Abstract
Purpose
To study the clinical effect and safety of two doses of low-dose perioperative dexamethasone on pain and recovery after total knee arthroplasty.
Methods A total of 108 patients were included in this randomized, double-blinded, placebo-controlled study. They received two doses of 10 mg IV dexamethasone (group Dexa) or IV isotonic saline (group Placebo). The CRP, IL-6 and pain levels, postoperative nausea and vomiting (PONV) incidence, nausea severity, postoperative fatigue, range of motion, length of stay, analgesic rescue and antiemetic rescue consumption, and complications were compared.
Results The CRP and IL-6 levels in group Dexa were lower than in group Placebo at 24, 48, and 72 h postoperatively (P < 0.001, P < 0.001, and P < 0.001, respectively). In group Dexa, patients had less pain at 24 h postoperatively, at rest (P < 0.001) and during walking (P < 0.001); they also had a lower PONV incidence (P = 0.002) and a lower nausea VAS score (P = 0.008). Postoperative fatigue (P < 0.001) was relieved and the analgesic and antiemetic rescue consumption was reduced. Length of stay (n.s.) and range of motion (n.s.) were similar in both groups. No early surgical wound infection or gastrointestinal haemorrhage occurred in either group.
Conclusions Administering two doses of low-dose perioperative dexamethasone for patients receiving total knee arthroplasty reduces postoperative CRP and IL-6 levels, provides additional analgesic effect, and reduces the PONV incidence and postoperative fatigue, without increasing the risk of early surgical wound infection and gastrointestinal haemorrhage. So two doses of low-dose perioperative dexamethasone are effective and safe for patients receiving TKA to decrease the inflammatory response, prevent PONV, relieve postoperative pain and fatigue, and enhance recovery.
Level of evidence I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enhanced recovery after surgery, put forward by Kehlet [26], has been introduced as a series of evidence-based interventions to enhance postoperative recovery; reduce perioperative stress response, length of stay (LOS) and complications; and increase satisfaction and safety after discharge [10, 14]. The surgical stress response includes inflammatory components after total knee arthroplasty (TKA) and is associated with postoperative nausea and vomiting (PONV) [25], pain [13, 27], fatigue [20], and decreased muscle strength [2, 26]. Thus, decreasing the inflammatory response after surgery is important for preventing PONV, relieving postoperative pain and fatigue, and enhancing recovery, which are the main components in Enhanced recovery after surgery for TKA.
Glucocorticoids, with its potent anti-inflammatory effects, have been widely used in various perioperative settings [6, 8, 9, 23], including TKA, for decreasing inflammatory markers, preventing PONV, and relieving postoperative pain and fatigue [7, 12, 15, 18, 19]. However, heterogeneity among studies regarding type, dosage and administration protocol of glucocorticoids, and the perioperative management of TKA makes it difficult to judge the practical value of glucocorticoids. In addition, in most previous studies, glucocorticoids were given as a single dose and at a low dose, and the number of patients studied was less than 25 patients per group [7, 12, 15, 18, 19]. Moreover, concerns regarding an increased risk of infection and gastrointestinal haemorrhage have hampered the widespread use of glucocorticoids during the perioperative period of TKA. Thus, prospective studies with sufficient power are required to provide additional evidence to determine the benefit and safety of glucocorticoids. In most studies, the administration protocol was a preoperative single dose of glucocorticoid. However, patients still have to deal with PONV, pain, and fatigue. In addition, higher doses of glucocorticoids may be associated with a higher risk of complications.
This prospective, randomized, controlled trial was conducted to evaluate the clinical effect of two doses of 10 mg IV dexamethasone (Dexa) during the perioperative period of TKA, which is expected to reveal (1) whether dexamethasone reduces the level of postoperative inflammatory markers; (2) whether the combined use of dexamethasone and mosapride further reduces PONV compared with mosapride alone; (3) whether the two doses of dexamethasone provide additional analgesic effect; (4) whether dexamethasone reduces postoperative fatigue; (5) whether dexamethasone improves the range of motion (ROM) after TKA; and (6) whether dexamethasone increases the risk of early surgical wound infection and gastrointestinal haemorrhage.
Materials and methods
The study protocol was approved by the Institutional Review Board of West China Medical Center of Sichuan University (No. 201302007) before patient enrolment, and the study was registered in the International Clinical Trial Registry (ChiCTR-IOR-16008865). Written informed consent and research authorization were obtained from all patients. From April to September 2016, all patients receiving elective, unilateral, primary TKA for end-stage osteoarthritis were consecutively screened for inclusion in the study. Exclusion criteria were alcohol or medical abuse, allergies to Dexa, age ≤ 18 years or ≥ 80 years, administration of any glucocorticoids during the past 3 months before surgery; administration of any strong opioids during the past 7 days; and history of severe heart disease (New York Heart Association [NYHA] Functional Classification > 2), liver or renal failure, and connective tissue diseases (rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus).
The recruited patients in this double-blinded study were randomly assigned to two groups: group Dexa and group Placebo. A random allocation sequence concealed in opaque-sealed envelopes was opened only before surgery. Patients in group Dexa (n = 54) received two doses of 10 mg IV dexamethasone (2 ml, Tianjin Kingyork group Co., Ltd., China), and patients in group Placebo (n = 54) received two doses of IV isotonic saline (2 ml). The first dose was administered just after the general anaesthesia was performed by an anaesthesiologist, and the second dose was administered by a nurse just after the patients returned to the inpatient unit. The anaesthesiologist and nurse were not involved in this trial, and the patients, surgeons, data controller and analyst were blinded.
Surgical procedure
All the operations were performed by one senior surgeon in the same laminar air flow operation room. All the operations were performed under general anaesthesia. We carried out operations using a midline skin incision, medial parapatellar approach. The patella was routinely everted during the operation with patella resurfacing. An intramedullary alignment system was used for femoral preparation, and an extramedullary alignment system was used for tibial preparation. All the total knee prostheses were posterior stabilized, cemented implants, without use of a tourniquet or drainage catheter. Before skin incision, a dose of 20 mg/kg IV tranexamic acid (TXA, Chongqing Lummy Pharmaceutical Co., Ltd. China) was administered. Before the incision was closed, an intra-articular injection of 1 g TXA was administered, and a peri-articular injection of 0.2% ropivacaine (100 ml) was administered. No patients received any nerve block and/or intravenous patient-controlled analgesia.
Postoperative care protocol
After the surgery, patients were transferred to the post-anaesthesia care unit for 2 h, and then returned to the inpatient unit. A cold pack was used on the surgical site immediately after the patient returned to the inpatient unit and applied for 12 h. Daily function training, including quadriceps strength training, active ROM training and walking training were carried out under the supervision and assistance of a physiotherapist.
Management of pain and PONV for all patients was the same. One day before the surgery, multimodal oral analgesic drugs (50 mg q12h diclofenac, 75 mg q8h pregabalin) were administered for preemptive analgesia. After the surgery, this analgesic management was restarted when patients resumed oral intake. Oral oxycodone (10 mg q8h) would be added when a patient reported pain greater than 4 on a 0–10 VAS. An intramuscular injection of parecoxib (40 mg) was given if a patient reported severe pain greater than 6. Oral mosapride (5 mg tid, before each meal) was started 1 day before the surgery. An intramuscular injection of metoclopramide (10 mg) was used as the first-line rescue treatment when patients had two or more times of PONV or had severe nausea (VAS > 4). An intramuscular injection of ondansetron (5 mg) was used as the second-line rescue treatment when severe nausea or vomiting persisted after two boluses of metoclopramide were administered in a 30-minute interval.
A combination of mechanical and chemical thromboprophylaxis was used to prevent venous thromboembolism. As mechanical prophylaxis, an intermittent foot slope pump system was used before walking. As chemical prophylaxis, a half-dose of low-molecular-weight heparin (LMWH; 2000 IU in 0.2 ml; Clexane, Sanofi-Aventis, France) was subcutaneously administered at 6 h postoperatively, and repeated at 24-hour intervals with a full dose (4000 IU in 0.4 ml). As prophylaxis after discharge, patients were instructed to take 10 mg of rivaroxaban (Xarelto, Bayer, Germany) orally once a day for 10–14 days if no bleeding events occurred.
Outcome measurements
Before the operation, demographic data, medical histories and concomitant medications were recorded. Inflammation markers (C-reactive protein [CRP], interleukin 6 [IL-6]) were tested pre-operatively and at 24, 48, and 72 h after the operation. Pain level and amount of analgesic rescue drugs (oxycodone and parecoxib) were recorded to evaluate the analgesic effect. Pain level was assessed using a visual analogue scale (VAS, 0 means no pain, 10 means most severe pain imaginable) and was performed pre-operatively and at 24, 48, and 72 h after the operation, both at rest (patients rested in bed for at least 30 min before the test) and walking training (patients walked 20 steps before the test). The incidence of PONV and the consumption of antiemetic rescue drugs (metoclopramide and ondansetron) were recorded postoperatively. The severity of nausea was assessed using VAS (0 means on nausea, 10 means severe nausea imaginable) in the first 6 h postoperatively. Fatigue was assessed using Identity-Consequence-Fatigue-Scale (ICFS) before surgery and at the third postoperative day (POD3). ROM was assessed pre-operatively and at POD3. The LOS and complications (early surgical wound infection, gastrointestinal haemorrhage) were carefully recorded. Two investigators independently measured and recorded pain level (VAS score), the severity of nausea (VAS score), ICFS score and ROM. The mean was calculated as the final result.
Statistical analysis
According to the results of previous studies [15, 18], we anticipated an average decrease in VAS (pain) score of 0.8 in group Dexa. With a desired power of 0.90 and the significant level of 0.05, a power analysis was performed (http://www.danielsoper.com/statcalc/calculator.aspx?id=47). It was estimated that a goal sample size of 41 patients per arm were need. If a 10% exclusion rate was expected, the minimum sample size was 46 in each group. We decided to include 60 patients in each group.
All data analyses were performed by SPSS version 22 (SPSS Inc. USA). Student’s t-test or Wilcoxon–Mann–Whitney U test was used to analyse quantitative data and Pearson Chi-squared test or Fisher’s exact test was used to analyse qualitative comparative data. Statistical significance was defined as P < 0.05.
Results
Patient demographics
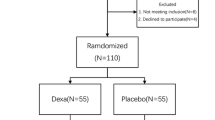
From April 2016 to September 2016, 120 patients were scheduled to take an elective, unilateral, primary TKA for end-stage osteoarthritis in our institution. Among these patients, 7 were ineligible and 5 declined to participate. Thus, 108 patients were included in the final study and analysis, and 54 were randomized into group Dexa and 54 were randomized into group Placebo (Fig. 1). We found no differences in demographic data between the groups (Table 1).
Inflammation markers
As acute inflammation markers, CRP and IL-6 increased postoperatively in all patients. The mean level of CRP peaked 48 h after the operation in both groups, and the level of CRP in group Dexa was lower than group Placebo at 24, 48, and 72 h after the operation (P < 0.001, P < 0.001, P < 0.001, respectively). The mean level of IL-6 in group Placebo peaked 24 h after the operation, and it peaked 48 h after the operation in group Dexa. The level of IL-6 in group Dexa was lower than group Placebo at 24, 48, and 72 h after the operation (P < 0.001, P < 0.001, P < 0.001, respectively) (Fig. 2).
Pain and analgesic rescue
Compared with the level of pain pre-operatively, pain declined after TKA. Patients in group Dexa had less pain than group Placebo at 24 h after the operation, both at rest (1.3 ± 0.9 vs. 2.0 ± 0.7, P < 0.001) and during walking (2.0 ± 0.7 vs. 2.5 ± 0.6, P < 0.001). The pain in both groups was similar at 48 or 72 h after the operation (n.s.) (Fig. 3).
Compared with group Placebo, the number of patients requiring oxycodone in group Dexa was smaller (P = 0.004), and the overall oxycodone consumption was less (P = 0.001). The number of patients requiring parecoxib was similar between the groups (n.s.). However, the overall parecoxib consumption in group Dexa was less (P = 0.042) (Table 2).
PONV and antiemetic rescue
Compared with group Placebo, the incidence of PONV (P = 0.002) was lower in group Dexa, and the VAS score (nausea) was lower (0.7 ± 0.9 vs. 1.4 ± 1.5, P = 0.008) (Table 3). The number of patients requiring metoclopramide was smaller in group Dexa (P = 0.018), and the overall metoclopramide consumption was less (P = 0.008). The number of patients requiring ondansetron (n.s.) and the overall ondansetron consumption (n.s.) were similar between the groups (Table 2).
Fatigue, knee ROM, LOS, and complications
In group Dexa, the postoperative ICFS score was lower than group Placebo (74.2 ± 9.9 vs. 85.1 ± 7.5); the difference was statistically significant (P < 0.001), including feelings of fatigue (16.0 ± 5.0 vs. 18.9 ± 3.9, P = 0.001), feelings of vigour (18.3 ± 3.5 vs. 20.4 ± 3.2, P = 0.002), impact on concentration (20.4 ± 3.2 vs. 23.6 ± 2.9, P < 0.001), and impact on energy (19.6 ± 3.1 vs. 22.4 ± 2.5, P < 0.001). At POD3, ROM (n.s.) was similar between the two groups. LOS (n.s.) was also similar between the two groups. No patient had early surgical wound infection or gastrointestinal haemorrhage in group Dexa or group Placebo (Table 3).
Discussion
The most important finding of this study was the administration of two doses of low-dose perioperative dexamethasone for patients receiving TKA can reduce the postoperative level of CRP and IL-6, provide additional analgesic effect and reduce the incidence of PONV, and reduce postoperative fatigue, without increasing the risk of early surgical wound infection and gastrointestinal haemorrhage.
With its potent anti-inflammatory effects, glucocorticoids have been widely used in TKA for decreasing the inflammatory response, preventing PONV, and relieving postoperative pain and fatigue [7, 12, 15, 18, 19]. The type of glucocorticoids includes dexamethasone, methylprednisolone, hydrocortisone, and triamcinolone, and a wide range of the doses was used (including high dose and low dose), and administration protocol of glucocorticoids includes systemic administration and local administration (intraarticularly or periarticularly) [17, 24].
In this randomized, double-blinded, placebo-controlled study, two doses of low-dose dexamethasone were intravenously administrated in the perioperative period of TKA, and they significantly decreased the postoperative level of CRP and IL-6. Our result was consistent with previous studies [17, 18]. It has been proven that high levels of CRP and IL-6 were associated with PONV, pain, fatigue, and decreased muscle strength [2, 3, 13, 17, 21, 25, 29].
The well-documented and recommended use of systemic glucocorticoids for PONV reduction is supported by the results in our study and in previous studies [15, 17, 18]. In our study, the incidence of PONV and the VAS nausea score were lower in group Dexa, and the consumption of metoclopramide was less, although the requirement of ondansetron was similar between the two groups. Thus, it was shown that the combined use of dexamethasone and mosapride further reduced PONV compared with mosapride alone. In addition, it has not been proven that local administration of glucocorticoids can reduce PONV.
Koh et al. reported that the mean reduction of pain VAS score was 1.6 after a single dose of 10 mg dexamethasone was administered to patients receiving TKA [15]. Backes et al. reported that the minimum mean reduction was 2 after dexamethasone was used [1]. In our study, the statistically significant reduction was 0.76 at rest and 0.58 during walking. A new concept, minimum clinically significant difference, has been put forward to determine if the reduction of VAS is clinically significant [4]. Jensen et al. reported that when VAS is applied to evaluate pain, the percentage of clinically significant reduction should be at least 33% [11]. Thus, in theory, the reduction should reach 0.67 at rest and 0.84 during walking. Based on this threshold of clinical significance, in our study, the reduction at rest was clinically significant, while the reduction during walking was not. However, we confirmed that along with a statistically significant reduction of pain VAS, the consumption of oxycodone and parecoxib in group Dexa was less than that in group Placebo, which makes it clinically significant.
The review of Lunn [17] supported the result from a multi-procedure meta-analysis [6] that dexamethasone ≥ 0.11 mg/kg (administered systemically) might be needed for opioid sparing and it might be superior for ameliorating postoperative pain compared with low doses for reducing PONV (dexamethasone < 0.11 mg/kg). In our study, the mean dose of dexamethasone was 0.33 mg/kg (when the mean weight was 61 kg), and we found that two doses of 10 mg IV dexamethasone can reduce overall pain at 24 h after TKA, and reduce the consumption of oxycodone. Koh et al. reported that pre-emptive use of a single dose of 10 mg dexamethasone reduced postoperative pain during the 6- to 24-hour period and reduced opioid consumption during the entire 72-hour period [15]. Lunn et al. reported that a single, high dose of 125 mg methylprednisolone reduced postoperative pain during the first 48 h [18]. However, we did not find an analgesic effect after 48 h, which may be due to the heterogeneity among studies regarding type, dosage and administration protocol of glucocorticoids and the perioperative management of TKA. In addition, as documented in the analysis performed by Lunn et al., the scientific quality was poor, and the risk of bias was high in most local administration studies. Thus, they considered that the effect of local glucocorticoids on pain and functional recovery should be appraised with certain reservations [17].
In our study, we assessed fatigue using ICFS. The ICFS is a validated multidimensional measure that has been designed specifically to measure fatigue and return to normal activity in surgical patients [20, 21, 26], including feelings of fatigue, feelings of vigour, impact on concentration, impact on energy, and impact on daily activities. The patients in our study were inpatients, and it was hard to assess the impact on daily activities (e.g. household chores, cooking, work, shopping, and making visits). Thus, we only assessed the former four subscales (120 scores in total). Various studies have shown that systemic administration of glucocorticoids (dexamethasone, methylprednisolone) could relieve postoperative fatigue [5, 18, 22, 26, 28], which was supported by the findings in our study. To the best of our knowledge, no study has reported that local administration of glucocorticoids could relieve postoperative fatigue.
In the study by Lunn et al., they found that no significant difference was observed in LOS after administration of high-dose preoperative methylprednisolone for patients receiving TKA [18]. Jules-Elyse et al. given two doses of hydrocortisone to patients receiving bilateral TKA, and found that LOS of the study group was similar to that of the control group [12]. In our study, we found that LOS was similar between the two groups. Backes et al. found a reduction in length of stay with dexamethasone administration after total joint arthroplasty (TKA and total hip arthroplasty) [1]. It may be due to that our study, as well as the studies of Lunn et al. and Jules-Elysee et al. included only patients receiving TKA, while the study of Backes et al. included patients receiving TKA and total hip arthroplasty. Another influencing factor in our study was that every patient needed to stay in hospital more than 3 days after the operation, which may prolong the LOS.
Despite chronic glucocorticoid use increasing the risk of infection and gastrointestinal haemorrhage, previous studies have not shown that a single dose or two doses of glucocorticoids perioperatively was associated with such severe complications [12, 15–18]. In our study, there was no patient who had early surgical wound infection or gastrointestinal haemorrhage in either group, a finding that supports the safety of the perioperative administration of glucocorticoids. However, 3 days is not enough to verify the presence or absence of infection in TKA. Therefore, studies with a large sample and long-term follow-up are required to meaningfully evaluate the safety of glucocorticoids. Thus, before the final recommendation can be made, such future studies are required to clarify the risk–benefit ratio [17].
There are several limitations in our study: (1) the follow-up period was only 3 days, and it was not sufficiently powered to verify safety, especially for infection in TKA; (2) the clinical effect was only evaluated during the first 3 days after TKA, and the long-term effect of glucocorticoids was not shown in this study; (3) only two doses of dexamethasone were administrated within the first 3 h postoperatively; the necessity and safety of low-dose dexamethasone within 24 or even 48 h after TKA remains unknown.
It was indicated that for patients receiving TKA, two doses of low-dose perioperative dexamethasone are effective and safe method for PONV, postoperative pain or fatigue because of the inflammatory response after surgery. The study provided a new treatment thought: for the patients with PONV, postoperative pain or fatigue lasting for 24 h or even more, low-dose dexamethasone may be an effective treatment.
Conclusions
The administration of two doses of low-dose perioperative dexamethasone can reduce the level of postoperative CRP and IL-6, reduce the incidence of PONV, provide additional analgesic effect, and reduce postoperative fatigue, but it cannot improve ROM or reduce LOS after TKA, and it did not increase the risk of early surgical wound infection and gastrointestinal haemorrhage.
Change history
19 June 2017
An erratum to this article has been published.
References
Backes JR, Bentley JC, Politi JR, Chambers BT (2013) Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. J Arthroplast 28(8 Suppl):11–17
Bautmans I, Njemini R, De Backer J, De Waele E, Mets T (2010) Surgery-induced inflammation in relation to age, muscle endurance, and self-perceived fatigue. J Gerontol A Biol Sci Med Sci 65(3):266–273
Bautmans I, Njemini R, Lambert M, Demanet C, Mets T (2005) Circulating acute phase mediators and skeletal muscle performance in hospitalized geriatric patients. J Gerontol A Biol Sci Med Sci 60(3):361–367
Bird SB, Dickson EW (2001) Clinically significant changes in pain along the visual analog scale. Ann Emerg Med 38(6):639–643
Bisgaard T, Klarskov B, Kehlet H, Rosenberg J (2003) Preoperative dexamethasone improves surgical outcome after laparoscopic cholecystectomy: a randomized double-blind placebo-controlled trial. Ann Surg 238(5):651–660
De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ (2011) Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 115(3):575–588
Fujii Y, Nakayama M (2005) Effects of dexamethasone in preventing postoperative emetic symptoms after total knee replacement surgery: a prospective, randomized, double-blind, vehicle-controlled trial in adult Japanese patients. Clin Ther 27(6):740–745
Gilron I (2004) Corticosteroids in postoperative pain management: future research directions for a multifaceted therapy. Acta Anaesthesiol Scand 48(10):1221–1222
Holte K, Kehlet H (2002) Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg 195(5):694–712
Husted H, Jensen CM, Solgaard S, Kehlet H (2012) Reduced length of stay following hip and knee arthroplasty in Denmark 2000–2009: from research to implementation. Arch Orthop Trauma Surg 132(1):101–104
Jensen MP, Chen C, Brugger AM (2003) Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain 4(7):407–414
Jules-Elysee KM, Lipnitsky JY, Patel N et al (2011) Use of low-dose steroids in decreasing cytokine release during bilateral total knee replacement. Reg Anesth Pain Med 36(1):36–40
Kehlet H, Jensen TS, Woolf CJ (2006) Persistent postsurgical pain: risk factors and prevention. The Lancet 367(9522):1618–1625
Kehlet H, Wilmore DW (2002) Multimodal strategies to improve surgical outcome. Am J Surg 183(6):630–641
Koh IJ, Chang CB, Lee JH, Jeon YT, Kim TK (2013) Preemptive low-dose dexamethasone reduces postoperative emesis and pain after TKA: a randomized controlled study. Clin Orthop Relat Res 471(9):3010–3020
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Lunn TH, Kehlet H (2013) Perioperative glucocorticoids in hip and knee surgery - benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol Scand 57(7):823–834
Lunn TH, Kristensen BB, Andersen LO et al (2011) Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth 106(2):230–238
Miyagawa Y, Ejiri M, Kuzuya T, Osada T, Ishiguro N, Yamada K (2010) Methylprednisolone reduces postoperative nausea in total knee and hip arthroplasty. J Clin Pharm Ther 35(6):679–684
Paddison JS, Booth RJ, Fuchs D, Hill AG (2008) Peritoneal inflammation and fatigue experiences following colorectal surgery: a pilot study. Psychoneuroendocrinology 33(4):446–454
Paddison JS, Booth RJ, Hill AG, Cameron LD (2006) Comprehensive assessment of peri-operative fatigue: development of the Identity-Consequence Fatigue Scale. J Psychosom Res 60(6):615–622
Romundstad L, Breivik H, Roald H et al (2006) Methylprednisolone reduces pain, emesis, and fatigue after breast augmentation surgery: a single-dose, randomized, parallel-group study with methylprednisolone 125 mg, parecoxib 40 mg, and placebo. Anesth Analg 102(2):418–425
Salerno A, Hermann R (2006) Efficacy and safety of steroid use for postoperative pain relief. Update and review of the medical literature. J Bone Joint Surg Am 88(6):1361–1372
Sean VW, Chin PL, Chia SL, Yang KY, Lo NN, Yeo SJ (2011) Single-dose periarticular steroid infiltration for pain management in total knee arthroplasty: a prospective, double-blind, randomised controlled trial. Singapore Med J 52(1):19–23
Wehner S, Vilz TO, Stoffels B, Kalff JC (2012) Immune mediators of postoperative ileus. Langenbecks Arch Surg 397(4):591–601
Wilmore DW, Kehlet H (2001) Management of patients in fast track surgery. BMJ 322(7284):473–476
Xie J, Ma J, Yao H, Yue C, Pei F (2016) Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss After primary total knee arthroplasty without tourniquet: a randomized clinical trial. J Arthroplast 31(11):2458–2464
Zargar-Shoshtari K, Paddison JS, Booth RJ, Hill AG (2009) A prospective study on the influence of a fast-track program on postoperative fatigue and functional recovery after major colonic surgery. J Surg Res 154(2):330–335
Zhang JM, An J (2007) Cytokines, inflammation, and pain. Int Anesthesiol Clin 45(2):27–37
Acknowledgements
The study was supported by Ministry of Health of the People’s Republic of China (201302007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the Institutional Review Board of West China Medical Center of Sichuan University (No. 201302007) before patient enrolment.
Funding
This study was funded by Ministry of Health of the People’s Republic of China (no. 201302007).
Informed consent
Written informed consent and research authorization were obtained from all patients.
Additional information
The original version of this article was revised: Unfortunately, the online published article has error in Table 2. In the Metoclopramide, Number of patients requiring, The P value should be 0.018.
Xu and Ma have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Xu, B., Ma, J., Huang, Q. et al. Two doses of low-dose perioperative dexamethasone improve the clinical outcome after total knee arthroplasty: a randomized controlled study. Knee Surg Sports Traumatol Arthrosc 26, 1549–1556 (2018). https://doi.org/10.1007/s00167-017-4506-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-017-4506-x