Abstract
Introduction and hypothesis
Limited existing evidence suggests that there is a high prevalence of female pelvic organ prolapse (POP) amongst Nepali women. However, to date, no comprehensive assessment of pelvic floor functional anatomy has been undertaken in this population. Our study aimed to determine functional pelvic floor anatomy in Nepali women attending a general gynaecology clinic.
Methods
One hundred and twenty-nine consecutive women attending the clinic were offered an interview, clinical examination [International Continence Society Pelvic Organ Prolapse Quantification system (ICS/POP-Q)] and 4D translabial ultrasound (TLUS). Most presented with general gynaecological complaints. Five were excluded due to previous pelvic surgery, leaving 124.
Results
A POP-Q exam was possible in 123 women, of whom 29 (24%) were diagnosed with a significant cystocele, 50 (41%) significant uterine prolapse and seven (6%) significant posterior compartment prolapse. Evaluation of 4D TLUS data sets was possible in 120 women, of whom 25 (21%) had a significant cystocele, 45 (38%) significant uterine prolapse and ten (8%) significant descent of the rectal ampulla. In 13 cases, there was a rectocele with a mean depth of 14 (10–28) mm. Of 114 women in whom uterine position could be determined, 68 (60%) had a retroverted uterus associated with significant uterine prolapse (P 0.038).
Conclusions
POP is common in Nepali women attending a general gynaecology clinic, with a high prevalence of uterine prolapse (40%). Uterine retroversion was seen in 60% and was associated with uterine prolapse. Patterns of POP in Nepal seem to be different from patterns observed in Western populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is the downward descent of pelvic organs, i.e. bladder, uterus or posthysterectomy vaginal cuff; small bowel; and/or rectal ampulla through the levator hiatus. The levator hiatus is formed by the puborectalis component of the levator ani muscle (LAM) and symphysis pubis (SP). It is the largest potential hernial portal in the human body, the size of which is associated with signs and symptoms of prolapse [1]. POP is a highly prevalent condition with a multifactorial and complex aetiology yet to be clearly understood [2]. The lifetime risk of surgery for POP in the general female population in Western Australia was estimated to be 19% [3].
Recently, studies reported that the prevalence and risk factors for POP and pelvic floor dysfunction (PFD) differ between ethnic groups. Ethnicity thus is likely to be a significant aetiological factor [4,5,6,7]. Stress urinary incontinence (SUI) and symptomatic POP seem to be less common among African American women compared with Caucasian women [4]. A study comparing nulliparous East Asian, Caucasian and Black South African women showed significant differences in levator biometry and pelvic organ mobility on translabial ultrasound (TLUS) [5]. Another imaging study comparing southeast Asian and Caucasian pregnant nulliparous women found that the former had a smaller levator hiatus, less pelvic organ mobility and a thicker levator muscle [6]. Another recent study compared Ugandan women with Caucasian women and found that all measurements of hiatal dimensions and pelvic organ descent were significantly higher among the Ugandans [7]. Such data is lacking for Nepali women, who are a particularly interesting group due to limited existing evidence for unusually high rates of prolapse [8, 9, 11].
POP is one of the most widespread reproductive health problems in Nepal, with >1 million women affected. A report on 2268 women showed a prevalence of 37% for uterine prolapse [8]. Another study examined 2072 women in western Nepal and detected that one in four had genital prolapse, of whom 95% had self-reported the prolapse [9]; presentation seems to be unusually early, mostly during the reproductive years [10, 11]. One of the potential explanations may be pelvic floor trauma [1], and another may be retroversion, which seems to be asssociated with uterine prolapse [12, 13].
We aimed to perform the first comprehensive assessment of functional pelvic floor anatomy in Nepali women. The protocol included an interview, clinical examination [International Continence Society/Pelvic Organ Prolapse Quantification system (ICS/POP-Q)], Oxford score and 4D TLUS. This was offered to all women attending a general gynaecology outpatient clinic at a tertiary hospital in the capital, Kathmandu.
Materials and methods
The first and second authors travelled to Nepal in November 2016 to conduct a study at the outpatient gynaecology clinic at Kathmandu Model Hospital. All nonpregnant women ≥18 years attending the clinic were invited to participate. Women were excluded if they were unable to communicate or if completion of a questionnaire was impossible due to illiteracy. A local Nepali intern assisted as interpreter in explaining the procedure, obtaining written consent and collecting questionnaire data covering general background and other demographic data, chief complaints, medical history, obstetric and gynaecological history, menopausal status, smoking and drinking, work background and symptoms of lower urinary tract dysfunction, prolapse, obstructed defecation and anal incontinence, including a St. Mark’s score. We used a visual analogue scale (VAS) bother score for SUI, urge urinary incontinence (UUI), anal incontinence (AI) and prolapse symptoms. Women were asked to rate the degree of bother caused by individual symptoms on a scale from 0 (no bother) to 10 (maximum possible bother). The VAS is an effective, simple and highly repeatable method to assess subjective bother of a symptom [14].
The first author performed pelvic and 4D TLS examinations on all patients while blinded to all other data. All participants were asked to empty their bladder prior to the examination, which was performed in dorsal lithotomy. The vaginal examination comprised assessment of the pelvic floor muscle (PFM) on contraction, avulsion and Oxford scoring [15]. The POP-Q exam was also performed by the first author on maximum Valsalva sustained for 6–8 s. As per previously published criteria, significant prolapse was defined as POP-Q stage ≥2 for anterior and posterior compartments and stage ≥1 for the central compartment [16]. Subsequently, participants underwent 4D TLUS using a GE Voluson-i System (GE Medical Systems, Zipf, Austria), with 8-4 MHz curved-array volume transducer. Volumes were acquired at rest, on maximum pelvic floor muscle contraction (PFMC) and on Valsalva manoeuvre at an acquisition angle set to the system maximum of 85°, as previously described [17, 19]. At least two volumes on maximum Valsalva and PFMC were acquired. Postimaging analysis of pelvic organ descent, hiatal area, LAM and external anal sphincter (EAS) integrity were performed by the first author at a later date using proprietary software (GE Kretz Medical 4D View Version 10.0) while blinded against all other data. As per previously published criteria, significant prolapse on TLUS is defined as bladder descent to ≥10 mm below the SP [17], uterine descent to ≤15 mm above the SP [18] or rectal ampulla descent to ≥15 mm below the SP on maximum Valsalva in the mid-sagittal plane, with the posteroinferior margin of the SP as reference line [17]. Hiatal area was determined by volume rendering, including the plane of minimal hiatal dimensions [1, 19] (Fig. 1). Levator integrity was determined by tomographic imaging at 2.5-mm interslice interval [20].
4D translabial ultrasound (TLUS) on maximum Valsalva manoeuvre. a Midsagittal image showing pelvic organ descent. A horizontal reference line is placed at the posteroinferior margin of the symphysis pubis, and organ descent (C cystocele, U uterus, R rectal ampulla) is measured against that line. b Volume rendering in the axial plane showing plane of minimal hiatal dimensions on maximum Valsalva. Dotted line indicates hiatal area. S symphysis pubis, B cystocele/ bladder, A anal canal, L levator ani muscle
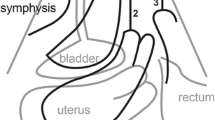
To remain blinded to prolapse assessment, anteversion/retroversion of the uterus was determined offline by the first author on a separate occasion while blinded against all clinical data. The uterus was anteverted if it was tilted anteriorly lying on the bladder roof and retroverted if tilted posteriorly, with bowel seen on the bladder roof (Fig 2). This method was highly repeatable in a test–retest series of 40 patients, yielding a Cohen’s kappa of 0.8.
Statistical analysis was performed using SPSS 20 (SPSS, Chicago, IL, USA). Categorical data is expressed as n (%). The study was approved by the Institutional Review Committee (IRC) of the Public Health Concern Trust Nepal (Phect-NEPAL) on 16 June 2016.
Results
Of 129 nonpregnant women seen in the clinic during the inclusion period, 43 (33%) presented with pelvic pain, 30 (23%) with vaginal discharge and 40 (32%) with other complaints such as vulval/vaginal itching, dysfunctional bleeding and request for contraceptive advice. Only 16 (12%) presented with symptoms of PFD: UI n = 3, obstructed defecation n = 3, prolapse n = 10) Five women were excluded due to previous pelvic surgery, leaving 124, to whom the following results pertain. For demographics, symptoms of PFD and bother scores, see Tables 1 and 2. An ICS/POP-Q exam was possible in 123 women. On POP-Q, a significant cystocele was seen in 29 (24%), 50 (41%) had significant uterine prolapse and seven (6%) had significant posterior compartment prolapse. Mean Ba was −0.95 (−3 to +3), C was −3.88 (−5 to +2.5) and Bp was −2.19 (−3 to +2). Expressed in POP-Q stages, 18 (15%) had stage 1, 64 (52%) had stage 2 and ten (8%) had stage ≥3 prolapse. Mean Gh was 4.05 (2.5–8), Pb was 3.11 (2–4.5) and Gh + Pb mean 7.16 (range 4.5–12) (Table 3).
Evaluation of 4D TLUS volume data sets by postprocessing on a PC was possible in 120 women: 25 (21%) had a significant cystocele, 45 (38%) significant uterine prolapse and ten (8%) significant posterior compartment prolapse. Mean hiatal area on Valsalva was 20.1 (range, 7.4–41) cm2. In 13 cases, there was a true rectocele (diverticulum of the rectal ampulla), with a mean depth of 14 (10–28) mm. Of the 107 women who had given birth vaginally, two (2%) showed an avulsion injury on tomographic imaging (Table 3). Symptoms of prolapse (n = 24) were associated with significant cystocele on POP-Q, significant cystocele, uterine descent and any significant prolapse on 4D TLUS (P = 0.038, 0.001, 0.047 and 0.028, respectively. Symptoms of obstructed defecation were not associated with any clinical or sonographic measures of pelvic organ descent. Of the 120 in whom evaluation of 4D TLUS data sets was possible, the bladder roof was not visible in six either because of significant residual urine or the volume not being deep enough. Of the remaining 114, 68 (60%) had a retroverted uterus. On Pearson’s chi-squared test, there was an association between retroversion and uterine prolapse on ICS/POP-Q and 4D TLUS (P 0.038 and 0.062, respectively).
Discussion
In summary, our study showed POP to be very common in Nepali women attending a general gynaecology clinic, confirming previous limited data. Significant uterine prolapse was diagnosed in 41 and 38%, significant cystocele in 24 and 21% and significant posterior compartment prolapse in 6 and 8% on POP-Q and TLUS, respectively. True rectocele was comparatively uncommon. Only 22% of women had significant hiatal area ballooning, with a mean hiatal area of only 20 cm2; major levator trauma (avulsion) was found in <2% of parous women. Retroversion was common and associated with uterine prolapse.
To date, published evidence on POP in Nepali women is limited and seems to vary among different regions [8,9,10,11, 22, 23]. Previous studies have either only included self- reported prolapse symptoms without other assessment, or the authors performed a vaginal examination for prolapse assessment but did not differentiate between the three compartments [9,10,11, 22, 23]. A recent study used POP-Q grading and found that 60.9% of women had stage ≥2 POP [21]. We recognise that some of these studies are of low methodological quality [8,9,10,11, 21,22,23]; only one used the POP-Q system, let alone imaging. However, even given these limitations, it seems clear that POP in Nepali women is common and presents early [8,9,10,11, 21,22,23].
Our study showed a high prevalence of POP in Nepali women attending a general gynaecology outpatient clinic, confirming such evidence. This is especially true for uterine prolapse, which we observed in ~40% on POP-Q and 4D TLUS. In a gynaecology clinic in the USA, the Pelvic Organ Support Study (POSST) study found the overall prevalence of POP to be 37% (35% stage 2, 2% stage 3) [24]. In comparison, we documented a much higher prevalence of stage 2 or 3 of 62%. Uterine retroversion was seen in 60% and was associated with uterine prolapse. Only 22% of women showed hiatal ballooning, and major levator trauma, or avulsion, was surprisingly uncommon.
Risk factors of POP in Nepali women have been studied and are reported as follows: extensive physical labour, especially during and immediately after pregnancy; low availability of skilled birth attendants; rapid succession of pregnancies; low maternal weight due to malnutrition; smoking while having chronic obstructive pulmonary disorder (COPD); number of vaginal deliveries; labour duration [8,9,10,11, 21,22,23]. However, the role of these factors in the aetiology of prolapse is not well defined and largely speculative. What seems clear is that age at presentation is younger than in Western populations: 40% of women with uterine prolapse were of reproductive age [10], with mean age at presentation being 27.9 years [11]. It is also evident that patterns of presentation are different from those seen in Western populations. Hence it seems important to investigate the aetiology and pathogenesis of POP in Nepali women.
In Western populations, both cystocele and uterine prolapse are strongly associated with levator avulsion, which is highly prevalent [25]. In the Nepali populations examined by us, major levator trauma was very uncommon, at <2%; hence, it cannot be a major aetiological factor in Nepal. This is in accord with low rates of hiatal ballooning, which is another potential indicator of childbirth-related trauma and strongly associated with prolapse and prolapse recurrence [1]. On the other hand, retroversion, a recognised risk factor for uterine prolapse [13], was much more common in our study than in Western populations [12] and was associated with uterine prolapse. The prevalence of uterine retroversion in a Western general gynaecology clinic was 19% [12], which agrees with other prevalence data from 15 studies summarised in [13]. In this context, the 60% retroversion prevalence we found in Nepali women attending a general gynaecology clinic seems extraordinarily high. On the basis of this finding, we hypothesise that (a) retroversion uteri is common in Nepali compared with in Western populations, and (b) retroversion is an important factor in the aetiology of prolapse in Nepali women.
While these hypotheses must be supported by additional work in nulliparous women, there are potentially significant implications for public health policy in Nepal. Surgical treatment of choice for any prolapse in Nepal is hysterectomy, which is offered both by indigenous and external health services [26, 27]. Such a primarily surgical approach may not be necessary in many Nepali women, since the absence of major levator trauma and low rates of hiatal ballooning make them perfect candidates for pessary management [19, 28]. Vaginal-ring pessaries are an effective, simple low-cost method of alleviating symptoms of POP and associated PFD [29]. Healthcare workers may be trained in vaginal pessary placement and management, with limited resource implications, which is particularly advantageous in rural areas where facilities are limited. However, it is understood that there may be obstacles to the use of pessaries in rural Nepal where proper sanitation and hygiene is not always available.
Strengths and limitations
To our knowledge, this is the first study to provide a comprehensive assessment of pelvic organ support and functional anatomy in Nepali women. Imaging was performed using a standardised, highly repeatable method, with postprocessing analysis allowing comprehensive blinding against all clinical data. However, there are some limitations: Our study was performed in a gynaecology clinic in an urban hospital in Kathmandu, which implies potential selection bias. Findings may not be representative of the general population. Secondly, it is conceivable that direct questioning about symptoms by a male Nepali intern may have impacted our ability to ascertain symptoms and history due to embarrassment. In addition, due to the nature of healthcare in Nepal, information on obstetric history was limited. Finally, direct comparisons with data obtained in Western populations is inherently difficult due to multiple potential confounders.
In conclusion, POP was very common in Nepali women attending a general gynaecology clinic, confirming literature data. This was especially true for uterine prolapse, which was observed in ~40%. In contrast to findings in Western populations, levator trauma and hiatal ballooning were uncommon and appear less likely as aetiological factors. Uterine retroversion, on the other hand, was very common at 60% and associated with uterine prolapse. Rectocele was comparatively uncommon.
POP patterns in Nepal seem to be different from those observed in Western populations and may require different approaches to prevention and treatment.
Abbreviations
- ICS/POP-Q:
-
International Continence Society, Pelvic Organ Prolapse Quantification system
- TLUS:
-
4D Translabial ultrasound
- POP:
-
Pelvic organ prolapse
- VAS:
-
Visual analogue scale
- PFM:
-
Pelvic floor muscle
- PFMC:
-
Pelvic floor muscle contraction
- LAM:
-
Levator ani muscle
- EAS:
-
External anal sphincter
- SP:
-
Symphysis pubis
- COPD:
-
Chronic obstructive pulmonary disease
References
Dietz HP, Franco AV, Shek KL, Kirby A. Avulsion injury and levator hiatal ballooning: two independent risk factors for prolapse? An observational study. Acta Obstet Gynecol Scand. 2012;91(2):211–4.
Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin N Am. 1998;25(4):723–46.
Smith FJ, Holman CA, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116(5):1096–100.
Whitcomb EL, Rortveit G, Brown JS, Creasman JM, Thom DH, Van Den Eeden SK, et al. Racial differences in pelvic organ prolapse. Obstet Gynecol. 2009;114(6):1271.
Abdool Z, Dietz HP, Lindeque BG. Ethnic differences in the levator hiatus and pelvic organ descent: a prospective observational study. Ultrasound Obstet Gynecol. 2017;50(2):242–6.
Cheung RY, Shek KL, Chan SS, Chung TK, Dietz HP. Pelvic floor muscle biometry and pelvic organ mobility in east Asian and Caucasian nulliparae. Ultrasound Obstet Gynecol. 2015;45(5):599–604.
Shek KL, Krause HG, Wong V, Goh J, Dietz HPI. Pelvic organ support different between young nulliparous African and Caucasian women? Ultrasound Obstet Gynecol. 2016;47(6):774–8.
Center for Agro-Ecology and Development (CAED) (2006) Uterine prolapse widespread. Post Report. Nepal. Available from: http://www.advocacynet.org/partners_archive/womens-reproductive-rights-program/
Bonetti TR, Erpelding A, Pathak LR. Listening to “felt needs”: investigating genital prolapse in western Nepal. Reprod health matters. 2004;12(23):166–75.
Subba B, Adhikari D, Bhattarai T. The neglected case of the fallen womb. Nepal: Himal South Asian; 2003.
Gurung G, Rana A, Amatya A, Bista KD, Joshi AB, Sayami J. Pelvic organ prolapse in rural Nepalese women of reproductive age groups: what makes it so common? Nepal J Obstet Gynaecol. 2007;2(2):35–41.
Haylen BT, McNALLY G, Ramsay P, Birrell W, Logan VA. Standardised ultrasonic diagnosis and an accurate prevalence for the retroverted uterus in general gynaecology patients. Aust NZ J Obstet Gynaecol. 2007;47(4):326–8.
Haylen BT. The retroverted uterus: ignored to date but core to prolapse. Int Urogynecol J. 2006;17(6):555.
Ulrich D, Guzman Rojas R, Dietz HP, Mann K, Trutnovsky G. Use of a visual analog scale for evaluation of bother from pelvic organ prolapse. Ultrasound Obstet Gynecol. 2014;43(6):693–7.
Dietz HP, Shek C. Validity and reproducibility of the digital detection of levator trauma. Int Urogynecol J. 2008;19(8):1097–101.
Dietz HP, Mann KP. What is clinically relevant prolapse? An attempt at defining cutoffs for the clinical assessment of pelvic organ descent. Int Urogynecol J. 2014;25(4):451–5.
Dietz HP, Lekskulchai O. Ultrasound assessment of pelvic organ prolapse: the relationship between prolapse severity and symptoms. Ultrasound Obstet Gynecol. 2007;29(6):688–91.
Shek KL, Dietz HP. What is abnormal uterine descent on translabial ultrasound? Int Urogynecol J. 2015;26(12):1783–7.
Chan SS, Cheung RY, Yiu KW, Lee LL, Leung TY, Chung TK. Pelvic floor biometry during a first singleton pregnancy and the relationship with symptoms of pelvic floor disorders: a prospective observational study. BJOG. 2014;121(1):121–9.
Dietz HP, Moegni F, Shek KL. Diagnosis of levator avulsion injury: a comparison of three methods. Ultrasound Obstet Gynecol. 2012;40(6):693–8.
Lien YS, Chen GD, Ng SC. Prevalence of and risk factors for pelvic organ prolapse and lower urinary tract symptoms among women in rural Nepal. Int J Gynecol Obstet. 2012;119(2):185–8.
Bodner-Adler B, Shrivastava C, Bodner K. Risk factors for uterine prolapse in Nepal. Int Urogynecol J. 2007;18(11):1343–6.
Thapa S, Angdembe M, Chauhan D, Joshi R. Determinants of pelvic organ prolapse among the women of the western part of Nepal: a case–control study. J Obstet Gynecol Research. 2014;40(2):515–20.
Swift S, Woodman P, O’boyle A, Kahn M, Valley M, Bland D, et al. Pelvic organ support study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192(3):795–806.
Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG. 2008;115(8):979–84.
Dhital R, Otsuka K, Poudel KC, Yasuoka J, Dangal G, Jimba M. Improved quality of life after surgery for pelvic organ prolapse in Nepalese women. BMC Womens Health. 2013;13(1):22.
Sah DK, Doshi NR, Das CR. Vaginal hysterectomy for pelvic organ prolapse in Nepal. Kathmandu University Medical J. 2010;8(2):281–4.
Pixton S, Caudwell Hall J, Turel F, Dietz HP. Predictors of ring pessary success in women with pelvic organ prolapse. Int Urogynecol J. 2017; in print.
Fernando RJ, Thakar R, Sultan AH, Shah SM, Jones PW. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108(1):93–9.
Acknowledgements
The authors thank the women who participated in this study. We also thank staff at Kathmandu Model Hospital for their assistance and generosity with their limited space. We are especially grateful to Dr. Vishal Kumar Trivedi for excellent assistance during data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H.P. Dietz has received unrestricted educational grants from GE Medical. F. Turel and D. Caagbay have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Turel, F., Caagbay, D. & Dietz, H.P. Functional pelvic floor anatomy in Nepali women attending a general gynaecology clinic. Int Urogynecol J 29, 1435–1440 (2018). https://doi.org/10.1007/s00192-017-3534-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-017-3534-x