Abstract
There is an increasing awareness on the importance in identifying early phases of the degenerative processes in knee osteoarthritis (OA), the crucial period of the disease when there might still be the possibility to initiate treatments preventing its progression. Early OA may show a diffuse and ill-defined involvement, but also originate in the cartilage surrounding a focal lesion, thus necessitating a separate assessment of these two entities. Early OA can be considered to include a maximal involvement of 50 % of the cartilage thickness based on the macroscopic ICRS classification, reflecting an OARSI grade 4. The purpose of this paper was to provide an updated review of the current status of the diagnosis and definition of early knee OA, including the clinical, radiographical, histological, MRI, and arthroscopic definitions and biomarkers. Based on current evidence, practical classification criteria are presented. As new insights and technologies become available, they will further evolve to better define and treat early knee OA.
Level of evidence IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an increasing awareness on the importance in identifying early phases of the degenerative processes in knee osteoarthritis (OA), the period of the disease when there might still be some regenerative ability of the articular cartilage, which is permanently lost in the advanced disease stages. A definition of the early OA phase is important to identify and properly treat patients at risk of progression, allowing to better design trials for the assessment of potential and indications of the available and new emerging treatments, and therefore to better allocate resources and manage patients affected by lesions of the knee articular surface in the clinical practice [34].
However, the diagnosis of early knee OA is more complicated compared with established OA. Once the disease has manifested itself, clear signs can be detected through history, clinical symptoms, and radiographic signs [34]. In its early phase, such characteristic signs and symptoms may still be limited and appear sporadic. Moreover, the radiographic evaluation cannot detect tissue-related phenomena characterising established OA. Magnetic resonance imaging (MRI) may allow detecting the whole spectrum of pathological joint tissue changes. Arthroscopy is seldom used as a sole diagnostic tool, but often is performed in patient populations that are suffering from symptomatic focal cartilage defects. Such patients are at risk of developing unicompartmental perilesional early OA as a result of a critical-size focal cartilage defect [52, 53]. These measures may help to define the early OA phase in the majority of patients in the daily clinical practice [34].
Early knee OA of a patient was classified [34] as being present if three criteria (Table 1) were fulfilled:
-
1.
Pain in the knee.
-
2.
Standard radiographs Kellgren and Lawrence (K&L) grade 0 or 1 or 2 (osteophytes only).
-
3.
At least one of the two following structural criteria.
-
Arthroscopic findings of cartilage lesions.
-
MRI findings demonstrating articular cartilage degeneration and/or meniscal degeneration, and/or subchondral bone marrow lesions (BMLs).
-
Risk factors
Identification of the risk factors in early OA is crucial to initiate adequate and prompt conservative treatments and to prevent the progression of the disease to the levels where reconstructive surgery becomes the only effective option. In addition to age, many risk factors have been identified. In general, any factor that alters the proper joint biomechanics triggers the onset or an acceleration of the degenerative process, facilitating the beginning of structural alterations and, subsequently, of the clinical symptoms. Also, the crucial role played by synovial inflammation both in the onset and in the progression of knee OA becomes clearer, although patients with early OA do not have typically high levels of systemic inflammatory markers.
Injuries involving knee ligaments may decrease joint stability and, therefore, could be responsible for joint degeneration. However, even after a successful anterior cruciate ligament (ACL) reconstruction, an increased risk of developing OA persists. Already after 1 year following ACL reconstruction, early signs of patellofemoral and tibiofemoral OA can be observed on MRI in some patients, with a direct correlation with associated meniscectomies and overweight [14]. Besides, even a physiological knee laxity has to be considered to be a risk factor [58]. Meniscal lesions, if left untreated or when treated with (partial) meniscectomy, alter its function of a shock absorber, expose the adjacent articular cartilage of both the tibial plateau and femoral condyle, and increase compressive and shear forces [35]. Axial malalignment of the lower limb results in increased loads at the medial (varus malalignment) or lateral (valgus malalignment) tibiofemoral compartment. Likewise, any additional load to the joint (due to overweight or increased physical activity) represents an increased risk of developing early OA. A body mass index (BMI) >30 determines a three-time greater risk of developing early OA compared to normal weight, both for an increased load on the articulation and for metabolic alterations with higher pro-inflammatory factors. Conversely, weight loss reduces the risk of developing symptomatic early OA of the knee and improves the function of the joint [57]. Furthermore, moderate exercise is a protective factor [4].
OA has a strong genetic component. Recent epidemiological and genetic research has shown that OA is a multifactorial disease with both environmental and genetic components [35]. Several genome-wide association scans have revealed associations between certain loci on the human genome and the development of OA [60]. Interestingly, evidence is supported for the ethnic-, sex-, and joint-specific effects in OA [35]. However, the roles and the potential clinical utility of genes identified are still unclear, and studies are needed integrating genetics, genomics, and epigenetics in the context of early OA development, together with the neuromuscular component [55].
Pain in early OA
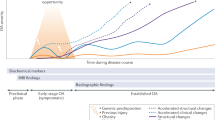
Previously, it was suggested that for diagnosing early OA, pain should be present at least in two episodes for more than 10 days in the last year [34]. Patients with early OA mostly describe intermittent diffuse joint pain typically worsening with mild swelling after excessive stress (e.g. sport), light crepitation, and/or an angle-dependent load pain (Fig. 1). The pain is relieved by rest or activities such as cycling with low resistance [6]. Pain while climbing stairs is often one of the first symptoms. Kneeling or squatting activities are associated with an increased sensation of pain and repetitive loading during or after sports exercise as well. Most affected are persons in their 30–50s, being forced to change their sports practice [17].
Illustration of the functional loss in relation to the symptoms in the various stages of OA. The correlation between the degree of OA and clinical symptoms is not always seen in clinical practise. Modified from [47]
In general, the majority of patients experience swelling early after exercise. Cartilage defects contribute to an impaired function of the organ ‘knee’. The symptom ‘pain’ in early OA thus has to be consecutively interpreted as a disturbed joint homeostasis [50]. The pain-eliciting movements as well as pain characteristics have to be investigated. In a cross-sectional case–control study of 100 early OA patients, 74 % of them with early radiographic OA had pain and function scores that were equal to or worse than individual patients with full-thickness tricompartmental final knee OA [27]. Thorstensson et al. studied 143 patients with chronic knee pain without trauma or other joint disease. They found that 86 % developed knee OA over the next 12 years. Thus, knee pain is very often the first true sign of knee OA [54]. Additional burden of the subchondral bone leading to a respective load pain can be also speculated, but no evidence has been published so far [35]. A more diffuse pain pattern is more often a signal of an inflammation of the Hoffa fat pad or irritation of ligaments. Chronic load pain during the triggering strain leads to myostatic imbalances and disturbances of sensomotoric functions [3]. An inhomogeneous muscular atrophy is the result and leads to secondary functional limitations.
A recent focus on changes in the peripheral and central nervous system suggests that changes occurred in many persons by the time they develop the chronic pain of OA. Caution should be exercised to correctly attribute pain, often requiring additional examinations [20].
Clinical definition of early OA
Clinically, early OA of the knee is characterised by signs and symptoms that may be limited and sporadic, compared to established OA. It foreruns radiographic knee OA at least of 2–3 years [10]. Some groups are at high risk of developing early OA [34], examples are young people who have sustained ACL and/or meniscal tears, patients with trochlear dysplasia and patellar instability [2] or other congenital lesions such as osteochondritis dissecans [13]. All patients with knee pain should be examined to confirm and characterise joint involvement, and to exclude pain and functional syndromes resulting from other causes.
As stated above, chronic knee pain alone or coupled with MRI findings suggesting OA is the first and predominant symptom. Patients with early OA also experience stiffness: in the morning, after a period of inactivity, or particularly in the evening. It generally disappears with exercise after a few minutes. Loss of joint movement and function is often a problem. Restricted passive movement can be the first and sole physical sign of the disease. Muscle weakness, poor knee joint proprioception, and high knee joint laxity have been hypothesised to contribute to the increase in activity limitations. Such weakness is also present in knees without pain or evidence of muscle atrophy, possibly due to arthrogenic inhibition of muscle contraction [25].
Clinically relevant frontal plane (varus or valgus) or patellofemoral malalignment increasing stress or loading across the affected region of the joint, decrease in walking speed, cadence, and lower knee flexion moment during the stance phase are often associated with early knee OA [34].
Macroscopic changes in the articular cartilage in early OA
Current regenerative treatment approaches in cartilage repair aim for the restoration of a localised focal (often traumatic) lesion. The clinical reality in patients eligible for regenerative cartilage repair reveals, however, that acute traumatic cartilaginous lesions are rare and it is mainly degenerative lesions that need regenerative treatment [53].
Patients differ in the underlying cause of the lesions (e.g. traumatic, overload, metabolic, and degenerative) and the duration of the disease with probably significant influence on outcome. In order to test and compare clinical trials with regenerative approaches, a more defined classification of traumatic, early and late OA is needed to characterise different patient populations. Especially the arthroscopic evaluation allows, besides clinical and radiological signs, a precise classification of early OA. The arthroscopic early OA classification can only be used when clinical symptoms according to the clinical early OA definition are present and radiological signs (standard X-ray) of late OA can be excluded.
The last definition and classification of early OA describes arthroscopic findings as lesions according to International Cartilage Repair Society (ICRS) grades 2–4 in one compartment with at least surrounding softening and swelling of the cartilage and as lesions according to ICRS grades 1–4 in at least two compartments [34]. According to this classification, it appears necessary to split the arthroscopic classification in two parts:
-
Part 1 The first one concentrates on the articular cartilage surrounding a focal lesion with analysis of the surrounding cartilage and the affected compartment. This would reflect the clinical fact that a focal articular cartilage defect may instigate the development of early osteoarthritis-like degenerative changes in the (and previously normal) articular cartilage surrounding the lesion, which may further extend and subsequently affect the entire compartment. It is thus a perilesional form of early OA that might continue to a (uni)compartmental and therefore advanced-stage OA. Practically, this involves a separate assessment of (1) the focal cartilage defect and (2) the degenerative changes in the perilesional cartilage (applying the ICRS system).
-
Part 2 This second part concentrates on the more classical definition, the one with diffuse and ill-defined early OA as a start for the disease affecting the whole joint (diffuse early OA).
An arthroscopic analysis would have to be performed in all three knee compartments, analysing the surfaces separately from each other (medial femoral, medial tibial, lateral femoral, lateral tibial, femoral trochlea, patella). The injured area has to be classified after the ICRS scoring system from grades 1–4 (www.cartilage.org/_files/contentmanagment/ICRS_evaluation.pdf).
Traumatic lesions of hyaline cartilage represent focal defects with sharp defect edges. The surrounding cartilage usually shows no sign of injury or degeneration (no perilesional early OA = grade 0). The injured area can be classified after the ICRS scoring system from grades 1–4 (www.cartilage.org/_files/contentmanagment/ICRS_evaluation.pdf). Early stages of cartilage injury may be addressed with conservative treatment, and cartilage defects (ICRS grades 3–4) can successfully be treated by regenerative cartilage therapy according to the national treatment guidelines [39].
Degeneration around the focal cartilage defect can be staged and graded also arthroscopically according to the histomorphometrical OARSI criteria [35]. The defect edges are mainly of round shape. Degeneration includes softening and swelling as well as fibrillation of the cartilage surface. The surrounded area should not show lesions greater than ICRS grade 2 in depth (grade of OA). Otherwise, this area should be included in the main cartilage defect and be treated with regenerative treatment approaches. In case of a second defect with ICRS grades 3 and 4 in the same compartment, which cannot be included in the primary defect, both focal defects should be scored separately and added to a final score. The size of the degenerated area around a focal cartilage defect seems to be clinical relevant. Therefore, the area of degeneration is staged according to the histopathological classification of the OARSI in five steps (stage of OA; Table 2). Additional cartilage lesions on the opposite surface of the same compartment or in other compartments are included in the staging, because they potentially influence the overall prognosis of the whole joint and of the regenerative treatment approach in particular. This additional scoring (A–D) depends on the grade and the location of the additional lesion (Table 3).
Diffuse early OA describes a joint damage without signs of a focal lesion, but with degeneration in at least two compartments of a knee joint. The classification is based on the grade (1–4) and the stage (A–E) of the degeneration (Table 3). According to the histopathological classification of early OA of the OARSI [35], diffuse cartilage damage is limited to ICRS ≤2. With higher grades of degeneration in the joint compartments (grades 3–4), signs of late OA with joint line narrowing may be present, which would exclude the use of the early OA classification.
Histopathological definition of early OA
Early OA affects the articular cartilage and the subchondral bone, besides the other structures of the joint. Following the phase of very early hypertrophic repair [1], early events in the disease involve the disruption of the articular chondrocyte pericellular matrix, linked to an abnormal activation of cell surface receptors [59]. The synthesis of superficial zone proteins (e.g. lubricin) is altered, resulting in an impaired surface lubrication [46, 49] and an increase in friction [15, 38]. The proteoglycan content of the surface is also reduced, and the superficial parts of the type 2 collagen network eroded and subsequently exposed. The superficial chondrocytes alter their spatial organisation and form clusters. Whether the loss of proteoglycans may be reversible, the erosion of the collagens is a critical point, since this damaged collagen network cannot be regenerated [23]. Small collagen fragments are released, activating an inflammatory cascade within the cartilage and causing inflammation of the synovial membrane and effusion [51]. Such a chronic low-grade inflammation, termed ‘microinflammation’, is often present for longer time periods than previously acknowledged, possibly further contributing to the early disruption of cartilage homeostasis [23]. Catabolic activities are increased [24, 28], leading to biochemical changes in the composition of the extracellular matrix (ECM), causing a change in its water-binding capacity with a reduced mechanical strength (chondromalacia), leading to a higher deformation of the cartilage under load [47]. Starting with superficial fibrillation, the superficial structure is progressively lost, fissures appear, and the cartilage thickness is reduced [35].
The subchondral bone plays an important role in early OA, as it is altered at very early stages [16, 26]. Early changes, among which a progressive increase in subchondral plate and subarticular spongiosa thickness [31, 41] occurs mainly in the regions of cartilage damage [12, 44], diminish its biomechanical properties [32]. The primacy of alterations in the cartilage versus subchondral bone remains yet to be determined, although a close crosstalk within the osteochondral unit exists [21, 41].
A comparison of the different grades of the macroscopic ICRS and the histological OARSI criteria in the context of early OA identifies corresponding grades (Table 4). In general, early OA can be considered to include a maximal involvement of 50 % of the cartilage thickness based on the macroscopic ICRS classification. This end point, which can be arthroscopically detected, reflects an OARSI grade 4 (e.g. erosion into the mid-zone of cartilage) [43]. If more than 50 % of its thickness is lost (either of the perilesional cartilage surrounding a deeper focal lesion or diffusely affected cartilage), the term ‘early OA’ may not be used anymore (Table 4). Practically, this necessitates a separate assessment of a focal cartilage defect (e.g. applying the ICRS criteria) and the cartilage surrounding the lesion.
Assuming that an involvement of deeper cartilage layers indicates advanced disease, the grade of OA reflects its depth progression into cartilage, while the stage describes its horizontal extent of involvement over a joint compartment [43]. The combined assessment of grade and stage is termed score. Since the stage relates to the horizontal extent of cartilage involvement, stages of 1 (<10 % of joint involvement) until stage 4 (more than 50 % of joint involvement) are possible.
Radiographic definition of early OA
Common radiological scores, such as the Fairbank or K&L score, are descriptive, and the individual items they contain are rather difficult to define, hence affecting their objectivity. The K&L score is currently considered as the gold standard for radiographic OA [8], despite the fact that it lacks precision, especially in the early stages of OA [19]. It considers the following four items for OA grading: height of the joint line, the presence or absence of osteophytes, subchondral sclerosis, and bone deformity. In the K&L score, joint space width is roughly graded as not altered (0 points), doubtfully narrowed (1 point), possibly narrowed (2 points), and definitively narrowed with possible bony deformity (3 points) compared to the normal joint. Because of its importance from a clinical and especially surgical perspective, the evolution of the process of joint space narrowing has been recently studied [11, 40]. However, the important relation between the structural damage and radiographic observation of the joint collapse as an irreversible functional end point has not received the deserved attention over many years [8]. Several factors, like the loss or the functional insufficiency of menisci and/or cartilage, may contribute to this phenomenon, but their individual contribution to the evolution of the disease is not really known. Especially for the early stages of OA, this might be of significant importance as it might help developing surgical strategies to prevent its progression [9, 18, 45]. Developing methods for an increased understanding of the process of joint line narrowing is therefore of utmost importance. As categorical methods such as the K&L score are statistically less powerful, the development of continuous methods is warranted [36]. Establishing a quantitative instead of a descriptive radiographic analysis of joint line narrowing with a precise measurement method should be a first step to allow for a better comparability and reproducibility of individual radiographs [11, 40]. To develop a radiological score for the individual knee compartments, radiographs in an anteroposterior weight-bearing view with the knee in full extension (anteroposterior; AP) and an AP weight-bearing view with the knee flexed at 45° (Schuss) may be helpful. Bony landmarks of the knee compartments, as well as a strategy to define an objective and quantitative measure of the joint space height, are needed. The height of the affected compartment could be measured in its centre and be normalised with respect to its width. Of note, determination of a cut-off value below of which the height of the joint space has to be considered as pathologic is mandatory. Such a score needs to yield a high reproducibility and a good correlation to long-established descriptive radiological scores. It would be useful for detecting early OA the affected tibiofemoral compartment and for a better follow-up of OA progression. Similar approaches could be developed for the patellofemoral compartment.
MRI definition of early OA
Imaging with MRI has a higher sensitivity for early OA than radiographs, where the disease is visible in a relatively late stage. In particular, studies with 3D MRI (MRI-based 3D bone shape) have been shown to effectively predict the development of a radiographic knee OA [37]. There is a relative consensus in the literature that MRI is an important tool to assess and to stage early knee OA. It allows to detect anatomical modifications such as bone marrow oedema, meniscal degeneration, synovial proliferation, and subchondral bone sclerosis which are typically non-detectable with traditional X-rays in early-stage OA. According to the European Society of Sports Traumatology, Knee Surgery and Arthroscopy (ESSKA), criteria for defining early OA MRI findings include [34]:
-
Cartilage Morphology Scores grade 3 or higher [Whole-Organ Magnetic Resonance Imaging Score (WORMS) grades 3–6].
-
Cartilage Regional loss Score grade 2 or higher (BLOKS grades 2 and 3).
-
Meniscal tears grade 3 or higher.
-
Bone marrow lesions graded by size (WORMS grades 2 and 3).
These criteria are still the standard reference in defining early OA and are applicable to the standard MRI images available in everyday practice. In the past few years, many cartilage-focused sequences have been presented, promoting MRI in assessing early and pre-symptomatic modifications of the OA joint [42]. Modern quantitative MRI techniques are promising as they allow evaluating structural and mechanical modifications of damaged cartilage and eventually comparing them with healthy cartilage, with the possibility of very precise defect measure using 3D MRI sequences. The role of MRI could be even more important in evaluating biological modifications: glycosaminoglycan content of cartilage can be estimated using delayed Gadolinium-enhanced MRI (dGEMRIC), and advanced techniques such as T2 mapping or diffusion-weighted imaging (DWI) are employable for quantitative and qualitative evaluation of damaged cartilage.
MRI may also detect subchondral bone alterations which are the increase in density, direct consequence of thickening of subchondral plate, and subsequent bone remodelling. Application of advanced compositional techniques in detecting early-stage OA has been extensively studied in experimental models, but there is a lack of clinical studies focusing on the application and feasibility of these techniques [17, 22]. T2 mapping and dGEMRIC can be relatively easily applied to standard platforms, while others such as T1 rho or sodium depletion studies require dedicated software and high-field MRI systems which still are not realistically applicable in everyday clinical practice. Although applicability and reliability of each of these techniques is not yet determined, MRI already plays a decisive role in diagnosis and in addressing both conservative and surgical treatments, and its role will surely be further implemented. For this reason, it is of the utmost importance to establish a broad consensus on the use of evolving MRI sequences in diagnostic protocols and staging of early OA in everyday practice.
Biomarkers in early OA
The limitations of the currently available diagnostic tests led to an increasing interest in new specific biomarkers for the diagnosis of early OA. A biomarker is ‘a characteristic that is objectively measured and evaluated as an indicator of normal biological process, pathogenic processes, or pharmacological responses to a therapeutic intervention’ [22]. Biochemical markers are the most promising disease markers currently explored for the identification of early OA predictors. Structural molecules or fragments linked to cartilage, bone, and synovium are likely the best candidates reflecting dynamic and quantitative changes in joint remodelling and therefore disease progression, such as biomarkers related to collagen and aggrecan metabolism, and other non-collagenous proteins. Alternatives are investigated among molecules associated with other processes, such as inflammation or fibrosis [33]. In order to provide a common framework for communication in the field, the Burden of Disease, Investigative, Prognostic, Efficacy of Intervention and Diagnostic (BIPEDS) classification has been proposed to categorise OA biomarkers according to their predictive potential [5].
Beside the diagnostic ability to distinguish between individuals with or without OA and to document its severity, biomarkers may have a prognostic value to determine the risk in patients without overt disease and the clinical outcome in those with symptoms of OA. In addition, biomarkers may be used to evaluate the efficacy of potential treatments. Finally, other categories include investigative (with still insufficient evidence to fit in other categories) and safety biomarkers for invasive investigations [30].
Sources for biomarkers are blood and other body fluids (plasma and/or serum, urine). However, secreted molecules are often diluted and may become undetectable. An even more important limitation is that systemic biomarkers may be confounded by other physiological or pathological processes originating from other tissues. On the other hand, also the evaluation of synovial fluid presents some limitations: while it bathes the intrinsic structures of diarthrodial joints offering the unique opportunity to study the entire joint, it is more difficult to obtain in the clinical practice, and its evaluation sometimes limited by the small quantity [7].
Despite the high research efforts, no single OA biomarker stands yet out as a gold standard and has been sufficiently validated for a systematic use, and only a few of them present robust evidence. Among these, uCTX-II (urinary C-terminal telopeptide of collagen type 2) holds possible promise as useful incidence and prognostic marker, and serum cartilage oligomeric protein (COMP) presents some supporting evidence to be an indicator of the presence, incidence, and progression of OA of the knee [48, 56]. Data on other inflammation markers such as C-reactive protein (considered linked to the disease progression) are not (yet) conclusive [48].
Some intrinsic limitations of biomarkers (e.g. no linear correlation with structural changes, not well-defined distinction from age-related changes, unrecognised heterogeneous disease phenotypes) make their use as primary end point unlikely. Conversely, there is increasing evidence that they could be valuable secondary end points, likely through the use of cluster of biomarkers, and even more with the combination of biomarker panels and other parameters (such as the new MRI technologies) into single diagnostic tests to better identify patients in the early OA phase.
Future directions
Musculoskeletal disorders and diseases are the leading cause of disability. Over half of adults 50 and older in the Western world have a chronic musculoskeletal condition. In the USA, the economic burden is considerable; the cost of musculoskeletal conditions is approaching $1 trillion annually, which represents over 7.4 % of the gross domestic product. The societal cost for the treatment for OA alone has surpassed that of both cardiovascular disease and cancer. Though still in its infancy, biological treatments for this burdensome disorder have been the focus of intense investigations. In spite of recent advances, significant divergence of opinion on the future of early detection and biological treatments for orthopaedic injuries remains. Even though new biomarkers for the early detection of OA are promising [29], there is a considerable need to improve scientific knowledge, expand technical capacities, and advance clinical practice through the acceleration of translational research and the identification of areas of high yield research topics in this area of detection and treatments.
It may be beneficial in view of the emerging new treatment approaches to clearly distinguish patient populations with knee pathology. For example, patients may be characterised as having tissue lesions (meniscus, cartilage, ligaments) but otherwise healthy joints, lesions in the context of early OA, displaying a number of changes in the joint tissues suggestive of emerging joint disease, and finally well-established OA as defined by the existing American College of Rheumatology (ACR) criteria. This will hopefully lead to improved treatment algorithms in specific patient populations. The above-presented classification criteria appear practical to the authors and applicable in daily practice. They will most probably further evolve as new insights and technologies become available.
References
Adams ME, Brandt KD (1991) Hypertrophic repair of canine articular cartilage in osteoarthritis after anterior cruciate ligament transection. J Rheumatol 18(3):428–435
Arendt EA, Dahm DL, Dejour D, Fithian DC (2014) Patellofemoral joint: from instability to arthritis. Instr Course Lect 63:355–368
Baert IA, Jonkers I, Staes F, Luyten FP, Truijen S, Verschueren SM (2012) Gait characteristics and lower limb muscle strength in women with early and established knee osteoarthritis. Clin Biomech (Bristol, Avon) 28(1):40–47
Batsis JA, Germain CM, Vasquez E, Zbehlik AJ, Bartels SJ (2015) Physical activity predicts higher physical function in older adults: the osteoarthritis initiative. J Phys Act Health. doi:10.1123/jpah.2014-0531
Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, Heinegard D, Jordan JM, Kepler TB, Lane NE, Saxne T, Tyree B, Kraus VB (2006) Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthr Cartil 14(8):723–727
Bijlsma JW, Berenbaum F, Lafeber FP (2011) Osteoarthritis: an update with relevance for clinical practice. Lancet 377(9783):2115–2126
Blanco FJ (2014) Osteoarthritis year in review 2014: we need more biochemical biomarkers in qualification phase. Osteoarthr Cartil 22(12):2025–2032
Brandt KD, Fife RS, Braunstein EM, Katz B (1991) Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum 34(11):1381–1386
Brouwer GM, van Tol AW, Bergink AP, Belo JN, Bernsen RM, Reijman M, Pols HA, Bierma-Zeinstra SM (2007) Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum 56(4):1204–1211
Case R, Thomas E, Clarke E, Peat G (2015) Prodromal symptoms in knee osteoarthritis: a nested case-control study using data from the osteoarthritis initiative. Osteoarthr Cartil 23(7):1083–1089
Chan WP, Huang GS, Hsu SM, Chang YC, Ho WP (2008) Radiographic joint space narrowing in osteoarthritis of the knee: relationship to meniscal tears and duration of pain. Skelet Radiol 37(10):917–922
Chiba K, Ito M, Osaki M, Uetani M, Shindo H (2011) In vivo structural analysis of subchondral trabecular bone in osteoarthritis of the hip using multi-detector row CT. Osteoarthr Cartil 19(2):180–185
Crawford DC, Safran MR (2006) Osteochondritis dissecans of the knee. J Am Acad Orthop Surg 14(2):90–100
Culvenor AG, Collins NJ, Guermazi A, Cook JL, Vicenzino B, Khan KM, Beck N, van Leeuwen J, Crossley KM (2015) Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheumatol 67(4):946–955
Desrochers J, Amrein MW, Matyas JR (2013) Microscale surface friction of articular cartilage in early osteoarthritis. J Mech Behav Biomed Mater 25:11–22
Dieppe P, Cushnaghan J, Young P, Kirwan J (1993) Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis 52(8):557–563
Felson DT, Hodgson R (2014) Identifying and treating preclinical and early osteoarthritis. Rheum Dis Clin N Am 40(4):699–710
Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, Lewis CE, Aliabadi P, Sack B, McCulloch C, Zhang Y (2008) A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol 35(10):2047–2054
Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P (2011) Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis 70(11):1884–1886
Fingleton C, Smart K, Moloney N, Fullen BM, Doody C (2015) Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil 23(7):1043–1056
Funck-Brentano T, Cohen-Solal M (2011) Crosstalk between cartilage and bone: when bone cytokines matter. Cytokine Growth Factor Rev 22(2):91–97
Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ (2015) Osteoarthritis. Lancet 386(9991):376–387
Goldring MB, Berenbaum F (2015) Emerging targets in osteoarthritis therapy. Curr Opin Pharmacol 22:51–63
Goldring MB, Marcu KB (2009) Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther 11(3):224
Hensor EM, Dube B, Kingsbury SR, Tennant A, Conaghan PG (2014) Toward a clinical definition of early osteoarthritis: onset of patient-reported knee pain begins on stairs. Data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 67(1):40–47
Hutton CW, Higgs ER, Jackson PC, Watt I, Dieppe PA (1986) 99mTc HMDP bone scanning in generalised nodal osteoarthritis. I. Comparison of the standard radiograph and four hour bone scan image of the hand. Ann Rheum Dis 45(8):617–621
Jones LD, Bottomley N, Harris K, Jackson W, Price AJ, Beard DJ (2014) The clinical symptom profile of early radiographic knee arthritis: a pain and function comparison with advanced disease. Knee Surg Sports Traumatol Arthrosc 24(1):161–168
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H (2010) Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 7(1):33–42
Karlsen TA, Jakobsen RB, Mikkelsen TS, Brinchmann JE (2013) microRNA-140 targets RALA and regulates chondrogenic differentiation of human mesenchymal stem cells by translational enhancement of SOX9 and ACAN. Stem Cells Dev 23(3):290–304
Kraus VB (2011) Osteoarthritis year 2010 in review: biochemical markers. Osteoarthr Cartil 19(4):346–353
Kraus VB, Feng S, Wang S, White S, Ainslie M, Graverand MP, Brett A, Eckstein F, Hunter DJ, Lane NE, Taljanovic MS, Schnitzer T, Charles HC (2013) Subchondral bone trabecular integrity predicts and changes concurrently with radiographic and magnetic resonance imaging-determined knee osteoarthritis progression. Arthritis Rheum 65(7):1812–1821
Li B, Aspden RM (1997) Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis 56(4):247–254
Lotz M, Martel-Pelletier J, Christiansen C, Brandi ML, Bruyere O, Chapurlat R, Collette J, Cooper C, Giacovelli G, Kanis JA, Karsdal MA, Kraus V, Lems WF, Meulenbelt I, Pelletier JP, Raynauld JP, Reiter-Niesert S, Rizzoli R, Sandell LJ, Van Spil WE, Reginster JY (2014) Republished: value of biomarkers in osteoarthritis: current status and perspectives. Postgrad Med J 90(1061):171–178
Luyten FP, Denti M, Filardo G, Kon E, Engebretsen L (2011) Definition and classification of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 20(3):401–406
Madry H, Luyten FP, Facchini A (2012) Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc 30(3):407–422
Muraki S, Akune T, En-Yo Y, Yoshida M, Suzuki T, Yoshida H, Ishibashi H, Tokimura F, Yamamoto S, Tanaka S, Nakamura K, Kawaguchi H, Oka H, Yoshimura N (2015) Joint space narrowing, body mass index, and knee pain: the ROAD study (OAC1839R1). Osteoarthr Cartil 23(6):874–881
Neogi T, Bowes MA, Niu J, De Souza KM, Vincent GR, Goggins J, Zhang Y, Felson DT (2013) Magnetic resonance imaging-based three-dimensional bone shape of the knee predicts onset of knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Rheum 65(8):2048–2058
Neu CP, Reddi AH, Komvopoulos K, Schmid TM, Di Cesare PE (2010) Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum 62(9):2680–2687
Niemeyer P, Andereya S, Angele P, Ateschrang A, Aurich M, Baumann M, Behrens P, Bosch U, Erggelet C, Fickert S, Fritz J, Gebhard H, Gelse K, Gunther D, Hoburg A, Kasten P, Kolombe T, Madry H, Marlovits S, Meenen NM, Muller PE, Noth U, Petersen JP, Pietschmann M, Richter W, Rolauffs B, Rhunau K, Schewe B, Steinert A, Steinwachs MR, Welsch GH, Zinser W, Albrecht D (2013) Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group “Tissue Regeneration” of the German Society of Orthopaedic Surgery and Traumatology (DGOU). Z Orthop Unfall 151(1):38–47
Oka H, Muraki S, Akune T, Mabuchi A, Suzuki T, Yoshida H, Yamamoto S, Nakamura K, Yoshimura N, Kawaguchi H (2008) Fully automatic quantification of knee osteoarthritis severity on plain radiographs. Osteoarthr Cartil 16(11):1300–1306
Orth P, Cucchiarini M, Wagenpfeil S, Menger MD, Madry H (2014) PTH [1-34]-induced alterations of the subchondral bone provoke early osteoarthritis. Osteoarthr Cartil 22(6):813–821
Palmer AJ, Brown CP, McNally EG, Price AJ, Tracey I, Jezzard P, Carr AJ, Glyn-Jones S (2013) Non-invasive imaging of cartilage in early osteoarthritis. Bone Joint J 95(6):738–746
Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB (2006) Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil 14(1):13–29
Radin EL, Paul IL, Tolkoff MJ (1970) Subchondral bone changes in patients with early degenerative joint disease. Arthritis Rheum 13(4):400–405
Reijman M, Pols HA, Bergink AP, Hazes JM, Belo JN, Lievense AM, Bierma-Zeinstra SM (2007) Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis 66(2):158–162
Ritter SY, Collins J, Krastins B, Sarracino D, Lopez M, Losina E, Aliprantis AO (2014) Mass spectrometry assays of plasma biomarkers to predict radiographic progression of knee osteoarthritis. Arthritis Res Ther 16(5):456
Ryd L, Brittberg M, Eriksson K, Jurvelin JS, Lindahl A, Marlovits S, Moller P, Richardson JB, Steinwachs M, Zenobi-Wong M (2015) Pre-osteoarthritis: definition and diagnosis of an elusive clinical entity. Cartilage 6(3):156–165
Saberi Hosnijeh F, Runhaar J, van Meurs JB, Bierma-Zeinstra SM (2015) Biomarkers for osteoarthritis: can they be used for risk assessment? A systematic review. Maturitas 82(1):36–49
Sakata R, McNary SM, Miyatake K, Lee CA, Van den Bogaerde JM, Marder RA, Reddi AH (2015) Stimulation of the superficial zone protein and lubrication in the articular cartilage by human platelet-rich plasma. Am J Sports Med 43(6):1467–1473
Saris DB, Dhert WJ, Verbout AJ (2003) Joint homeostasis. The discrepancy between old and fresh defects in cartilage repair. J Bone Joint Surg Br 85(7):1067–1076
Scanzello CR, Goldring SR (2012) The role of synovitis in osteoarthritis pathogenesis. Bone 51(2):249–257
Schinhan M, Gruber M, Vavken P, Dorotka R, Samouh L, Chiari C, Gruebl-Barabas R, Nehrer S (2012) Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J Orthop Res 30(2):214–220
Steinwachs MR, Engebretsen L, Brophy RH (2012) Scientific evidence base for cartilage injury and repair in the athlete. Cartilage 3(1 Suppl):11S–17S
Thorstensson CA, Andersson ML, Jonsson H, Saxne T, Petersson IF (2009) Natural course of knee osteoarthritis in middle-aged subjects with knee pain: 12-year follow-up using clinical and radiographic criteria. Ann Rheum Dis 68(12):1890–1893
Tsezou A (2014) Osteoarthritis year in review 2014: genetics and genomics. Osteoarthr Cartil 22(12):2017–2024
Van Spil WE, Welsing PM, Bierma-Zeinstra SM, Bijlsma JW, Roorda LD, Cats HA, Lafeber FP (2015) The ability of systemic biochemical markers to reflect presence, incidence, and progression of early-stage radiographic knee and hip osteoarthritis: data from CHECK. Osteoarthr Cartil 23(8):1388–1397
Vincent HK, Heywood K, Connelly J, Hurley RW (2012) Obesity and weight loss in the treatment and prevention of osteoarthritis. PM R 4(5 Suppl):S59–S67
Wada M, Imura S, Baba H, Shimada S (1996) Knee laxity in patients with osteoarthritis and rheumatoid arthritis. Br J Rheumatol 35(6):560–563
Xu L, Polur I, Servais JM, Hsieh S, Lee PL, Goldring MB, Li Y (2011) Intact pericellular matrix of articular cartilage is required for unactivated discoidin domain receptor 2 in the mouse model. Am J Pathol 179(3):1338–1346
Zeggini E, Panoutsopoulou K, Southam L, Rayner NW, Day-Williams AG, Lopes MC, Boraska V, Esko T, Evangelou E, Hoffman A, Houwing-Duistermaat JJ, Ingvarsson T, Jonsdottir I, Jonnson H, Kerkhof HJ, Kloppenburg M, Bos SD, Mangino M, Metrustry S, Slagboom PE, Thorleifsson G, Raine EV, Ratnayake M, Ricketts M, Beazley C, Blackburn H, Bumpstead S, Elliott KS, Hunt SE, Potter SC, Shin SY, Yadav VK, Zhai G, Sherburn K, Dixon K, Arden E, Aslam N, Battley PK, Carluke I, Doherty S, Gordon A, Joseph J, Keen R, Koller NC, Mitchell S, O’Neill F, Paling E, Reed MR, Rivadeneira F, Swift D, Walker K, Watkins B, Wheeler M, Birrell F, Ioannidis JP, Meulenbelt I, Metspalu A, Rai A, Salter D, Stefansson K, Stykarsdottir U, Uitterlinden AG, van Meurs JB, Chapman K, Deloukas P, Ollier WE, Wallis GA, Arden N, Carr A, Doherty M, McCaskie A, Willkinson JM, Ralston SH, Valdes AM, Spector TD, Loughlin J (2012) Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet 380(9844):815–823
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madry, H., Kon, E., Condello, V. et al. Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 24, 1753–1762 (2016). https://doi.org/10.1007/s00167-016-4068-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-016-4068-3