Abstract
Purpose
Contemporary data on mortality of hematological patients admitted to the intensive care unit (ICU) are missing. In a Danish nationwide set-up, we assessed 30-day and 1-year mortality in this population including impact of age and comorbidity, with non-hematological patients as reference.
Methods
This population-based cohort study included all non-surgical patients > 15 years of age admitted to an ICU in Denmark between 2010 and 2015. Data on hematological malignancies were obtained from the Danish Hematological Database, and information on the Charlson Comorbidity Index was obtained from the Danish National Patient Registry. Thirty-day and 1-year mortality was estimated using the Kaplan–Meier method. We used Cox proportional hazards regression to estimate hazard ratios.
Results
We included 2122 ICU patients with a hematological malignancy and 88,951 non-hematological ICU patients. The 30-day mortality was 44% (95% confidence interval: 42–47%) among hematological patients and 27% (27–27%) among non-hematological patients. Similarly, 1-year mortality was 66% (64–68%) and 37% (37–37%), respectively. The corresponding hazard ratio with adjustment for age, sex, and comorbidity was 1.62 (1.54–1.71). Excess mortality was observed in all subgroups of age or of comorbidity. For example, the 1-year mortality for patients with Charlson Comorbidity Index Score > 3: 70% (66–74%) among hematological patients and 62% (61–63%) among non-hematological patients.
Conclusion
ICU patients with hematological malignancy had higher mortality than other ICU patients. However, one third of critically ill patients with a hematological malignancy is alive 1 year after ICU admission
Similar content being viewed by others
Avoid common mistakes on your manuscript.
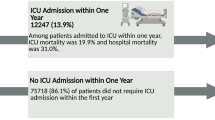
In this population-based cohort of more than 2000 patients with hematological malignancies admitted to the intensive care unit, one third was still alive 1 year after admission. Mortality was higher than in non-hematological patients, a difference that was reduced but did not disappear with increasing age and comorbidity. |
Introduction
Survival is improving for hematological malignancies in general. In Europe, 5-year survival increased by more than 10% for a number of hematological malignancies during a 10-year period from 1997 to 2008 [1]. Similarly, for critically ill hematological patients, a recent publication found an annual mortality reduction of 7% [2]. The reduction was found even though severity of critical illness increased in the same period. Nevertheless, admissions to the intensive care unit (ICU) for patients with hematological malignancies are still subject to controversies, and robust contemporary evidence is needed to reduce reluctance to admit hematological patients to the ICU. Up-to-date information is becoming increasingly important with the explosion of new treatments available for some of the most common hematological malignancies [3, 4].
Despite increased focus on critically ill patients with hematological malignancies, the currently available data have several limitations. Many reports do not distinguish patients with solid tumors and hematological malignancies [5, 6]. Restriction to hematological patients is important because of the different nature of the malignancy often involving immune paresis, either as an effect of the malignancy itself or as an effect of high-dose combination immuno-chemotherapy. Second, studies that include follow-up time beyond ICU discharge have substantial drop-out rates, for example almost 10% at 1 year follow-up in an otherwise well performed study in France and Belgium [7]. Third, so far, no studies include non-hematological patients, which allows for direct comparison of mortality. Lastly, detailed comorbidity data are often lacking.
The aim of the current study was to assess characteristics and outcome among patients with hematological malignancies treated on contemporary treatment protocols admitted to the ICU in a population-based hospital setting with complete follow-up. Specifically, we investigated mortality up to 1 year from ICU admission and ICU admission characteristics for patients with hematological malignancies overall, in subgroups of hematological diagnosis, age and comorbidity, and with non-hematological ICU patients as reference.
Methods
Setting
The study was performed in Denmark with inclusion of data from 2010 to 2015. The Danish health care system is tax-supported with equal access to care regardless of economic status. A unique personal identification number allows linkage of a number of different nationwide registries with complete coverage and information that have been validated also for research purposes [8].
Study population
Our study population comprised all patients admitted to the ICU at a Danish hospital from January 1, 2010 to December 31, 2015. Specifically, we used the Danish Intensive Care Database, which is a nation-wide registry. The coverage of the registry is considered to be ~ 95% [9]. For the current study, we included only first-time ICU admissions. We excluded patients under age 15 years. Patients coded with a surgical procedure on the day of or 1 day prior to ICU admission were considered admitted for a surgical reason and were excluded. Patients included had to have a Danish personal identification number to allow for linkage between registries [8]. A flowchart showing exclusion is given in Supplementary Fig. 1.
Exposure status
Patients with hematological malignancies were identified from the Danish Hematological Database. The database contains data on all incidences of hematological malignancies in Denmark. The content of the database has been described in detail in the four different parts of the registry: The Danish National Acute Leukemia Registry [10], the Danish National Lymphoma Registry [11], the Danish National Multiple Myeloma Registry [12], and the Danish National Chronic Myeloproliferative Neoplasia Registry [13]. The coverage of the registries varies but are generally high; 99.6% for acute leukemias [14], ~ 90% for myelodysplastic syndrome [10], 94.9% for lymphomas [11], ~ 100% for multiple myeloma [12], and > 90% for chronic myeloproliferative neoplasms (CMPD) [13]. The database includes data on the specific malignancy, date of diagnosis, and WHO performance score. For each of the registries, a number of clinical and biochemical data from time of diagnosis are collected. However, although some treatment information is reported, the completeness of these data is too low for general use. For hematological patients, only ICU admissions after the date of diagnosis of their malignancy was included. In cases where individual patients received more than one hematological diagnosis, the most recent was used in the analysis. Our main analysis included all patients with hematological malignancies but in subsequent analyses we stratified by type of hematological malignancy into acute leukemia, multiple myeloma, CMPD, and lymphoma. Low-risk myelodysplastic syndrome was included in the CMPD group and chronic lymphocytic leukemia was included in the lymphoma group. We further stratified by disease aggressiveness. High-grade disease included acute leukemia, Burkitt lymphoma, diffuse large B-cell lymphoma, other high-grade B-cell lymphomas, Hodgkin lymphoma, and T-cell lymphomas, and low-grade disease included all other hematological malignancies.
Outcome and follow-up
The outcome of interest in this study was all-cause mortality. Individuals were followed from the time of admission to the ICU to 1 year from admission, emigration, or 31 December 2015 using the Danish Civil Registration System. The information in this registry is virtually complete with a prevalence of disappeared persons of only 0.3%. The registry also contains information on date of birth and sex [8]. In a landmark analysis to assess outcome following UCI discharge, follow-up started at the time of hospital discharge.
Covariates
From the Danish Intensive Care Database, we collected information on the date and time of ICU admission, type of admission, organ-supportive treatments, and Simplified Acute Physiology Score (SAPS) II. For most variables, the completeness is 100% throughout the study period, for other variables like mechanical ventilation, the completeness increased from 64% in the beginning of the study period to 95% in 2015. SAPS II completeness ranged from 24% in 2010 to ≈ 75% in the following years [9]. From the Danish Hematological Database, we collected WHO performance status at the time of diagnosis of hematological malignancy (completeness ≈99%) [10,11,12,13]. To quantify the level of comorbidity, we used Charlson Comorbidity Index score. The score was generated using the Danish National Patients Registry and to obtain a meaningful comparison of the hematological and non-hematological patient cohort, the hematological malignancy was omitted when calculating the comorbidity index [15]. The Danish National Patients Registry includes information on all hospital admissions since 1977. Each hospital admission initiates a record, which includes the personal identification number of the patient, admission and discharge dates, a primary discharge diagnosis, and supplementary diagnoses coded according to the International Classification of Diseases (ICD), 7th (1977–1993) and 10th revision (1994–today). No specific diagnosis code is reported for ICU admissions; hence, we report the primary diagnosis as the primary diagnosis for the entire admission. For specific diagnosis codes, see Supplementary Table 1. Patients with non-hematological malignancies were defined as patients registered with a cancer diagnosis (excluding non-melanoma skin cancers and hematological cancer) in the National Registry of Patients within 5 years of the ICU admission.
Statistical analysis
Categorical variables are presented as frequencies and continuous variables as medians [with 1st and 3rd quartiles (Q25–Q75)]. Mortality probabilities were plotted using 1-Kaplan–Meier survival curves and reported at 30 days and 1 year with 95% confidence intervals (CI). We compared mortality between hematological and non-hematological patients using Cox proportional hazards with five different models. In addition to a crude model, we ran four different adjusted models accounting also for clustering by ICU admission unit (see below). In model 1, we adjusted for sex, age (continuous, linear), year of admission (categorical), and Charlson Comorbidity Index Score (categorical). In model 2 and 3, we investigated how the hazard ratio changed with inclusion of information on the primary cause of admission and SAPS II, respectively. Model 4 was a sensitivity analysis only including patients without missing SAPS II. Model 1 was considered the primary analysis because it did not include information obtained after ICU admission and therefore more informative in relation to the main aim of the study. The proportional hazards assumption was checked using Log–Log plots and found to be met. More sophisticated modelling of the continuous variable age such as spline or fractional polynomial approaches did not alter the estimates, and therefore simple linearity was preferred (results not shown). SAPS II was modelled as a spline (natural cubic, 8 knots) due to a moderate effect on the estimates. Missing information on the SAPS II variable was handled by multiple imputation (30 imputations) under the assumption that these data were missing at random. The predictive mean matching method was used to produce the imputed values [16]. The estimates of each imputation were combined using Rubin’s rules [17]. The justification of the missing at random assumption was investigated by inspecting the patient characteristics of putative imputation variables by missing status of SAPS II. Subsequently, logistic regression was used to confirm that the variables predicted missing SAPS II to some extent (results not shown). The variables included in the imputation were all of those entered in the Cox proportional hazards models (exposure, outcome, and covariates). We further included the following auxiliary variables to aid in the prediction of SAPS II: hematological malignancy, indicator of whether the patient was alive at discharge, the Nelson–Aalen cumulative hazard of all-cause mortality [18], hospital type, need for mechanical ventilation, renal replacement therapy, and vasopressors, admission time (daytime/nighttime), prior surgery, and geographic region of hospital. We used odds ratios with 95% CI to compare characteristics between patients with hematological and non-hematological malignancies. We specified ICU admission unit as a clustering variable (specified in an ID statement in the PHREG procedure in SAS) and used robust variance estimation to account for clustering in the adjusted models (clustered Cox models). In accordance with Danish law regarding handling of registry data, there was no requirement of an ethics committee approval [19]. The study was approved by the Danish Data Protection Agency (record number: 1-16-02-500-15). All analyses were performed in SAS 9.4 (SAS Institute).
Results
Characteristics
Our cohort included 91,073 adult patients admitted for the first time to an ICU during the study period; 2122 patients with a hematological malignancy and 88951 without a hematological malignancy. Table 1 gives basic demographic information. The median age was 69 years (interquartile range: 55—83) among hematological patients and 67 years (interquartile range: 44–90) among non-hematological patients. At diagnosis, most hematological patients had a WHO performance score of 0 or 1 (65%). The median time from diagnosis of hematological malignancy to ICU admission was 0.9 years (Interquartile range: 0.2–2.8). Supplementary Table 2 gives differences between patients with hematological and non-hematological malignancies. The distribution of age and sex were similar in the two groups, but more patients with non-hematological malignancies than hematological patients had a Charlson Comorbidity index score of 0.
ICU characteristics
Table 2 gives ICU admission characteristics. For 40% of the hematological patients, the primary diagnosis for the hospitalization (including ICU stay) was registered as neoplasm. The hematological patients treated at the ICU were most commonly admitted more than 3 months from diagnosis, with patients with acute leukemia admitted closest to diagnosis; 42% were admitted during the early disease phase (under 3 months from date of diagnosis). This was more uncommon among patient with myeloma (28%), lymphoma (24%), and CMPD (19%). Use of vasopressors, mechanical ventilation, and renal replacement therapy was more common among hematological patients compared to patients with non-hematological malignancies (Supplementary Table 2).
Mortality after ICU admission
Figures 1 and 2 illustrate mortality for hematological and non-hematological patients overall and stratified by age and comorbidity. The 30-day mortality was 44.4% (95% CI: 42.3%–46.6%) among hematological patients and 27.1% (95% CI 26.8–27.4%) among non-hematological patients; 1-year mortality was 65.6% (95% CI 63.5–67.7%) and 37.1% (95% CI 36.8–37.4%), respectively. Among hematological patients who were discharged from hospital, mortality 30 days from discharge was 24.8% (95% CI 22.6–27.2%) and 1-year mortality was 51.7% (95% CI 49.0–54.5%). The corresponding mortality for non-hematological patients was 13.1% (95% CI 12.9–13.4%) and 24.1% (23.8–24.5%). Mortality differed substantially between the four groups of hematological malignancies with 1-year mortality for acute leukemia being highest: mortality of 73.6% (95% CI 68.7–78.3%) compared to lymphoma [61.6% (95% CI 58.6–64.7%)], CMPD [71.3% (66.0–76.3%)], and multiple myeloma [64.7% (95% CI 59.9–69.4%)] (see Supplementary Table 3). For high-grade disease, 1-year mortality was 68.7% (95% CI 65.5–71.9%) compared to 63.5% (95% CI 60.8–66.2%) for low-grade disease. The mortality difference between the hematological and non-hematological patients was reduced with increasing age and/or comorbidity. Nevertheless, mortality for hematological patients never reached mortality for non-hematological patients, e.g., for patients with Charlson Comorbidity Index Score > 3, the 1-year mortality among hematological patients was 70.0% (95% CI 65.7–74.2%) and 61.8% (95% CI 60.9–62.8%) among non-hematological patients. The hazard ratio for death was increased among hematological patients (Table 3). After inclusion of information on primary cause of admission [HR 1.47 (1.29–1.68)] and SAPS II [HR: 1.21 (1.06–1.38)] in the regression models, the hazard ratios were reduced but showed an increased mortality among hematological patients throughout (Supplementary Table 4). Supplementary Table 5 gives differences in characteristics for patients surviving the index admission and patients deceased before hospital discharge.
Kaplan–Meier curve for hematological and non-hematological patients from admission to 1 year after the admission the intensive care unit stratified by age and by comorbidity. a Individuals 15–59 years, b Individuals 60–69 years, and c > 70 years, d Charlson Comorbidity Index score = 0, e Charlson Comorbidity Index score = 1—3, f Charlson Comorbidity Index score > 3. In the calculation of Charlson Comorbidity Index, the hematological malignancy is omitted
Discussion
We found that mortality 30 days after ICU admission was 44% among patients with a hematological malignancy and 27% among patients without a hematological malignancy, and 1-year mortality was 66% and 37%, respectively. After adjustment for key variables including comorbidity, mortality was 1.6-fold higher among patients with hematological malignancies. The difference was reduced with increasing co-morbidity but never disappeared. The population-based design with virtually no loss to long-term follow-up and complete and validated information on outcome and exposure is unparalleled. With inclusion of 2122 patients with hematological malignancies, it is the largest study of outcomes among critically ill hematological patients. Due to the cohort size we could restrict inclusion to the most recent treatment period where most current therapeutical changes have been implemented.
Selection of patients that will benefit from ICU admission is a complex process that has to balance many inputs. This process will naturally differ between different populations and health care systems and therefore also give rise to different cohorts of ICU-admitted patients. One of the most important determinants of the composition of the ICU cohort is the availability of ICU beds. With numbers ranging from 25 ICU beds per 100,000 inhabitants in Germany, 20 in the US, and 8.2 in Spain, the variation is large among high-income countries; however, even larger in middle- and low-income countries, e.g., an estimated number of 1.8 in Mexico [20, 21]. With 6.7 ICU beds per 100,000 inhabitants, Denmark has a low to medium ICU bed capacity compared to other European countries [22]. Empirically, it would be expected that the patients included in this study were sicker and hence had a higher mortality. This could explain the slightly higher mortality compared to recent Dutch data [2]. In their study, the overall 1-year mortality was 62%, however, with restriction to the period used in this study (2010–2015) the 1-year mortality was < 60%. A number of other differences may also be important. In the study by de Vries et al., the median age in the most recently included period (mid 2009–2015) was 57 years compared to 67 years in our study. A difference that may be related to the restriction of patients treated at university hospitals. The distribution of specific hematological malignancies was comparable. One-year mortality was also slightly lower in another comparable multicenter study performed in France and Belgium [7]. Among the 1011 patients with a median age of 60 years from 17 university hospitals treated during 2010 and 2011, the 1-year mortality was 57%. Other studies report ICU-mortality estimates ranging from 34–46% [23,24,25,26], and 30-day mortality ranging from 38–49% [27, 28]. Mortality estimates from low- and middle-income countries differ but generally show substantially poorer outcomes than high-income countries [29,30,31].
Improvement in outcomes for hematological patients admitted to the ICU has long been a matter of debate [32,33,34]. Encouragingly, recent data have indicated a 7% reduction in mortality per year since 2003 [2], and overall survival increase for hematological patients during the last 15 years of 10% [1]. Confirmation of such results is central to reduce reluctance to admit hematological patient to the ICU primarily among ICU physicians. Importantly, such reluctance may in itself lead to a higher mortality, and recent consensus statements recommend early ICU admission and early intervention [35, 36]. With no studies randomizing between usual practice and an early intervention strategy, such recommendations are based on observational studies. For example, among 219 patients with cancer and acute respiratory failure the only factor that predicted 28-day mortality after multivariable adjustment was time between onset of respiratory symptoms and ICU admission [6]. Similarly, Lengline et. al assessed a strategy of early ICU admission in which newly diagnosed high-risk AML patients were admitted to the ICU without immediate need for life support including no organ dysfunction. Despite the transferal of half of the control patients to the ICU at a later point, outcomes improved by this strategy, including decreased mortality [37]. Denmark does not have an early ICU admission policy for hematological patients, and based on the current data in our study, it was not possible to distinguish between specific hematological reasons for admission, e.g., sepsis, respiratory failure, tumorlysis syndrome, etc. Nevertheless, certain important characteristics emerge. First, the hematological patients treated at the ICU were most commonly admitted more than 3 months from diagnosis, with patients with acute leukemia admitted closest to diagnosis; almost half was admitted within the 3 months from diagnosis. Overall, 51% of the hematological patients received vasopressor use, 45% needed mechanical ventilation, and 13% needed renal replacement therapy. Frequencies substantially higher than non-hematological patients but comparable to other cohorts of hematological patients with regard to mechanical ventilation and use of vasopressor use. A notable difference between studies, however, is the frequency of patients needing renal replacement therapy. Here, frequencies ranged from 15% [2], like in our study, to much more common use reported in other studies: 41% [38], 36% [25], and 26% [7].
Certain limitations apply to our study. Despite high completeness, hematological patients may be missed in the clinical hematological databases. This misclassification for exposure status would underestimate the difference in mortality between the two groups. Yet, the potential for classifying a hematological patient as non-hematological is small with the completeness of the registries well above 90% and in most instances close to 100% [10,11,12,13]. Of importance for the interpretation of the study is the composition of the comparison group of non-hematological patients. Here, we only excluded individuals that were admitted to the ICU post-surgery, all other patient groups are included. The comparison groups thereby reflected the diversity of different admission types and many different types of comorbidity, e.g., other non-hematological malignancies. We investigated main differences between these patient groups and found comparable basic characteristics but a more common use of vasopressors, mechanical ventilation, and renal replacement therapy. Lastly, reliable outcome information is pivotal in a register-based study like this. The information on vital status and date of death is > 99% complete in the Civil Registration System and therefore of no issue in this study. It can, however, be discussed what is the most reasonable follow-up time. The mortality rate for hematological patients follows a different pattern than non-hematological patients, and therefore the most meaningful follow-up period has been debated [39]. Often studies report ICU mortality, i.e., the proportion of patients diseased during ICU stay. Focus is now increasingly on longer term mortality, which may be more informative in the decision process of ICU referral. Nevertheless, with many hematological malignancies being incurable, focusing on mortality too far into the future may result in exaggerated mortality differences. To be specific, when both hematological and non-hematological patients have reconstituted after ICU admission, patients with incurable hematological malignancies will invariably have a higher mortality rate, which is unrelated to the ICU admission. Here, we decided to focus on 1-year mortality.
In conclusion, the current study underscores that with contemporary treatment regimens hematological patients admitted to the ICU have a reasonable chance of surviving past the first month and even year despite their combined critical illness and malignancy. The study also shows that mortality is still substantially higher than non-hematological patients, and that the difference tends to be reduced with increasing age and comorbidity.
References
M Sant P Minicozzi M Mounier 2014 Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study Lancet Oncol 15 931 942 https://doi.org/10.1016/s1470-2045(14)70282-7
VA Vries MC Müller SM Arbous 2018 Time trend analysis of long term outcome of patients with haematological malignancies admitted at dutch intensive care units Br J Haematol 181 68 76 https://doi.org/10.1111/bjh.15140
M Hallek 2017 Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment Am J Hematol 92 946 965 https://doi.org/10.1002/ajh.24826
JL Warren LC Harlan J Stevens 2013 Multiple myeloma treatment transformed: a population-based study of changes in initial management approaches in the United States J Clin Oncol 31 1984 1989 https://doi.org/10.1200/jco.2012.46.3323
R Rosa T Tonietto B Duso 2017 Mortality of adult critically ill subjects with cancer Respir Care 62 615 622 https://doi.org/10.4187/respcare.05210
D Mokart J Lambert D Schnell 2013 Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure Leuk Lymphoma 54 1724 1729 https://doi.org/10.3109/10428194.2012.753446
E Azoulay D Mokart F Pène 2013 Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium a groupe de recherche respiratoire en réanimation onco-hématologique study J Clin Oncol 31 2810 2818 https://doi.org/10.1200/jco.2012.47.2365
M Schmidt L Pedersen H Sørensen 2014 The Danish Civil Registration System as a tool in epidemiology Eur J Epidemiol 29 541 549 https://doi.org/10.1007/s10654-014-9930-3
C Christiansen M Møller H Nielsen S Christensen 2016 The Danish Intensive Care Database Clin Epidemiol 8 525 530 https://doi.org/10.2147/clep.s99476
L Østgård J Nørgaard K Raaschou-Jensen 2016 The Danish National Acute Leukemia Registry Clin Epidemiol 8 553 560 https://doi.org/10.2147/clep.s99460
B Arboe T El-Galaly M Clausen 2016 The Danish National Lymphoma Registry: coverage and data quality Plos One 11 e0157999 https://doi.org/10.1371/journal.pone.0157999
P Gimsing MO Holmström T Klausen 2016 The Danish National Multiple Myeloma Registry Clin Epidemiol 8 583 587 https://doi.org/10.2147/clep.s99463
M Bak E Ibfelt T Larsen 2016 The Danish National Chronic Myeloid Neoplasia Registry Clin Epidemiol 8 567 572 https://doi.org/10.2147/clep.s99462
L Østgård J Nørgaard M Severinsen 2013 Data quality in the Danish National Acute Leukemia Registry: a hematological data resource Clin Epidemiology 5 335 344 https://doi.org/10.2147/clep.s48411
SK Thygesen CF Christiansen S Christensen 2011 The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients Bmc Med Res Methodol 11 83 https://doi.org/10.1186/1471-2288-8-25
N Schenker JM Taylor 1996 Partially parametric techniques for multiple imputation Comput Stat Data Anal 22 425 446 https://doi.org/10.1016/0167-9473(95)00057-7
Rubin D (1987) Multiple Imputation for Nonresponse in Surveys.
IR White P Royston 2009 Imputing missing covariate values for the Cox model Stat Med 28 1982 1998 https://doi.org/10.1002/sim.3618
J Ludvigsson M Nørgaard E Weiderpass 2015 Ethical aspects of registry-based research in the Nordic countries Clin Epidemiol 7 491 18 https://doi.org/10.2147/clep.s90589
SA Ñamendys-Silva EP Plata-Menchaca E Rivero-Sigarroa A Herrera-Gómez 2015 Opening the doors of the intensive care unit to cancer patients: a current perspective World J Crit Care Med 4 159 162 https://doi.org/10.5492/wjccm.v4.i3.159
H Wunsch DC Angus DA Harrison 2008 Variation in critical care services across North America and Western Europe and ast Crit Care Med 36 2787 e8 https://doi.org/10.1097/ccm.0b013e318186aec8
A Rhodes P Ferdinande H Flaatten 2012 The variability of critical care bed numbers in Europe Intens Care Med 38 1647 1653 https://doi.org/10.1007/s00134-012-2627-8
M Vliet van IW Verburg M Boogaard van den 2014 Trends in admission prevalence, illness severity and survival of haematological patients treated in Dutch intensive care units Intens Care Med 40 1275 1284 https://doi.org/10.1007/s00134-014-3373-x
PA Hampshire CA Welch LA McCrossan 2009 Admission factors associated with hospital mortality in patients with haematological malignancy admitted to UK adult, general critical care units: a secondary analysis of the ICNARC Case Mix Programme Database Crit Care 13 R137 https://doi.org/10.1186/cc8016
DD Benoit KH Vandewoude JM Decruyenaere 2003 Outcome and early prognostic indicators in patients with a hematologic malignancy admitted to the intensive care unit for a life-threatening complication Crit Care Med 31 104 112 https://doi.org/10.1097/00003246-200301000-00017
S Horster JH Stemmler PC Mandel 2012 Mortality of patients with hematological malignancy after admission to the intensive care unit Oncol Res Treat 35 556 561 https://doi.org/10.1159/000342672
E Beers van M Müller A Vlaar 2016 Haematological malignancy in the intensive care unit: microbiology results and mortality Eur J Haematol 97 271 277 https://doi.org/10.1111/ejh.12721
E Faucher M Cour V Jahandiez 2016 Short- and long-term outcomes in onco-hematological patients admitted to the intensive care unit with classic factors of poor prognosis Oncotarget 7 22427 22438
J Liu Q Cheng Q Yang 2015 Prognosis-related factors in intensive care unit (ICU) patients with hematological malignancies: a retrospective cohort analysis in a Chinese population Hematology 20 494 503 https://doi.org/10.1179/1607845414y.0000000216
S Maqsood F Badar A Hameed 2017 Characteristics and outcomes of patients with hematological malignancies admitted for intensive care - a single centre experience Asian Pac J Cancer Prev Apjcp 18 1833 1837
SA Ñamendys-Silva MO González-Herrera FJ García-Guillén 2013 Outcome of critically ill patients with hematological malignancies Ann Hematol 92 699 705 https://doi.org/10.1007/s00277-013-1675-7
F Pène JI Salluh T Staudinger 2014 Has survival increased in cancer patients admitted to the ICU? No Intens Care Med 40 1573 1575 https://doi.org/10.1007/s00134-014-3412-7
DD Benoit M Soares E Azoulay 2014 Has survival increased in cancer patients admitted to the ICU? We are not sure Intens Care Med 40 1576 1579 https://doi.org/10.1007/s00134-014-3480-8
D Mokart SM Pastores M Darmon 2014 Has survival increased in cancer patients admitted to the ICU? Yes Intens Care Med 40 1570 1572 https://doi.org/10.1007/s00134-014-3433-2
M Kiehl G Beutel B Böll 2018 Consensus statement for cancer patients requiring intensive care support Ann Hematol 97 1271 1282 https://doi.org/10.1007/s00277-018-3312-y
E Azoulay F Pène M Darmon 2015 Managing critically Ill hematology patients: time to think differently Blood Rev 29 359 367 https://doi.org/10.1016/j.blre.2015.04.002
E Lengline E Raffoux V Lemiale 2012 Intensive care unit management of patients with newly diagnosed acute myeloid leukemia with no organ failure Leukemia Lymphoma 53 1352 1359 https://doi.org/10.3109/10428194.2011.649752
G Bird P Farquhar-Smith T Wigmore 2012 Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: a 5 year study Bja Br J Anaesth 108 452 459 https://doi.org/10.1093/bja/aer449
I Moors DD Benoit 2014 Time to look beyond one-year mortality in critically ill hematological patients? Crit Care 18 107 https://doi.org/10.1186/cc13722
Acknowledgements
The study was supported by a grant from the Danish Program for Clinical Research Infrastructure (PROCRIN). All authors have approved the final manuscript. Anders Kjærsgaard had full access to all the data in the study, performed the analysis, and takes responsibility for the integrity of the data and the accuracy of the analysis. Peter Kamper, Steffen Christensen, Peter Asdahl, and Christian Christiansen designed the study. Peter Asdahl wrote the first draft of the manuscript and completed the manuscript with input from all listed authors. No authors have any financial or other conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Asdahl, P.H., Christensen, S., Kjærsgaard, A. et al. One-year mortality among non-surgical patients with hematological malignancies admitted to the intensive care unit: a Danish nationwide population-based cohort study. Intensive Care Med 46, 756–765 (2020). https://doi.org/10.1007/s00134-019-05918-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05918-1