this model on urethral function and on the continence mechanism as well as its potential applicability in the investigation of preventive and therapeutic modalities for urethral regeneration and SUI such as pharmacotherapy, electrical therapy, surgical intervention and cellular therapy.
References
Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4–20.
Rud T, Andersson KE, Asmussen M, et al. Factors maintaining the intraurethral pressure in women. Investig Urol. 1980;17(4):343–7.
Cannon TW, Wojcik EM, Ferguson CL, et al. Effects of vaginal distension on urethral anatomy and function. BJU Int. 2002;90:403–7.
Phull HS, Pan HQ, Butler RS, et al. Vulnerability of continence structures to injury by simulated childbirth. Am J Physiol Renal Physiol. 2011;301(3):F641–9.
Huang J, Cheng M, Ding Y, et al. Modified vaginal dilation rat model for postpartum stress urinary incontinence. J Obstet Gynaecol Res. 2013;39(1):256–63.
Damaser MS, Broxton-King C, Ferguson C, et al. Functional and neuroanatomical effects of vaginal distension and pudendal nerve crush in the female rat. J Urol. 2003;170:1027–31.
Pan HQ, Kerns JM, Lin DL, et al. Dual simulated childbirth injury delays anatomic recovery. Am J Physiol Renal Physiol. 2009;296:F277–83.
Prantil RL, Jankowski RJ, Kaiho Y, et al. Ex vivo biomechanical properties of the female urethra in a rat model of birth trauma. Am J Physiol Renal Physiol. 2007;292:F1229–37.
Hong SH, Piao S, Kim IG, et al. Comparison of three types of stress urinary incontinence rat models: electrocauterization, pudendal denervation and vaginal distension. Urology. 2013;81:465.e1–6.
Rocha MA, Sartori MGF, Simões MJ, et al. The impact of pregnancy and childbirth in the urethra of female rats. Int Urogynecol J. 2007;18:645–51.
Pan HQ, Kerns JM, Lin DL, et al. Increased duration of simulated childbirth injuries results in increased time to recovery. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1738–44.
Hofer MD, Cheng EY, Bury MI, et al. Analysis of primary urethral wound healing in the rat. Urology. 2014;84(1):246.e1–7.
Sengupta P. The laboratory rat: relating its age with Human’s. Int J Prev Med. 2013;4(6):624–30.
Jiang HH, Damaser MS. Animal models of stress urinary incontinence. Handb Exp Pharmacol. 2011;202:45–67.
Phillips JI, Davies I. The comparative morphology of the bladder and urethra in young and old female C57BL/Icrfat mice. Exp Geront. 1980;15:551–62.
Woo LL, Hijaz A, Kuang M, et al. Over expression of stem cell homing cytokines in urogenital organs following vaginal distention. J Urol. 2007;177:1568–72.
Lenis AT, Kuang M, Woo LL, et al. Impact of parturition on chemokine homing factor expression in the vaginal distention model of stress urinary incontinence. J Urol. 2013;189:1588–94.
Birot OJG, Koulmann N, Peinnequin A, et al. Exercise-induced expression of vascular endothelial growth factor mRNA in rat skeletal muscle is dependent on fibre type. J Physiol. 2003;552(1):213–21.
Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–81.
Gerdes J, Schwab U, Lemke H, et al. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31(1):13–20.
Wood HM, Kuang M, Woo L, et al. Cytokine expression after vaginal distention of different durations in virgin Sprague-Dawley rats. J Urol. 2008;180:753–9.
Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84-A(5):822–32.
Bornemann A, Schmalbruch H. Desmin and vimentin in regenerating muscles. Muscle Nerve. 1992;15:14–20.
Beamish JA, He P, Kottke-Marchant K, et al. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:467–91.
Schiaffino S, Rossi AC, Smerdu V, et al. Developmental myosins: expression patterns and functional significance. Skelet Muscle. 2015;5:22.
Birk DE, Fitch JM, Babiarz JP, et al. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95:649–57.
Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210.
Acknowledgements
For excellent assistance, the authors are grateful to Eloísa D. Castro, Gabriel A. Alves and Graciele A. Oliveira.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Financial support
This research was supported by Fundação de Apoio à Pesquisa do Estado de São Paulo FAPESP (no. 20.254/2011).
Rights and permissions
About this article
Cite this article
Bortolini, M.A.T., Feitosa, S.M., Bilhar, A.P.M. et al. Molecular and histomorphological evaluation of female rats’ urethral tissues after an innovative trauma model of prolonged vaginal distention: immediate, short-term and long-term effects. Int Urogynecol J 30, 465–476 (2019). https://doi.org/10.1007/s00192-018-3634-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-018-3634-2