Abstract
Introduction and hypothesis
The objective of this study was to assess the safety and efficacy of the Elevate Apical and Posterior single-incision mesh system (SIMS) with IntePro Lite for pelvic organ prolapse repair.
Methods
This prospective multicenter study included 139 women with ≥ stage II posterior vaginal prolapse and/or apical descent who underwent placement of type I polypropylene mesh through a single transvaginal incision with no external needle passes. Primary endpoint was the percent of patients with posterior and/or apical stage ≤ I (“cure”) at follow-up. Secondary endpoints included, but were not limited to, rate of mesh extrusion and disease-specific quality of life outcomes.
Results
At 12 months, objective posterior wall and apical cure rates were 92.5 and 89.2 %, respectively, with an extrusion rate of 6.5 %.
Conclusions
The SIMS appears to be effective and safe in treating patients with posterior vaginal and/or apical prolapse. The risks and benefits of transvaginal synthetic mesh insertion should be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is a common condition for which over 200,000 women in the USA undergo pelvic reconstructive surgery each year [1]. This clinical entity may be associated with multiple site endopelvic fascial defects [2], with Richardson et al. [3] first introducing the concept of prolapse borne of discrete breaks or tears in supportive connective tissue, as opposed to the presumption of generalized weakening. Further, it is commonly accepted that disruption of the uterosacral-cardinal ligament complex is responsible for apical descent [4]. As women with POP often have a combination of defects, concomitant correction of compromised apical support and/or endopelvic fascia must be considered at the time of pelvic floor reconstruction for a comprehensive repair.
Nonabsorbable synthetic mesh has been employed by vaginal surgeons in an effort to provide greater anatomic durability in patients with single or multiple compartment POP. Type I [5] polypropylene is most often described for use in the vagina as it possesses the in vivo characteristics of good tissue integration with minimal inflammatory response [6]. The placement of polypropylene mesh transvaginally has shown anatomic benefit in anterior vaginal repair [7], and more recently, has been shown to provide greater anatomic durability than native tissue in repair of the posterior compartment [8]. Since 2004, several trocar-based transvaginal mesh systems have been available to treat posterior vaginal prolapse and apical descent concomitantly, including Apogee (American Medical Systems, Minnetonka, MN, USA), posterior Prolift (Ethicon, Somerville, NJ, USA), and Avaulta Posterior (CR Bard, Covington, GA, USA). In 2007, the Elevate Apical and Posterior single-incision mesh system (SIMS, American Medical Systems, Minnetonka, MN, USA) was introduced for the same indications, with a design to reduce complications seen with trocar-dependent systems [9, 10]. The SIMS allows for insertion of synthetic mesh independent of transperineal trocars, with apical fixation provided by low-profile anchors embedded bilaterally into the sacrospinous ligaments. This “kit” also offers the potential benefit of placement without tension to maintain a normal vaginal axis, and to preserve normal visceral and sexual function.
Our objective was to determine the safety and efficacy of the SIMS for posterior vaginal and/or apical prolapse.
Materials and methods
We performed a prospective multicenter study conducted at 16 US and 6 European urogynecologic, urologic, or gynecologic sites, enrolling women with posterior vaginal wall prolapse (≥ stage II) and/or apical (cuff or cervix) descent (≥ stage II). Primary endpoint was the percent of patients with posterior and/or apical “cure” (stage ≤ I) at follow-up. Assessment of anatomic durability was performed by a single practitioner at each site, employing the Pelvic Organ Prolapse Quantification (POP-Q) system according to International Continence Society (ICS) guidelines [11]. Subjects were seen postoperatively at 6 weeks, 3, 6, and 12 months. All sites received Institutional Review Board or Ethics Committee approval prior to the conduct of any study activities. Study oversight was carried out by the lead investigator’s academic Conflict of Interest Committee to ensure the absence of bias.
Secondary endpoints included procedure time, estimated blood loss (EBL), Wong-Baker FACES pain scores, adverse events (AEs), and change in validated quality of life (QOL) questionnaires including the Pelvic Floor Distress Inventory (PFDI), Pelvic Floor Impact Questionnaire Short Form (PFIQ-7), and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ-12) [12–14]. The PFDI and PFIQ each have three subscales (POPDI and POPIQ for prolapse; UDI and UIQ for lower urinary tract; CRADI and CRAIQ for colorectal) in measuring site-specific symptom distress and life impact, respectively. Both instruments are valid, reliable, and responsive to intervention [15]. A patient satisfaction survey was also employed.
IntePro Lite was employed in all cases. It is a type I macroporous polypropylene mesh with the same knit as IntePro (as seen in Apogee), possessing a smaller fiber diameter (3/1,000 of an inch for IntePro Lite versus 4/1,000 of an inch for IntePro) and less mesh density (25.5 g/m2 for IntePro Lite versus 50 g/m2 for IntePro).
Preoperatively, patients received intravenous cephalosporin or quinolone within an hour of surgery. Operative technique was standardized and included a vertical midline incision ≤5 cm in length along the posterior vaginal wall. A full-thickness dissection was performed, using local anesthesia or saline to develop the rectovaginal space. An IntePro mesh arm was anchored into the sacrospinous ligament on either side at a point 2 cm medial to the ischial spine (Fig. 1). Extended absorbable suture (2-0 polydioxanone) or permanent stitch (2-0 polypropylene) was used to secure the IntePro Lite graft proximally to the vaginal apex or posterior cervix, respectively. The apical mesh arms were then passed through the mesh body lateral eyelets ipsilaterally, and a positioning tool was used to elevate the graft and attached apex. Final IntePro Lite placement was without posterior deviation, in the presumptive prevention of defecatory dysfunction and maintenance of a normal anatomic vaginal orientation. Each mesh arm was then trimmed at a point 1–2 cm beyond a locking islet. The distal portion of the graft was trimmed at the discretion of the surgeon to fit vaginal length and attached to the perineal body and rectovaginal septa bilaterally with delayed absorbable suture. Digital rectal examinations were performed throughout each case to ensure the absence of trauma to the rectum, and also to confirm the absence of over tensioning of the graft onto the apical mesh arms. There was no routine trimming of redundant vaginal wall. Closure of the incision was with delayed absorbable suture. Additional reconstructive procedures were performed as indicated with the exception of concomitant repairs in the posterior compartment and/or apex. Vaginal packing and a transurethral catheter were placed and removed within 12–24 h.
Descriptive statistical analysis was performed with continuous variables summarized using mean ± SD or median (range), and discrete variables reported using numbers and percentages. Data in regard to anatomic success by compartment were analyzed using the last failure carried forward (LFCF) method, which carries forward a patient’s objective failure at 6 months if their 12-month results are missing. The LFCF analysis also considers subjects to be failures if they were reoperated for recurrent prolapse in the posterior or apical segments within 12 months from the initial implant. Changes in QOL scores and POP-Q measurements between follow-up and baseline were evaluated by a paired t test or Wilcoxon signed rank test as appropriate. Only those subjects who completed both baseline and follow-up were included in paired analyses. Proportions were compared through Fisher’s exact test. Statistical analyses were performed using SAS Version 9.1.3 (SAS Institute, Inc., Cary, NC, USA). P < 0.05 was considered statistically significant.
Results
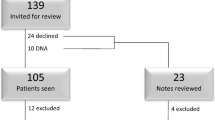
A total of 141 subjects were taken to the operating room for placement of a SIMS. Two were not implanted as per protocol. One required the use of a suture to replace one of the locking anchors that had been dropped. The other subject was not implanted due to a sacrospinous ligament that would not support the fixation tip. One hundred thirty-nine women were successfully implanted. Baseline demographics are provided in Table 1. At baseline, 134 (96.4 %) patients presented with posterior vaginal prolapse ≥ stage II and 42 (30.2 %) had apical descent ≥ stage II. Previous compartment-specific surgery was recorded in 21 (15 %) and 8 (6 %) of those with posterior vaginal and apical prolapse, respectively. Hysterectomy was performed at the time of mesh placement in 20 (14.4 %) subjects. Posterior enterocele was identified intraoperatively in 66 (47.5 %) patients.
Average procedure time for the SIMS only was 45.4 ± 18.6 min. Mean EBL was 55.4 ± 45.7 cc (median 50.0 cc with a range of 5–340 cc) and no patient required transfusion. Mean Wong-Baker FACES pain scores were reduced from 1.9 ± 2.3 at baseline to 0.6 ± 1.3 and 0.3 ± 0.9 at 6 weeks and 3 months, respectively.
Twelve-month follow-up data were available for 126 (90.6 %) subjects. Of the 13 without follow-up, 8 missed the 12-month visit (all showing cure at 6 months) and 5 withdrew consent. Objective posterior wall cure rate was 92.5 % (111/120) and apical cure was 89.2 % (33/37) (Table 2). Results stratified by baseline stage (II versus III/IV) showed no difference in cure of the apex (93.8 vs 85.7 %, P = 0.618) or in cure of the posterior vaginal wall (95.0 vs 87.5 %, P = 0.260). When anatomic data were analyzed by intent to treat analysis, success was found to be 82.8 and 78.6 % for the posterior and apical compartments, respectively. Significant improvements were seen in POP-Q points Ap, Bp, and C, with no significant change seen in total vaginal length (TVL) (Table 3). One patient receiving the device for posterior vaginal prolapse without an apical defect at baseline exhibited advanced stage cervical descent at 6 months, attributed to cervical elongation.
Of the 57 subjects who did not receive a concomitant anterior repair, 51 had anatomic data at baseline and at 12 months. The mean Aa score changed from −1.8 ± 1.3 at baseline to −2.3 ± 0.8 at follow-up (P = 0.002). The mean Ba score changed from −1.2 ± 2.4 at baseline to −2.2 ± 0.9 at follow-up (P < 0.001). One subject had an asymptomatic de novo anterior vaginal prolapse (stage II).
Device- and/or procedure-related AEs are shown in Table 4. A total of 30 subjects (21.6 %) experienced at least one AE determined to be “possibly,” “probably,” or “definitely” related to the device and/or procedure. No device- or procedure-related dyspareunia was reported. Extrusion (vaginal exposure of the mesh) was seen in nine (6.5 %) subjects, with a median onset of 94 days (range 19–371). Two of the nine were apical in location (one following concomitant hysterectomy) and seven were along the posterior vaginal wall (four distal, two central, one lateral). Three subjects required revision in the operating room for treatment of mesh exposure, one of whom exhibited a persistent extrusion at 12 months. Six were treated conservatively (local estrogen and/or trimming in the office), with three showing continued exposure at 1 year. None of the patients in our study required total mesh removal.
All QOL scores were improved significantly at 12 months (Table 5). Subjects noted less bother in both the prolapse and colorectal-anal subscales of the PFDI and PFIQ-7 questionnaires postoperatively. Sexual symptom scores as per the PISQ-12 also improved significantly following surgery. Fifty-three patients were sexually active at baseline, with 48 having completed the PISQ-12 both before surgery and at follow-up; 6 (12.5 %) of the 48 reported baseline dyspareunia (defined as “always” or “usually” on question 5 of the PISQ-12, “Do you feel pain during sexual intercourse?”), with 4 (66.7 %) showing resolution at 12 months. Regarding de novo dyspareunia, 5 (11.9 %) of 42 who had no pain with intercourse at baseline answered “usually” or “always” at follow-up per PISQ-12 question 5. Additionally, 13 (18.1 %) of the 72 women who were not sexually active at baseline were sexually active at 1-year follow-up, and 5 (9.4 %) of the 53 who were sexually active at baseline were not active at 12 months. Patient satisfaction was such that 96.8 % (119/126) felt that they were “some” or “a lot” improved and 81.0 % (102/126) were “very” or “extremely” satisfied. Overall, 96.0 % (121/126) of subjects responded that they would recommend the procedure to a friend.
Discussion
Data from our prospective multicenter study on the use of SIMS in the treatment of posterior compartment and/or apical prolapse suggest anatomic durability through 12 months, low morbidity, infrequent reoperation for mesh extrusion, improved functional outcomes, and high patient satisfaction.
Results for posterior vaginal correction are consistent with prospective, noncomparative free graft data (suture secured mesh) from Rutman et al., who reported 6-month follow-up on 50 subjects receiving uterosacral ligament anchored polypropylene for apical/posterior wall reconstruction [16]. Postoperative mean POP-Q measurements for the posterior wall were similar to those seen in our sample, with subjects exhibiting values of −3.0 for Ap and −2.9 for Bp (versus −2.7 and −2.6 for SIMS). Apical indices following free graft placement were better with values of −9.7 and 9.7 for point C and TVL, respectively (versus −6.6 and 8.7 for SIMS).
Our results in regard to anatomic cure are in the range of published trocar-based data. Gauruder-Burmester et al. reported retrospectively on 48 subjects receiving Apogee, [17] with all women showing POP-Q stage III posterior vaginal prolapse prior to surgery, and 16 exhibiting a concomitant stage II vaginal vault descensus. Postoperatively, all subjects were cured (stage ≤ I) of posterior vaginal and apical prolapse at 1-year follow-up. Withagen et al. reported prospective anatomic results at 1 year in 77 subjects who underwent posterior Prolift, citing 90 and 94 % success (stage ≤ I) in the posterior and apical compartments, respectively. However, 16 (25 %) of 65 patients who did not receive concomitant anterior vaginal wall support developed de novo prolapse in the anterior compartment [18]. Only 2 % of patients in our sample without an anterior wall repair exhibited de novo anterior prolapse. Withagen et al. offered more recent prospective comparative data with 12-month follow-up, citing posterior wall and apical success rates of 95.9 and 93.7 % after posterior Prolift versus 75.5 and 97.5 % following native tissue repair [8].
Our objective posterior vaginal wall cure rate of 92.5 % is comparable to trocar-free data reported by Zyczynski et al. [19], who determined 29 (93.5 %) of 31 patients to exhibit ≤ stage I prolapse 12 months after receiving the Prosima Pelvic Floor Repair System (Ethicon, Somerville, NJ, USA) in the posterior compartment. No subjects with advanced apical prolapse were included in their study. Shortened wear (<21 days) of a vaginal support device postoperatively was associated with significantly inferior anatomic support. Total vaginal length was found to be reduced at 12 months following posterior Prosima, with a baseline value of 8.7 ± 1.2 and a 1-year value of 7.3 ± 0.9. No significant change in total vaginal length was seen in our sample.
Average procedure time for placement of the SIMS alone was longer than the 35 min reported by Gauruder-Burmester for Apogee [17] and shorter than the 57.6 min required for insertion of posterior Prolift in a sample of 71 patients [9]. Our mean intraoperative blood loss was less than the 85 cc reported in the aforementioned Prolift study.
A distinct advantage of the SIMS is that of a design that obviates the need for transperineal trocar passage, given the attendant risk of neurovascular trauma or damage to viscus from such maneuvers. In data from the Nordic Transvaginal Mesh Group registry, Altman and Falconer reported 4 cases of rectal perforation (4.4 %) in a series of 91 Prolift repairs with bilateral transgluteal passage of trocars through the sacrospinous ligament [9]. One (0.7 %) rectal injury was observed in our study, deemed not to be device related, however, may represent the sequela of the thicker dissection typically employed in placing the SIMS. Abdel-Fattah et al. reported an intraoperative blood loss of more than 400 cc in 2 (2.9 %) of 70 subjects undergoing posterior Prolift, with 1 patient requiring blood transfusion (1.4 %) [10]. In our study, blood loss did not exceed 400 cc in any patient, and no transfusions were administered. No neurologic sequelae were recorded.
There was no increase in the mean Wong-Baker FACES pain score at 6 weeks and 3 months compared to baseline in those receiving SIMS. Pain or discomfort localized to the buttock, pelvis, or back was reported by a total of four (2.9 %) patients in our sample, of whom two exhibited resolution without intervention. Favorable pain outcomes in our study may be associated with the low-profile anchor, reducing the likelihood of coccygeus muscle strangulation upon sacrospinous ligament capture. Additionally, as the SIMS mesh is secured at a distance from the ligament, tension on the musculature may be less.
Our extrusion rate was higher than that generated from free graft data, with Rutman et al. and de Tayrac et al. reporting an incidence of posterior vaginal wall mesh exposure of 2 and 1.3 % at a mean follow-up of 6 and 13 months, respectively [16, 20]. Regarding device-specific data, our extrusion rate was better than the 11 % reported by Withagen et al. in subjects undergoing posterior vaginal prolapse repair with Prolift [18]. No extrusions were reported by Gauruder-Burmester at 1 year in the aforementioned Apogee study [17]. Three (10 %) and five (7.1 %) of subjects were found by Abdel-Fattah et al. to exhibit extrusion in 30 and 70 patients receiving Apogee and posterior Prolift, respectively [10]. Revisit to the operating room due to mesh extrusion following SIMS was infrequent, with no subject undergoing complete mesh explant. Favorable results in this regard may be the result of employing a lighter weight mesh with a density of 25.5 g/m2. Additionally, surgeon experience and improvement upon technique may have been contributory.
Significant improvements in the PFDI and PFIQ-7 in addition to those seen in the mean PISQ-12 score are consistent with overall data (anterior, posterior, and combined mesh repairs) reported by Zyczynski et al. in the aforementioned Prosima study [19]. Data were also comparable in regard to resolution of dyspareunia (9/11 patients or 81.8 % for Prosima versus 4/6 patients or 66.7 % for SIMS) and percentage of patients with new sexual activity (12/73 patients or 16.4 % for Prosima versus 13/72 or 18.1 % for SIMS). Sentilhes et al. reported less favorable results in regard to sexual function 1 year following anterior and/or posterior repair with or without vaginal hysterectomy employing one of three different synthetic meshes [21], as no significant improvements were seen in mean PISQ-12 score. Sexual function scores were also less by PISQ-12 at 1 year (mean 15.5 ± 8.0 at baseline versus 11.7 ± 6.9 at follow-up) in 105 patients who received anterior, posterior, or combined Prolift [22]. Overall patient satisfaction in our study was high.
Study limitations include the absence of a randomized comparator and the use of an unblinded practitioner to perform postoperative anatomic assessments of stage. Additionally, the number of patients with ≥ stage II apical prolapse at baseline was disproportionately less than the number of those with posterior vaginal prolapse, so a conclusion in regard to apical effectiveness may not be as meaningful as one for the posterior compartment. Finally, as most of our patients were primary repairs, observed anatomic success following SIMS may not be fully generalized. Strengths include a prospective study design with the use of validated instruments for outcome analysis.
Although prospective comparative evidence for the use of synthetic mesh in the posterior compartment is limited, presumptive benefit includes enhanced durability for those with a deficient vaginal muscularis, for those with a large posterior enterocele that requires bridging between the rectovaginal fascia and apex, and for those with significant defecatory dysfunction in the absence of concomitant pelvic outlet obstruction. More comparative trials between a trocar-free system versus native tissue repair with evaluation of long-term anatomic durability, QOL, and change in defecatory function are needed.
The single-incision Elevate system appears to be safe and effective through 12 months, yielding good anatomic durability, low morbidity, infrequent reoperation for mesh extrusion, improved QOL, and high patient satisfaction. The device allows for the concomitant correction of posterior vaginal prolapse and/or apical descent through a single incision without the need for external trocars. Transvaginal mesh implantation should be performed following a thorough consideration of its risks and benefits.
References
Boyles SH, Weber AM, Meyn L (2003) Procedures for pelvic organ prolapse in the United States, 1979-1997. Am J Obstet Gynecol 188:108–115
Monga A (1996) Fascia—defects and repair. Curr Opin Obstet Gynecol 8:366–371
Richardson AC, Lyon JB, Williams NL (1976) A new look at pelvic relaxation. Am J Obstet Gynecol 126:568–573
DeLancey JO (1992) Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 166:1717–1724
Amid PK (1997) Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1:15–21
Cosson M, Debodinance P, Boukerrou M et al (2003) Mechanical properties of synthetic implants used in the repair of prolapse and urinary incontinence in women: which is the ideal material? Int Urogynecol J Pelvic Floor Dysfunct 14:169–178
Sung VW, Rogers RG, Schaffer JI et al (2008) Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol 112:1131–1142
Withagen MI, Milani AL, den Boon J, Vervest HA, Vierhout ME (2011) Trocar-guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstet Gynecol 117:242–250
Altman D, Falconer C, Nordic Transvaginal Mesh Group (2007) Perioperative morbidity using transvaginal mesh in pelvic organ prolapse repair. Obstet Gynecol 109:303–308
Abdel-Fattah M, Ramsay I, West of Scotland Study Group (2008) Retrospective multicentre study of the new minimally invasive mesh repair devices for pelvic organ prolapse. BJOG 115:22–30
Bump RC, Mattiasson A, Bø K et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–17
Barber MD, Kuchibhatla MN, Pieper CF, Bump RC (2001) Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol 185:1388–1395
Barber MD, Walters MD, Bump RC (2005) Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol 193:103–113
Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C (2003) A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Int Urogynecol J Pelvic Floor Dysfunct 14:164–168, discussion 168; Erratum in: Int Urogynecol J Pelvic Floor Dysfunct 2004;15:219
Barber MD, Walters MD, Cundiff GW, PESSRI Trial Group (2006) Responsiveness to the Pelvic Floor Distress Inventory (PFDI) and Pelvic Floor Impact Questionnaire (PFIQ) in women undergoing vaginal surgery and pessary treatment for pelvic organ prolapse. Am J Obstet Gynecol 194:1492–1498
Rutman MP, Deng DY, Rodriguez LV, Raz S (2005) Repair of vaginal vault prolapse and pelvic floor relaxation using polypropylene mesh. Neurourol Urodyn 24:654–658
Gauruder-Burmester A, Koutouzidou P, Rohne J, Gronewold M, Tunn R (2007) Follow-up after polypropylene mesh repair of anterior and posterior compartments in patients with recurrent prolapse. Int Urogynecol J Pelvic Floor Dysfunct 18:1059–1064
Withagen MI, Vierhout ME, Milani AL (2010) Does trocar-guided tension-free vaginal mesh (Prolift) repair provoke prolapse of the unaffected compartments? Int Urogynecol J 21:271–278
Zyczynski HM, Carey MP, Smith AR et al (2010) One-year clinical outcomes after prolapse surgery with nonanchored mesh and vaginal support device. Am J Obstet Gynecol 203:587, e1–e8
de Tayrac R, Devoldere G, Renaudie J, Villard P, Guilbaud O, Eglin G, French Ugytex Study Group (2007) Prolapse repair by vaginal route using a new protected low-weight polypropylene mesh: 1-year functional and anatomical outcome in a prospective multicentre study. Int Urogynecol J Pelvic Floor Dysfunct 18:251–256
Sentilhes L, Berthier A, Sergent F, Verspyck E, Descamps P, Marpeau L (2008) Sexual function in women before and after transvaginal mesh repair for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 19:763–772
Altman D, Elmér C, Kiilholma P, Kinne I, Tegerstedt G, Falconer C, Nordic Transvaginal Mesh Group (2009) Sexual dysfunction after trocar-guided transvaginal mesh repair of pelvic organ prolapse. Obstet Gynecol 113:127–133
Acknowledgements
The authors would like to thank the other investigators who contributed subjects to this study including: Roger D. Beyer, Kalamazoo, MI; Seth Herbst and Moises Virelles, Wellington, FL; Christophe Courtieu, France; Christopher Mayne, UK; John Nguyen, Downey, CA; James Flaherty, Portland, ME; Philip Hoekstra, Grand Rapids, MI; Rainer Lange, Germany; and Bernhard Liedl, Germany.
Conflicts of interest
This study was funded by American Medical Systems, Minnetonka, MN, USA.
Dr. Lukban has served as consultant, facilitator, and speaker for AMS; consultant and speaker for Novasys; and consultant for Coloplast. Dr. Roovers has served as consultant for AMS. Dr. VanDrie has served as consultant and speaker for AMS, CR Bard, Coloplast, Ethicon, Johnson & Johnson, and Kimberly Clark. Dr. Erickson has served as consultant and speaker for AMS, Coloplast, and Ethicon. Dr. Zylstra has served as consultant for AMS. Dr. Patel has served as consultant and speaker for Coloplast and Medtronic. Dr. Moore has served as consultant and speaker for AMS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lukban, J.C., Roovers, JP.W.R., VanDrie, D.M. et al. Single-incision apical and posterior mesh repair: 1-year prospective outcomes. Int Urogynecol J 23, 1413–1419 (2012). https://doi.org/10.1007/s00192-012-1692-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1692-4