Abstract
Purpose
Little is known about the arthroscopic or radiographic outcomes after arthroscopic microfracture of osteochondral lesions of the talus (OLTs). The purpose of this study was to investigate tissue growth after arthroscopic microfracture of OLTs using computed tomography arthrography (CTA) and to identify the relationship between CTA findings and clinical outcomes. We hypothesized that the morphology of the repaired tissue would be similar to that of normal anatomy and correlate with the clinical outcomes.
Methods
Forty-two ankles treated using arthroscopic microfracture of OLTs between 2009 and 2014 were monitored. CTA was performed post-operatively at 6 months and at 1 and 2 years after surgery. The post-operative thickness of the repaired tissue associated with OLT (grade) and the volume of the subchondral cystic lesions were evaluated using CTA. Clinical outcomes, including the pain visual analog scale (VAS) and American Orthopaedic Foot and Ankle Society (AOFAS) ankle functional scores, were evaluated and correlated with CTA.
Results
The proportion of fully grown tissue (grade 3) increased over time; specifically, the rates were 12/40 (33.3%) at 6 months, 11/18 (61.1%) at 1 year, and 8/10 (80%) at 2 years after surgery (p = 0.005). The VAS pain (p < 0.001) and AOFAS scores (p < 0.001) were also improved at the final follow-up; however, they were not associated with repaired tissue thickness as shown by CTA (n.s.).
Conclusions
After microfracture of OLTs, tissue growth in the osteochondral defects was well visualized using CT arthrography and was observed in most cases. However, the CTA findings were not related to the clinical outcomes.
Level of evidence
IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteochondral lesions of the talus (OLTs) are subchondral and cartilage lesions that cause recurrent ankle pain, swelling, and functional limitations. Increasingly, OLTs have been detected using magnetic resonance imaging (MRI), and various techniques are available to address these lesions [1, 9, 10]. One technique is arthroscopic microfracture, which has gained popularity as the first-line treatment for OLTs [18, 23, 25].
Although the potential clinical outcome of tissue growth after arthroscopic microfracture of OLTs has been a major topic of interest, few studies have reported this outcome in conjunction with radiographic imaging evaluations or second-look arthroscopy after arthroscopic microfracture [17, 29]. Performing second-look arthroscopy for uncomplicated cases merely to assess arthroscopic microfracture outcomes might be unethical because it is an invasive procedure [14]. MRI is also not a favourable imaging modality because it is expensive and time-consuming; furthermore, it cannot precisely demarcate the exact morphology of the articular surface for serial OLT follow-up assessments as well as the subchondral bony consolidation. Nevertheless, many previous imaging follow-up reports regarding microfracture of OLTs have used MRI [8, 13, 17, 19, 29].
To the best of our knowledge, no OLT studies have used computed tomography arthrography (CTA) to radiographically evaluate arthroscopic microfracture treatments. Therefore, this study sought to verify the tissue growth of OLTs after arthroscopic microfracture using CTA and to evaluate the correlations with clinical outcomes. The hypothesis of this study was that CTA could be used to evaluate not only the tissue growth but also the joint congruency and subchondral changes after arthroscopic microfracture of OLTs and that the clinical outcomes would be proportionally related to the degree of tissue growth revealed by CTA.
Materials and methods
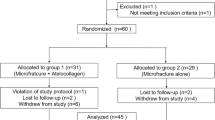
A single senior author treated 60 OLT patients (61 ankles) at Konkuk Medical Center using arthroscopic microfracture from 2009 to 2014. OLTs were confirmed using preoperative MRI and ankle arthroscopy. The inclusion criteria were patients with a diagnosis of OLT whose pain remained unresponsive to conservative management, including rest, splints, such medications as non-steroidal anti-inflammatory drugs, and physical therapy, for at least 6 months. Patients were included only if they agreed to undergo CTA at 6-month, 1-year, and 2-year follow-ups. Any psychotic disorders, diabetes, or immune diseases, e.g. rheumatoid arthritis, a previous history of ankle surgery, or a follow-up less than 6 months after surgery, were excluded. All the patients were instructed to undergo CTA post-operatively and to complete a survey during the follow-up period; a total of 42 patients (42 ankles) were enrolled.
A total of 26 men and 16 women with a mean age of 39.8 ± 15.7 years and a mean body mass index (BMI) of 25.6 ± 3.8 kg/m2 were included in the sample at the time of surgery. Twenty-one of the ankles of interest were on the right side. Thirty patients (71.4%) had evident previous trauma. All patients complained of more than one symptom, including chronic ankle pain, swelling, functional limitations, or weakness. The median duration of preoperative ankle pain was 20.0 months (range 6–120 months), and the median follow-up period was 13.0 months (range 6–54 months).
Nine patients had ten concomitant diseases at the time of surgery, including three cases of lateral ankle instability, three cases of sinus tarsi syndrome, one case of painful accessory navicular, one case of ankle soft tissue impingement, and two cases of tibial osteophytes. To eliminate confounding effects, these nine patients were excluded from the analysis of the relationships between the CTA findings and the clinical outcomes.
Post-operative ankle CTA was performed on 40, 18, and 10 ankles at 6 months, 1 year, and 2 years after the surgery, respectively. Clinical surveys were completed preoperatively and then post-operatively at 6 months, 1 year, and each year thereafter.
Operative technique and post-operative rehabilitation
All operative procedures were performed by a single senior surgeon. After cartilage flap or osteochondral fragment removal, the subchondral crater was debrided with a shaver and curette. Multiple microfractures 3–4 mm deep were made perpendicular to the subchondral bone with approximately 2–4 mm between the holes. After the input saline was turned off, bleeding and marrow fat droplet leakage from the subchondral microfracture holes were confirmed. A short-leg splint and a partial weight-bearing state were maintained for 2 weeks post-operatively, after which weight-bearing and range-of-motion ankle exercises were allowed as tolerated.
Clinical evaluation
Demographic variables known as “possible prognostic factors” were collected and co-analysed. These variables included sex, age, BMI, symptom duration, history of trauma, and the presence of osteophytes [3, 11]. Functional outcomes were evaluated using scores on the American Orthopaedic Foot and Ankle Society (AOFAS) Ankle-Hindfoot Scale [15]. Subjective pain was represented using a 10-point visual analog scale (VAS), by which 10 points indicated the maximum pain imaginable.
Radiological evaluation
To avoid potential bias, two musculoskeletal radiologists with 10 years of experience who were uninvolved with the treatment of patients and blinded to the purpose of this study assessed all the radiographs, including those from MRI and CTA. All measurements were taken twice by two observers with a 2-week interval and included one decimal value. As a result, the intra- and interobserver reliability was indicated by kappa values of 0.85 and 0.81, respectively. The intra- and interobserver reliability of the region-of-interest (ROI) measurements was indicated by intra- and interclass correlation values of 0.78 and 0.72, respectively.
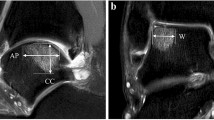
Preoperatively, all lesions were classified using MRI, as described by Hepple et al. [12]. Regarding the OLT size and the volume of the subchondral lesions, coronal width (W) was measured via the maximum extension within the coronal images. The anteroposterior length (AP) of the OLT and the depth of the subchondral lesions (D) were measured within the sagittal images. Then, the area of the OLT was calculated using the ellipse formula (W × AP × 0.79), and the volume of the subchondral lesions was determined using the standard volume of an ellipsoid (W × AP × D × 4/3π) [3, 29].
At the follow-up assessment, the post-operative OLT was assessed using CTA. The thickness of the repaired tissue was compared with the thickness of adjacent normal talar dome cartilage and classified into the following four grades: (0) no growth; (1) growth less than 50% of the adjacent normal cartilage thickness; (2) more than 50% of the adjacent normal cartilage thickness; and (3) similar to the thickness of adjacent normal talar dome cartilage (Table 1) [21, 32].
Changes in the OLT area and the subchondral cystic lesion volume were also calculated using the methods described above.
For the quantitative analysis of the degree of bone consolidation, the mean Hounsfield unit (HU) was obtained for the subchondral lesion of the OLT on one representative coronal or sagittal reconstruction CTA image [27]. The HU value was measured using manually drawn regions of interest (ROIs) on the CTA image [20].
CTA imaging protocol
A 22-gauge needle was inserted into the anterolateral joint space. The 20 ml of injection material consisted of 12 ml of non-ionic iodine contrast (Telebrix 30 Meglumine, Guerbet, Aulnay-sous-Bois, France) mixed with 8 ml of normal saline. The average total injected volume was 5 ml. After injection, all patients were immediately scanned using a 64-row multi-detector CT scanner (LightSpeed VCT XT; GE Healthcare, Milwaukee, WI, USA). The institutional review board at our hospital approved this study (Konkuk Medical Center, KUH1060110).
Statistical analyses
Correlation analyses were performed to correlate the AOFAS functional score and the VAS pain score with the thickness and repaired area of each affected lesion. Improvements in the AOFAS functional score and the VAS pain score, which followed a normal distribution, were assessed using a paired-sample T test. Radiographic changes at 6 months and 1 year after surgery were validated using the Wilcoxon test. Univariate analyses were performed using Pearson’s or Spearman’s correlations. For the analysis of the relationship between the thickness of repaired tissue and clinical scores, repaired tissue thickness grades of 0 and 1 were coded as “0”, whereas 2 and 3 were coded as “1”. For this correlative analysis, the clinical score at the final follow-up of each patient was used. Thus, the data from all 42 patients were analysed for correlations between tissue thickness determined by CTA and clinical outcomes. To detect the factors that affect tissue growth, a logistic regression analysis was performed on the same assumption. Patient sex, age, symptom duration, BMI, and trauma history were used as independent factors in this analysis; p values less than 0.05 were considered significant in all situations. To estimate the required sample size, the VAS pain score was used as the primary outcome and an attempt was made to distinguish differences of 0.5 in patients prior to surgery and at the final follow-up with a standard deviation of 0.5 points and an effect size of 1. Based on these parameters, the power analysis indicated that a sample size of 34 would provide 80% power to detect an effect of this magnitude (α = 0.05, β = 0.2).
Results
Clinical outcomes
Overall, the mean VAS pain score improved from 7.2 ± 1.6 preoperatively to 2.0 ± 1.8 at the final follow-up assessment (p < 0.001). The mean AOFAS score significantly increased from 70.9 ± 9.7 preoperatively to 88.4 ± 4.7 at the final follow-up assessment (p < 0.001). Although one patient was recommended for osteochondral autograft transfer due to persistent ankle pain, none of the patients underwent surgical revision after primary arthroscopic microfracture.
Radiographic outcomes
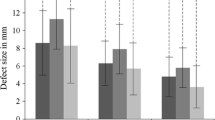
Thirty-six patients had a medial lesion, four had a lateral lesion, and two had both medial and lateral lesions. According to the preoperative MRI data, stage IIA was the most common (24 ankles 57.1%), followed by stage III (8 ankles 19%). The median OLT areas are listed in Table 2.
The proportion of repaired tissue of OLT with grade 3 increased over time (Table 2). At the final follow-up of each ankle, tissue growth of grades 1, 2, and 3 was observed in 10, 9, and 21 ankles, respectively. As a result, defect filling was observed in 40 ankles (95.2%).
Tissue growth and subchondral bony consolidation increased over time in most cases (Figs. 1, 2). Hypertrophic tissue and poor tissue growth were also observed. Uncontained shoulder lesions either restored the previous shape of the talus with cartilaginous signal or showed in situ tissue growth (Fig. 3). One lesion even achieved congruency of the joint by forming tissue growth at the confronting plafond surface (Fig. 3c). These lesions were classified as given in Table 3. A debrided OLT before microfracture is shown in Fig. 4.
Arthroscopic image showing the defect after debridement for microfracture. This image and the CTA images in Fig. 1 were obtained from the same patient
The ROIs and tissue growth were significantly improved at the 1-year follow-up compared with those at six months (p = 0.001 and 0.007, respectively); however, the overall size of the subchondral cystic lesions did not decrease [not significant (n.s.)].
Relationship between clinical outcomes and CTA findings
Neither VAS pain nor AOFAS scores were associated with repaired tissue thickness (n.s.). Patients with trauma histories had an approximately fivefold greater chance of tissue growth than did patients without such histories (odds ratio = 5.133, p = 0.028). Most patients with an uncontained shoulder lesion showed a positive clinical outcome at post-operative 2 years. However, four patients with large buttress defects showed unfavourable outcomes regardless of the morphology of the repaired tissue, and one of these patients required an autogenous bone graft.
Discussion
The most important finding of the present study was that CTA was useful for monitoring tissue growth and subchondral lesion remodelling, which showed progression for up to 2 years after surgery, albeit in the small group of patients who completed the follow-up examinations. Subchondral consolidation partially improved, but overall cyst size did not decrease within the study period. Patient trauma history affected whether the defect could be filled after microfracture. However, the presence or thickness of grown tissue determined by CTA was not associated with short-term clinical outcomes. OLTs without a lateral pillar led to either in situ tissue growth or moulded reformation with connective tissue. Patients with a large lateral pillar defect had unfavourable clinical outcomes.
Since CTA was first used to detect cartilage defects, such as chondromalacia patellae, in the early 1980s [24], many cartilage imaging studies have used CTA to investigate other joints [2, 21, 22, 30, 32]. Since the introduction of multi-detector technology, CTA has been widely used for cartilage imaging to acquire isotropic data, which allows for multi-planar reconstructions and thin sectioning [4, 6, 16, 26]. The interface between the surface of the cartilage and the joint space appears as a sharp line on CTA [32]. Thus, CTA might be the most appropriate modality for evaluating ankle joint cartilage because its articular cartilage is relatively thin compared with that of other joints. Nevertheless, the performance of CTA for the ankle joint remains poorly understood, other than for soft tissue impingement or post-traumatic arthritis [4,5,6, 16, 26, 28]. To the best of our knowledge, this comprehensive study is the first to post-operatively evaluate OLT progression after arthroscopic microfracture using CTA.
Pain does not originate from the cartilage lesion itself but from the stimulation of the highly innervated subchondral bone [7, 31]. From that perspective, an evaluation of the surface or thickness of cartilage that covers the subchondral bone might be valuable and favour CTA over MRI when monitoring OLTs after arthroscopic microfracture. Moreover, bone assessment using CTA is superior to that using MRI because it is advantageous for evaluating subchondral bony changes after microfracture. Although minor complications, such as transient ankle pain or a tingling sensation, may occur after intra-articular dye injection, CTA still has other advantages over MRI, including a brief examination time, high spatial resolution, multi-planar capability, low sensitivity, and limited number of imaging artefacts.
Recently, researchers correlated the morphologies on MRI with clinical outcomes after microfracture for OLTs [29]. These authors suggested that the volume of bone marrow oedema was associated with clinical symptoms. However, bone consolidation, which is well defined by CTA, can be a more useful marker for long-term monitoring than bone marrow oedema. CTA findings have not been reported to be associated with clinical outcomes after the surgical fixation of malleolar fractures, which is similar to our results [16]. Once the osteochondral defect is covered with tissue that forms after microfracture, the subchondral bone is likely protected from painful stimuli regardless of the overlying tissue thickness. We believe that this could explain our finding that the thickness of newly grown tissue did not correlate with clinical outcomes. Therefore, the clinical relationship between CTA and OLTs after microfracture requires more long-term follow-up investigation with larger data mass and the analysis of other factors in addition to the thickness of grown tissue, such as joint congruency, to confirm our results.
Uncontained shoulder lesions likely result in unfavourable outcomes; however, few morphological studies have been conducted. In our study, most uncontained shoulder lesions were restored to their previous shape, and these patients showed favourable outcomes at short-term follow-up assessments. However, poor clinical outcomes, regardless of morphology without moulding into the previous shape or in situ tissue growth, were found among patients who lacked a considerable lateral/medial buttress. This finding suggests that clinical outcomes are affected not only by the congruency or tissue growth but also by the mechanical stability of the buttress structures.
Our study had several limitations. First, biopsy and microscopic examinations were not performed; thus, we cannot conclude whether the repaired lesions were true fibro- or hyaline cartilage. However, several techniques, such as the T2 mapping used in previous studies, are also limited regarding their cartilage evaluation and clinical use during follow-up examinations. Second, the preoperative and post-operative cartilage states could not be compared because the preoperative evaluation was performed using MRI and not CTA. Finally, the follow-up period using CTA was relatively brief, and there was a loss of data; thus, long-term follow-up assessments with CTA might reveal long-term outcomes, such as treatment survival after arthroscopic microfracture of OLTs, in future studies. Nevertheless, we believe that the current study of post-operative CTA for OLTs demonstrates the viability of this non-invasive imaging modality for visualizing the tissue growth in the osteochondral defect after arthroscopic microfracture and can thus be used to replace post-operative MRI or second-look arthroscopy.
Conclusions
Tissue growth after arthroscopic microfracture of OLTs was detected in 96% of patients at each final follow-up examination using ankle CTA. CTA is a viable option for the follow-up evaluation of OLTs after microfracture regarding articular tissue growth and subchondral bone consolidation. However, the thickness of tissue as determined by CTA was not associated with short-term clinical outcomes.
References
Badekas T, Takvorian M, Souras N (2013) Treatment principles for osteochondral lesions in foot and ankle. Int Orthop 37:1697–1706
Choi BH, Kim NR, Moon SG, Park JY, Choi JW (2015) Superior labral cleft after superior labral anterior-to-posterior tear repair: CT arthrographic features and correlation with clinical outcome. Radiology 278:441–448
Choi WJ, Park KK, Kim BS, Lee JW (2009) Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med 37:1974–1980
Cochet H, Pele E, Amoretti N, Brunot S, Lafenetre O, Hauger O (2010) Anterolateral ankle impingement: diagnostic performance of MDCT arthrography and sonography. AJR Am J Roentgenol 194:1575–1580
De Filippo M, Bertellini A, Pogliacomi F, Sverzellati N, Corradi D, Garlaschi G, Zompatori M (2009) Multidetector computed tomography arthrography of the knee: diagnostic accuracy and indications. Eur J Radiol 70:342–351
De Filippo M, Pogliacomi F, Bertellini A, Araoz PA, Averna R, Sverzellati N, Ingegnoli A, Corradi M, Costantino C, Zompatori M (2010) MDCT arthrography of the wrist: diagnostic accuracy and indications. Eur J Radiol 74:221–225
Elias I, Jung JW, Raikin SM, Schweitzer MW, Carrino JA, Morrison WB (2006) Osteochondral lesions of the talus: change in MRI findings over time in talar lesions without operative intervention and implications for staging systems. Foot Ankle Int 27:157–166
Giannini S, Battaglia M, Buda R, Cavallo M, Ruffilli A, Vannini F (2009) Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med 37:112S–118S
Giannini S, Buda R, Ruffilli A, Cavallo M, Pagliazzi G, Bulzamini MC, Desando G, Luciani D, Vannini F (2014) Arthroscopic autologous chondrocyte implantation in the ankle joint. Knee Surg Sports Traumatol Arthrosc 22:1311–1319
Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G (2006) Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy 22:1085–1092
Hannon CP, Smyth NA, Murawski CD, Savage-Elliott I, Deyer TW, Calder JD, Kennedy JG (2014) Osteochondral lesions of the talus: aspects of current management. Bone Joint J 96:164–171
Hepple S, Winson IG, Glew D (1999) Osteochondral lesions of the talus: a revised classification. Foot Ankle Int 20:789–793
Higashiyama I, Kumai T, Takakura Y, Tamail S (2000) Follow-up study of MRI for osteochondral lesion of the talus. Foot Ankle Int 21:127–133
Kim YS, Park EH, Koh YG, Lee JW (2014) Supramalleolar osteotomy with bone marrow stimulation for varus ankle osteoarthritis: clinical results and second-look arthroscopic evaluation. Am J Sports Med 42:1558–1566
Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M (1994) Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int 15:349–353
Kraniotis P, Maragkos S, Tyllianakis M, Petsas T, Karantanas AH (2012) Ankle post-traumatic osteoarthritis: a CT arthrography study in patients with bi-and trimalleolar fractures. Skeletal Radiol 41:803–809
Kuni B, Schmitt H, Chloridis D, Ludwig K (2012) Clinical and MRI results after microfracture of osteochondral lesions of the talus. Arch Orthop Trauma Surg 132:1765–1771
Lee KB, Bai LB, Chung JY, Seon JK (2010) Arthroscopic microfracture for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc 18:247–253
Mintz DN, Tashjian GS, Connell DA, Deland JT, O’Malley M, Potter HG (2003) Osteochondral lesions of the talus: a new magnetic resonance grading system with arthroscopic correlation. Arthroscopy 19:353–359
Nakaura T, Nakamura S, Maruyama N, Funama Y, Awai K, Harada K, Uemura S, Yamashita Y (2012) Low contrast agent and radiation dose protocol for hepatic dynamic CT of thin adults at 256-detector row CT: effect of low tube voltage and hybrid iterative reconstruction algorithm on image quality. Radiology 264:445–454
Nishii T, Tanaka H, Nakanishi K, Sugano N, Miki H, Yoshikawa H (2005) Fat-suppressed 3D spoiled gradient-echo MRI and MDCT arthrography of articular cartilage in patients with hip dysplasia. Am J Roentgenol 185:379–385
Omoumi P, Michoux N, Thienpont E, Roemer FW, Vande Berg BC (2015) Anatomical distribution of areas of preserved cartilage in advanced femorotibial osteoarthritis using CT arthrography (part 1). Osteoarthritis Cartilage 23:83–87
Park HW, Lee KB (2015) Comparison of chondral versus osteochondral lesions of the talus after arthroscopic microfracture. Knee Surg Sports Traumatol Arthrosc 23:860–867
Reiser M, Karpf PM, Bernett P (1982) Diagnosis of chondromalacia patellae using CT arthrography. Eur J Radiol 2:181–186
Ross KA, Hannon CP, Deyer TW, Smyth NA, Hogan M, Do HT, Kennedy JG (2014) Functional and MRI outcomes after arthroscopic microfracture for treatment of osteochondral lesions of the distal tibial plafond. J Bone Joint Surg Am 96:1708–1715
Schmid MR, Pfirrmann CW, Hodler J, Vienne P, Zanetti M (2003) Cartilage lesions in the ankle joint: comparison of MR arthrography and CT arthrography. Skeletal Radiol 32:259–265
Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG (2011) Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am 93:1057–1063
Tan TC, Wilcox DM, Frank L, Shih C, Trudell DJ, Sartoris DJ, Resnick D (1996) MR imaging of articular cartilage in the ankle: comparison of available imaging sequences and methods of measurement in cadavers. Skeletal Radiol 25:749–755
Tao H, Shang X, Lu R, Li H, Hua Y, Feng X, Chen S (2014) Quantitative magnetic resonance imaging (MRI) evaluation of cartilage repair after microfracture (MF) treatment for adult unstable osteochondritis dissecans (OCD) in the ankle: correlations with clinical outcome. Eur Radiol 24:1758–1767
Thienpont E, Schwab PE, Omoumi P (2014) Wear patterns in anteromedial osteoarthritis of the knee evaluated with CT-arthrography. Knee 21:S15–S19
van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ (2010) Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc 18:570–580
Vande Berg BC, Lecouvet FE, Poilvache P, Jamart J, Materne R, Lengele B, Maldague B, Malghem J (2002) Assessment of knee cartilage in cadavers with dual-detector spiral CT arthrography and MR imaging. Radiology 222:430–436
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Funding
The project was supported by Konkuk University.
Ethical approval
This study was approved by institutional review board at Konkuk Medical Center (KUH1060110).
Informed consent
Patients gave written informed consent.
Rights and permissions
About this article
Cite this article
Jung, HG., Kim, NR., Jeon, JY. et al. CT arthrography visualizes tissue growth of osteochondral defects of the talus after microfracture. Knee Surg Sports Traumatol Arthrosc 26, 2123–2130 (2018). https://doi.org/10.1007/s00167-017-4610-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-017-4610-y