Abstract
Purpose
To evalute the efficacy and safety of two low-dose peri-operative dexamethasone on pain and recovery following total hip arthroplasty (THA).
Methods
One hundred ten patients received two-dose of 10 mg IV-dexamethasone (group dexa) or IV-isotonic saline (group placebo). The level of C-reactive protein (CRP) and interleukin-6 (IL-6), pain at rest and during mobilization, incidence of post-operative nausea and vomiting (PONV), intensity of nausea, post-operative fatigue, consumption of analgesic and antiemetic rescue, range of motion (ROM), post-operative length of stay (post-operative LOS), wound problems and complications were recorded and compared.
Results
The level of inflammation markers (CRP, IL-6) in group dexa was lower than group placebo at 24, 48, 72 hours post-operatively. Dynamic pain VAS score at 24 hours was lower in group dexa (P = 0.002), however, there was no significant effect on pain at rest. In group dexa, patients had a lower incidence of PONV (P = 0.003), as well as a lower VAS score of nausea (P = 0.044). The post-operative fatigue (P < 0.001) was relieved and the consumption of analgesic and antiemetic rescues were reduced. Furthermore, patients had better maximum hip flexion (P < 0.001) and abduction (P = 0.017), with shorter post-operative LOS (P = 0.006). There is no difference between groups in wound problems. No surgical site infection or gastrointestinal haemorrhage was detected in both groups.
Conclusions
The administration of two low-dose peri-operative dexamethasone can effectively reduce the post-operative level of CRP and IL-6, provide additional pain and nausea control, ameliorate post-operative fatigue, enhance mobility, and shorten post-operative LOS following THA, without increasing the risk of infection and gastrointestinal hemorrhage.
Level of evidence: I
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total hip arthroplasty (THA) is one of the most effective treatment options for end-stage osteoarthritis and other hip diseases, which can greatly improve mobility and quality of life [1]. However, surgical trauma during THA often results in severe post-operative inflammation [2] which might contribute to intensive post-surgical pain and fatigue, increased incidence of post-operative nausea and vomiting (PONV), limited joint movement, and prolonged hospital stays [3]. In addition, inadequately peri-operative management has been directly correlated with poor patient satisfaction and can lead to delays in the early recovery period [4]. Consequently, it is essential to control peri-operative inflammation in THA.
Glucocorticoid is a class of steroid hormone with considerable anti-inflammatory properties, which has been commonly adopted in several surgical fields including THA [5,6,7]. As reported before, glucocorticoids are able to reduce PONV, meliorate fatigue and can be a portion of multimodal analgesic regimes in THA [8,9,10]. However, due to clinical heterogeneity, the optimal timing, way, and dosage of glucocorticoids in THA have not been clearly defined, which can lead to a great deal of variation in clinical results. According to previous studies, glucocorticoids were frequently administered pre-operatively considering the 2 h of onset time [11]. Moreover, the majority of regimes were single short-dose and utilized intravenously on account of the relatively lower adverse side effects [12,13,14]. Nevertheless, some of the patients still suffer from pain, fatigue, and PONV [10, 15]. Thus, it was hypothesized that the action time and dose can not meet the needs of anti-inflammatory.
Therefore, this prospective randomized controlled trial was conducted to clarify the effectiveness and safety of two low-dose of 10 mg IV-dexamethasone (Dexa) during the peri-operative period of THA, which is expected to determine: (1) whether dexamethasone reduces the level of post-operative inflammatory marks; (2) whether the combined use of dexamethasone and mosapride further reduces PONV compared with mosapride along; (3) whether the two low-dose of dexamethasone provide additional analgesic effect; (4) whether dexamethasone reduces post-operative fatigue; (5) whether dexamethasone improves the function and range of motion (ROM) after THA; (6) whether dexamethasone shortens post-operative LOS; (7) whether dexamethasone increases the risk of infection and gastrointestinal hemorrhage.
Materials and methods
Patients and design
The trial was approved by the institutional review board and registered in the International Clinical Trial Registry (ChiCTR-IOR-16008865). All subjects gave their oral and written informed consent for participation in the study before surgery. From May to October 2016, all patients receiving elective, unilateral, primary THA were consecutively screened for recruitment into the trial. Exclusion criteria included alcohol or medical abuse, allergies to Dexa, age ≤ 18 years or ≥80 years, administration of any glucocorticoids during the past three months before surgery; administration of any strong opioids during the past 7 days; history of severe heart disease (NYHA > 2), liver or renal failure, rheumaimmune systemic diseases (rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus).
Consecutive patients (n = 110) were randomly assigned into two groups, including group dexa and group placebo. A random allocation sequence concealed in opaque sealed envelopes only opened before surgery. Patients in group dexa (n = 55) received two-dose of 10 mg IV-dexamethasone (2 ml, Tianjin Kingyork group Co., Ltd., China), and patients in group placebo (n = 55) received two-dose of IV-isotonic saline (2 ml). The first dose was administered just after the general anesthesia was well performed by an analgesist, and the second dose was adopted just when the patients returned to inpatient unit by a nurse. The analgesist and nurse were not involved in this trial, and the patients, surgeons, data controller and analyst were blinded.
Surgery procedure
All the THAs were operated in the same laminar flow operating room and performed by a single experienced orthopaedic surgeon. We carried out operations using the posterolateral approach and cementless cups and stems. General anesthesia were selected by anesthetists in our medical centre with blood pressure controlled within 90–110 mmHg/60–70 mmHg throughout the procedure. Before skin incision, a dose of 20 mg/kg IV- tranexamic acid (TXA, Chongqing Lummy Pharmaceutical Co., Ltd. China; DAIICHI SANKYO PROPHARMA CO., LTD., Japan) was administered. Before the incision was closed, an intra-articular injection of 1 g TXA was administered, and a peri-articular injection of 0.2% ropivacaine (100 ml) was administered. Moreover, no nerve block and/or intravenous patients-controlled analgesia (PCA) had been utilized peri-operatively.
Post-operative care protocol
Patients were transferred to the post-anesthesia care unit after the surgery for two hours, then return to the inpatient unit. A cold pack was used on the surgical sites for 24 hours at the time backing to ward. Daily function training, including active ROM training, strength training, and walking training were followed out under the supervision and assistance of a physiotherapist.
The strategies to restrain pain and PONV for all patients were the same. Multimodal oral analgesic drugs (50 mg q12h diclofenac, 75 mg q8h pregabalin) were administered for pre-emptive analgesia one day before the surgery. The analgesic process was adopted again when patients resumed oral intake after the surgery. Once patients reported the pain greater than 4 on a 0–10 VAS, the oral oxycodone (10 mg q8h) would be utilized. An intramuscular injection of parecoxib (40 mg) was used if a patient claimed severe pain greater than 6. One day before the surgery, oral mosapride (5 mg tid, before each meal) was started. An intramuscular injection of metoclopramide (10 mg) was selected as a first-line rescue option if patients had two or more occurrences of PONV or had severe nausea (VAS > 4). An intramuscular injection of ondansetron (5 mg) could be a second-line rescue option under the condition that severe nausea or vomiting persevered after two boluses of metoclopramide has been administered in a 30 minute interval.
A combination of mechanical and chemical thromboprophylaxis was adopted to prohibited venous thromboembolism. As chemical prophylaxis, all patients received a half-dose of low-molecular-weight heparin (LMWH; 2000 IU in 0.2 ml; Clexane, Sanofiaventis, France) starting at six hours post-operatively, followed by a full dose (4000 IU in 0.4 ml) at 24 hour intervals. As mechanical prophylaxis, an intermittent foot slope pump system was utilized before walking. As prophylaxis after discharge, patients were instructed to take 10 mg Rivaroxaban (Xarelto, Bayer, Germany) orally once a day for 14 days.
Outcome measurements
Patient demographics, medical histories and concomitant medication were registered pre-operatively. Inflammatory factors (CRP, IL-6) were tested in pre-operation and at 24, 48, 72 hours after operation. In order to evaluate the analgesic effect, pain level and the quantity of analgesic rescue drugs (oxycodone and parecoxib) were recorded. Pain level was evaluated using a visual analogue scale (VAS, 0 means no pain, 10 means severe pain imaginable) and was conducted in pre-operation and at 24, 48, 72 h postoperatively, both at rest (rest in bed at least for 30 minutes before test) and walking training (walk 20 steps before test). The occurrence of PONV and the consumption of antiemetic rescue drugs (metoclopramide and ondansetron) were collected after the surgery. The intensity of nausea was assessed using VAS (0 means no nausea, 10 means severe nausea imaginable) at the first six hours after operation. Fatigue was evaluated choosing Identity-Consequence-Fatigue-Scale (ICFS) before surgery and at POD3. Hip ROM was measured by extension, flexion and abduction with a goniometer one day pre-operatively as a baseline, post-operatively on day three. Wound problems (wound leakage, redness or swelling around the wound) were also assessed. The complications (surgical site infection, gastrointestinal hemorrhage) were carefully recorded during the inpatient hospital stay and two weeks follow-up. In this current trial, prolonged post-operative LOS is defined as the duration which was greater than the 75th percentile for the entire cohort, and the reasons of delayed discharge were recorded.
Statistical analysis
Sample size calculations were performed using PASS 2011 (NCSS, LLC. Kaysville, Utah, USA) software on the basis of a two-sample t-test. According to the results of previous studies [8, 16], we anticipated an average decrease in VAS (pain) score of 0.7 in group dexa. With a power of 0.90 and the significant level of 0.05, 40 patients per arm were needed. If a 10% exclusion rate was expected, the minimum sample size was 45 in each group. Thus, we decided to include 60 patients in each group.
All data analysis was performed by SPSS version 24 (SPSS Inc. USA). Student’s t test or Wilcoxon Mann-Whiney U test was used to analyze quantitative data and Pearson Chi-square test or Fisher exact test was used to analyze qualitative comparative data. Statistical significance was defined as P < 0.05.
Results
Patients’ demographics
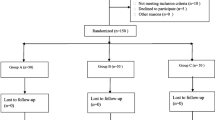
One hundred twenty patients recruited from May 2016 to October 2016 were scheduled to receive an elective, unilateral, primary THA at our institution. Among these patients, six were ineligible, and four were rejected from participation. Hence, the trial was completed in 110 patients. Fifty five were randomized into group dexa, while the others were randomized into group placebo (Fig. 1), and the two groups were comparable in terms of their baseline characteristics (Table 1).
Inflammation marks
As acute inflammatory factors, CRP and IL-6 revealed a rapid increase in all patients after the operations. The peak level of CRP was observed on 48 hours in both groups, and the mean serum level in the placebo group was significantly greater in comparison with the dexa group at 24, 48, and 72 hours post-operatively (P1 = 0.038, P2 < 0.001, P3 < 0.001). The mean serum concentrations of IL-6 peaked 24 hours after the surgery in group dexa, and peaked 48 hours post-operatively in group placebo. In addition, the level of IL-6 in group dexa was generally lower than group placebo 24, 48, 72 hours after operation (P1 < 0.001, P2 < 0.001, P3 < 0.001) (Fig. 2).
Pain and analgesic rescue
The overall pain was reduced after THA compared with pre-operation. Patients in group dexa had lower pain VAS score than group placebo at 24 hours during walking (P1 = 0.002), however, the dynamic pain of both groups was similar at 48 or 72 hours after the operation (P2 = 1.000, P3 = 0.698). While there was no significant effect on pain at rest at any time period (P1 = 0.578, P2 = 0.786, P3 = 0.658) (Fig. 3).
Compared with group placebo, the number of patients requiring parecoxib showed a remarkable decrease in group dexa (P = 0.002), and the overall parecoxib consumption was less (P = 0.005). However, no significant intergroup differences were observed in the number of patients requiring oxycodone (P = 1.000) and the overall oxycodone consumption (P = 1.000) (Table 3).
PONV and antiemetic rescue
The occurrence of PONV (P = 0.003) and the VAS score for nausea (1.25 ± 1.32 vs 2.16 ± 2.00, P = 0.044) in group dexa was lower compared with group placebo (Table 2). The number of patients requiring metoclopramide was smaller in group dexa (P = 0.001), and the overall metoclopramide consumption was less (P = 0.003). The number of patients requiring ondansetron was similar between groups (P = 0.118). However, the overall ondansetron consumption in group dexa was less (P = 0.010) (Table 3).
Fatigue, post-operative LOS, ROM, wound problems and complications
In group dexa, patients had lower post-operative ICFS score (73.91 ± 5.75 vs 85.49 ± 9.10, P < 0.001) and shorter post-operative LOS (4.07 ± 1.22 vs 4.84 ± 1.60, P = 0.006) than group placebo. At POD3, the maximum hip flexion (112.82 ± 6.86 vs 102.18 ± 4.59, P < 0.001) and abduction (38.91 ± 3.43 vs 37.09 ± 4.38, P = 0.017) in group dexa was better than that in group placebo. Although the occurrence of wound problems was lower in group dexa compared with group placebo, the differences were not statistically significant (P = 0.716). No surgical site infection or gastrointestinal hemorrhage was observed in both groups (Table 2).
Prolonged post-operative LOS and causes
In this trial, the 75th percentile for the entire cohort was five days, which was used to define the prolonged post-operative LOS. Fifteen patients in group placebo and six in group dexa had post-operative hospital stays over five days, with a significant intergroup difference (p = 0.029). Among the 15 patients in group placebo, ten were due to nausea and five to wound problems. Among the six patients in group dexa, three were attributed to nausea and the rest were caused by wound problems. This result indicates a significant effect on nausea of dexamethasone (p = 0.039). However, no significant difference was observed in wound problems between groups (p = 0.716) (Table 4).
Discussion
Dexamethasone is a kind of synthetic glucocorticoid with higher potency, greater bioavailability, and longer acting time [17, 18]. It can be administered through different routes, including systemical and topical application, with a great range of doses, given either pre-operatively, intra-operatively, or post-operatively. However, the ideal dose, way and timing of administration still remain controversial [12].
It has been demonstrated that the local and systemic inflammatory response following THA is closely related to the early rehabilitation and post-operative complications [3, 12, 19]. In this study, two low-dose dexamethasone had been administered in the peri-operative time for early recovery and patient satisfaction. Both the CRP and IL-6 were significantly lower in group dexa compared with the placebo group, which is in agreement with previous studies [8, 20].
The sufficient antiemetic efficacy of short-dose systemic dexamethasone has been well elucidated in previous studies [12, 14]. It can play an efficient role by inhibiting prostaglandin synthesis or endogenous opioids release [4]. Mathiesen et al. adopted a single-dose of 8 mg dexamethasone pre-operatively [21], however, the incidence rate of PONV still reached 19.0% [8]. In this randomized trial, an extra one low-dose of dexamethasone has been administered at the time of returning to ward, which have shown a notable reduction of PONV. Furthermore, patients in the dexa group had lower VAS-nausea score and fewer antiemetic rescue. The main results of our study could indicate that an additive dose of dexamethasone combined with mosapride could provide prolonged antiemetic effects.
Many recent studies have reported that postoperative pain after THA can be ameliorated with high-dose systemic glucocorticoid [12]. Mathiesen and colleagues found no additional effects on pain or opioid requirements with a single short-dose of 8 mg dexamethasone [21]. In our study, we found dexamethasone, administered in two-dose of 10 mg IV, can effectively meliorate dynamic pain at 24 hours after THA, and reduce the consumption of parecoxib, which has the imposed similar effect with a single dose of dexamethasone 40 mg dispensed pre-operatively [14]. This result may indicate that an additive adoption of intravenous dexamethasone can cover the shortage of dosage. Moreover, higher dose (dexamethasone ≥ 0.21 mg/kg) might not provide additional benefits and may increase the risk of side effects [12]. Thus, further studies about effective multiple boluses for analgesia would be of interest.
Post-operative fatigue is a common symptom following systemic or local inflammation, with decreased strength and a feeling of exhaustion [19], which can lead to delays in the early recovery. Severe fatigue is most often accompanied by grievous pain, and they seem to be isochronous [19]. Content fatigue control may contribute to satisfactory pain management, which may in turn meliorate the post-operative fatigue. Thus, the virtuous cycle would be established. In our study, we adopted ICFS to assess fatigue which is designed specifically for a general surgical population [22]. This kind of multidimensional measure usually consists of fatigue feelings, vigor feelings, impacts on concentration, impacts on energy, and impacts on daily activities. The review of Rubin reported that glucocorticoids may attenuate fatigue after surgery [23], which was in accordance with our study.
Inpatient hospital stay is deemed as an important outcome due to its economic factors [24]. Our study has well demonstrated that two low-dose peri-operative dexamethasone effectively shortens postoperative LOS by approximately 1 day. Backes et al. observed a statistically significant decrease of LOS with peri-operative dexamethasone by nearly 1.2 days [9], which is in keeping with our finding. In our trial, ten patients in the placebo group had post-operative LOS over five days, which were caused by nausea. By contrast, there were only three patients in the dexa group with post-operative hospital stays over five days because of nausea. It indicates that patients in the placebo group were more likely to be influenced by nausea and then delayed hospital discharge (p = 0.029). This result shows a persistent effect on nausea of dexamethasone (p = 0.039), and the consistent finding could support our thesis. Moreover, the effect on the ROM has also been observed in this study, which may contribute to the time to meet discharge criteria.
Infection and gastrointestinal hemorrhage are severe complications with increased morbidity and mortality in THA. Considering the fact that chronic glucocorticoid utilization could raise the possible risks and side effects, the safety of dexamethasone has yet to be completely clarified [12]. Richardson et al. conducted a large-scale study with a long term follow-up, and they found no significant increases in the incidence of infection with a single low-dose (4–10 mg) of dexamethasone [4]. However, the study is retrospective and there is no consensus of anesthetic and surgical protocols. Thus, a prospective study with standardized process is urgently wanted. In our study, no surgical site infection or gastrointestinal hemorrhage were detected in both groups, which might provide an additional evidence to evaluate the safety of glucocorticoids.
There are several limitations to be noted in this study: 1) This study solely focused on a short follow-up period, and it could be short on statistical power to sufficiently assess the clinical effect and safety; 2) No comparison about the dosage was shown in our study, further studies may need to determine the minimum effective dose; 3) The second dose was administered within the first post-operative three hours, and if it is necessary and safe to give an additive dose of dexamethasone within 24 or even 48 hours following THA is still unknown.
In conclusion, the administration of two low-dose peri-operative dexamethasone for patients can reduce the level of post-operative CRP and IL-6, provide significant additional pain and nausea control, reduce post-operative fatigue, enhance mobility, and shorten post-operative LOS following THA, without increasing the risk of infection and gastrointestinal hemorrhage. However, further studies with large-scale and long-term follow-up on efficacy and safety are still in demand for fully assessing the effects of peri-operative dexamethasone administration.
References
Yue C, Kang P, Yang P, Xie J, Pei F (2014) Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplast 29(12):2452–2456. doi:10.1016/j.arth.2014.03.032
Wasko MK, Bobecka-Wesolowska K, Tomasiuk R, Kowalczewski J (2015) Measurement of the inflammatory response in the early postoperative period after hip and knee arthroplasty. Clin Chem Lab Med 53(11):1785–1792. doi:10.1515/cclm-2014-1055
Aasvang EK, Luna IE, Kehlet H (2015) Challenges in postdischarge function and recovery: the case of fast-track hip and knee arthroplasty. Br J Anaesth 115(6):861–866. doi:10.1093/bja/aev257
Richardson AB, Bala A, Wellman SS, Attarian DE, Bolognesi MP, Grant SA (2016) Perioperative dexamethasone administration does not increase the incidence of postoperative infection in Total hip and knee Arthroplasty: a retrospective analysis. J Arthroplast 31(8):1784–1787. doi:10.1016/j.arth.2016.01.028
Bolac CS, Wallace AH, Broadwater G, Havrilesky LJ, Habib AS (2013) The impact of postoperative nausea and vomiting prophylaxis with dexamethasone on postoperative wound complications in patients undergoing laparotomy for endometrial cancer. Anesth Analg 116(5):1041–1047. doi:10.1213/ANE.0b013e318276cf58
Chen CC, Siddiqui FJ, Chen TL, Chan ES, Tam KW (2012) Dexamethasone for prevention of postoperative nausea and vomiting in patients undergoing thyroidectomy: meta-analysis of randomized controlled trials. World J Surg 36(1):61–68. doi:10.1007/s00268-011-1343-9
Romundstad L, Breivik H, Roald H, Skolleborg K, Haugen T, Narum J, Stubhaug A (2006) Methylprednisolone reduces pain, emesis, and fatigue after breast augmentation surgery: a single-dose, randomized, parallel-group study with methylprednisolone 125 mg, parecoxib 40 mg, and placebo. Anesth Analg 102(2):418–425. doi:10.1213/01.ane.0000194358.46119.e1
Lunn TH, Andersen LO, Kristensen BB, Husted H, Gaarn-Larsen L, Bandholm T, Ladelund S, Kehlet H (2013) Effect of high-dose preoperative methylprednisolone on recovery after total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Br J Anaesth 110(1):66–73. doi:10.1093/bja/aes345
Backes JR, Bentley JC, Politi JR, Chambers BT (2013) Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. J Arthroplast 28(8 Suppl):11–17. doi:10.1016/j.arth.2013.05.041
Miyagawa Y, Ejiri M, Kuzuya T, Osada T, Ishiguro N, Yamada K (2010) Methylprednisolone reduces postoperative nausea in total knee and hip arthroplasty. J Clin Pharm Ther 35(6):679–684. doi:10.1111/j.1365-2710.2009.01141.x
Romundstad L, Breivik H, Niemi G, Helle A, Stubhaug A (2004) Methylprednisolone intravenously 1 day after surgery has sustained analgesic and opioid-sparing effects. Acta Anaesthesiol Scand 48(10):1223–1231. doi:10.1111/j.1399-6576.2004.00480.x
Lunn TH, Kehlet H (2013) Perioperative glucocorticoids in hip and knee surgery - benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol Scand 57(7):823–834. doi:10.1111/aas.12115
De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ (2011) Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 115(3):575–588. doi:10.1097/ALN.0b013e31822a24c2
Kardash KJ, Sarrazin F, Tessler MJ, Velly AM (2008) Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg 106(4):1253–1257, table of contents. doi:10.1213/ANE.0b013e318164f319
Bergeron SG, Kardash KJ, Huk OL, Zukor DJ, Antoniou J (2009) Perioperative dexamethasone does not affect functional outcome in total hip arthroplasty. Clin Orthop Relat Res 467(6):1463–1467. doi:10.1007/s11999-009-0733-x
Cui Z, Liu X, Teng Y, Jiang J, Wang J, Xia Y (2015) The efficacy of steroid injection in total knee or hip arthroplasty. Knee Surg Sports Traumatol Arthrosc 23(8):2306–2314. doi:10.1007/s00167-014-3049-7
Spies CM, Strehl C, van der Goes MC, Bijlsma JW, Buttgereit F (2011) Glucocorticoids. Best Pract Res Clin Rheumatol 25(6):891–900. doi:10.1016/j.berh.2011.11.002
Coutinho AE, Chapman KE (2011) The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335(1):2–13. doi:10.1016/j.mce.2010.04.005
Louati K, Berenbaum F (2015) Fatigue in chronic inflammation — a link to pain pathways. Arthritis Res Ther 17:254. doi:10.1186/s13075-015-0784-1
Sculco PK, McLawhorn AS, Desai N, Su EP, Padgett DE, Jules-Elysee K (2016) The effect of perioperative corticosteroids in Total hip Arthroplasty: a prospective double-blind placebo controlled pilot study. J Arthroplast 31(6):1208–1212. doi:10.1016/j.arth.2015.11.011
Mathiesen O, Jacobsen LS, Holm HE, Randall S, Adamiec-Malmstroem L, Graungaard BK, Holst PE, Hilsted KL, Dahl JB (2008) Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth 101(4):535–541. doi:10.1093/bja/aen215
Nostdahl T, Bernklev T, Raeder J, Sandvik L, Fredheim O (2016) Postoperative fatigue; translation and validation of a revised 10-item short form of the Identity-Consequence fatigue scale (ICFS). J Psychosom Res 84:1–7. doi:10.1016/j.jpsychores.2016.03.002
Rubin GJ, Hotopf M (2002) Systematic review and meta-analysis of interventions for postoperative fatigue. Br J Surg 89(8):971–984. doi:10.1046/j.1365-2168.2002.02138.x
Sibia US, MacDonald JH, King PJ (2016) Predictors of hospital length of stay in an enhanced recovery after surgery program for primary total hip arthroplasty. J Arthroplast 31(10):2119–2123. doi:10.1016/j.arth.2016.02.060
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Funding
This study was funded by the National Health and Family Planning Commission of the People’s Republic of China (CN) program (201302007).
Ethical approval
The trial was approved by the institutional review board and registered in the International Clinical Trial Registry (ChiCTR-IOR-16008865).
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Lei, Yt., Xu, B., Xie, Xw. et al. The efficacy and safety of two low-dose peri-operative dexamethasone on pain and recovery following total hip arthroplasty: a randomized controlled trial. International Orthopaedics (SICOT) 42, 499–505 (2018). https://doi.org/10.1007/s00264-017-3537-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3537-8