Abstract
Purpose
To describe the modern incidence and predictors of ICU admission for adult patients newly diagnosed with a hematologic malignancy.
Methods
We conducted a population-based cohort study of adults with a new diagnosis of hematologic malignancy (April 1, 2006–March 31, 2017) in Ontario, Canada. We described the baseline demographic, clinical and laboratory predictors of ICU admission and subsequent mortality. The primary outcome was the incidence of ICU admission within 1 year of hematologic malignancy diagnosis. We assessed the predictors of ICU admission using Cox-proportional models that accounted for the competing risk of death and reported as subdistribution hazard ratios (sHR) with 95% confidence intervals (CI).
Results
A total of 87,965 patients (mean [SD] age, 67.8 (15.7) years) were included. The 1-year incidence of ICU admission was 13.9% (median time 35 days), ranging from 7.3% (indolent lymphoma) to 22.5% (acute myeloid leukemia). After multivariable adjustment, compared to indolent lymphoma, acute myeloid leukemia (sHR, 3.09; 95% CI 2.84–3.35), aggressive non-Hodgkin lymphoma (sHR, 2.47; 95% CI 2.31–2.65) and acute lymphoblastic leukemia (sHR, 2.46; 95% CI 2.15–2.80) had the highest risk of ICU admission. Comorbidities such as cardiovascular disease (sHR, 2.09; 95% CI 2.01–2.19), chronic obstructive pulmonary disease (sHR, 1.33; 95% CI 1.26–1.39) and baseline laboratory abnormalities (anemia, thrombocytopenia and high creatinine) were also associated with ICU admission. Among ICU patients, 36.7% required invasive mechanical ventilation and in-hospital mortality was 31%.
Conclusion
Critical illness in patients with a newly diagnosed hematologic malignancy is frequent, occurring early after diagnosis. Certain baseline characteristics can help identify those patients at the highest risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Critical illness occurs frequently after a new diagnosis of hematologic malignancy and has high associated mortality. Baseline characteristics at diagnosis can help identify those patients at the highest risk of critical illness. |
Introduction
Treatment of hematologic malignancy has changed dramatically in recent years [1, 2]. Novel diagnostic strategies permit earlier detection, and the elucidation of molecular disease pathways has allowed the development of new therapeutic strategies [3, 4]. These advances along with improvement in supportive care have led to an increase in survival for patients with hematologic malignancy [2, 5, 6]. However, these changes have also been accompanied by an increased number of patients susceptible to life-threatening complications [7, 8]. Critical illness can occur as a direct consequence of the underlying disease or its treatment, requiring admission to an intensive care unit (ICU) [9,10,11,12,13,14]. Historically, ICU mortality across this cohort has been reported as high as 90%; however, more recent reports from high-volume centers have demonstrated an improvement to 50% or lower [13, 15,16,17,18].
Information on the incidence and predictors of ICU admission in patients with hematologic malignancy has mostly been provided by subspecialized cancer centers [7, 9, 19,20,21]. Prior studies may be limited by historic data that may not reflect contemporary practices, low precision of the incidence estimates, lack of information surrounding hematologic malignancy subtypes, limited details regarding baseline patient factors and lack of generalizability [22, 23]. Population-based research using cancer registries captures all cases in a region and thus reflects disease trajectories across a broader spectrum of the population [24, 25].
The objective of this study was to characterize the epidemiology of critical illness after a new diagnosis of hematologic malignancy in a population-based cohort, describing the cumulative incidence and predictors of ICU admission. Furthermore, we also described the use of invasive mechanical ventilation and related mortality over time.
Patients and methods
Study design and setting
We conducted a population-based cohort study including all adult patients who had an incident diagnosis of a hematologic malignancy between April 1, 2006 and March 31, 2017 in the province of Ontario, Canada’s largest province (population 14.7 million). The study was conducted using relevant provincial administrative databases available at ICES in Toronto, Canada. ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health-care and demographic data, without consent, for health system evaluation and improvement. The use of this data is authorized under section 45 of Ontario’s Personal Health Information Protection Act (PHIPA) and does not require review by a research ethics board.
Data sources
The datasets assembled for this study and their details are available in eTable 1 in the Supplement. These include the Ontario Cancer Registry [26, 27], Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD), Ontario Health Insurance Plan database, Cancer Level Activity Reporting database, New Drug Funding Program database, the Registered Persons Database and the Ontario Laboratories Information Services database. These datasets were linked using unique encoded identifiers.
Study population
We included all adult patients (> 17 years old) who had a new diagnosis of a hematologic malignancy between April 1, 2006 and March 31, 2017, with follow-up of all patients until March 31, 2018. Hematologic malignancy cases were identified by specific codes present in the Ontario Cancer Registry, a validated and prospectively created dataset which collects information on incident cancer diagnosis in the province [26, 27].
Classification of hematologic malignancy
The type of hematologic malignancy was classified into the following categories: aggressive non-Hodgkin lymphoma, indolent lymphoma, multiple myeloma, chronic lymphocytic leukemia, myeloproliferative neoplasm, myelodysplastic syndrome, acute myeloid leukemia, Hodgkin lymphoma and acute lymphoblastic leukemia. Each category was defined based on the International Classification of Disease for Oncology (ICD-O3) coding system as contained in the Ontario Cancer Registry (eTable 2).
Measurements and variables
We included baseline characteristics of study participants, including age, sex, neighborhood income quintile, urban versus rural status, comorbidities (e.g., cardiovascular disease, diabetes, chronic obstructive pulmonary disease, neurologic condition, chronic kidney disease, other oncologic disease), type of underlying hematologic malignancy, and treatments for the hematologic malignancy provided during the year after diagnosis but prior to ICU admission (systemic therapies/receipt of autologous or allogeneic hematopoietic stem cell transplant). We also described the baseline laboratory parameters (hemoglobin, white cell count and differential, platelet count and creatinine), between 60 days before and 30 days following the index date of hematologic malignancy diagnosis. If multiple laboratory values were present, we used the value closest to the index date. Only laboratory parameters measured before the ICU admission were considered. Since blood work parameters were systematically available only in more recent years in the province of Ontario, these data were limited to 2012 and onward.
The primary outcome of this study was admission to the ICU during the first year after a new diagnosis of a hematologic malignancy, identified by the presence of special care unit codes and using an algorithm shown to have very high accuracy [28, 29]. For patients with more than one ICU admission during the first year, only the first one was considered. We also described 1-year mortality for the overall cohort. Secondary outcomes restricted to those admitted to the ICU included receipt of mechanical ventilation, dialysis and ICU and hospital mortality. Furthermore, we described the cumulative incidence in use of invasive mechanical ventilation, ICU and hospital mortality for each study year.
Statistical analysis
We summarized patients’ baseline characteristics using proportions for categorical variables and means (standard deviation [SD]) or medians (interquartile range [IQR]) as appropriate for continuous variables. We described the cumulative incidence of ICU admission during the year after diagnosis of hematologic malignancy for all patients and for each subtype of hematologic malignancy. We compared the baseline characteristics of patients who were admitted versus those who were not admitted to the ICU during the year after diagnosis. To better quantify the difference in baseline characteristics between these two groups in a large sample, we reported standardized mean differences, considering a threshold of 10% as clinically relevant [30].
The association between patients’ baseline characteristics and the primary outcome of time to ICU admission within the first year was analyzed using a multivariable proportional hazards model that accounted for the competing risk of death using the approach described by Fine and Gray [31]. The criteria for including variables in the multivariable model were based on our conceptual model and guided by biological plausibility and previous literature (eFigure 1). Hematopoietic cell transplant during follow-up was included as a time varying covariate in the model, and only the occurrence of transplant before ICU admission was considered. Estimates of association for each predictor were reported as subdistribution hazard ratios (sHR) with 95% confidence intervals (CI).
Secondary analysis: baseline laboratory
Due to the high proportion of missing data for laboratory variables in the early years of the study (2006–2011), we did not include laboratory characteristics in the primary analysis. As a secondary analysis, we re-fitted our model in a restricted cohort with an index diagnosis of hematologic malignancy in the year 2012 and onward. This model included the same variables for the primary analysis with the addition of available laboratory parameters (hemoglobin, white blood cell count, platelet count and creatinine). Platelet count and creatinine were included in the model as categorical variables considering clinically relevant thresholds. We assessed for interaction between baseline laboratory variables and subtype of malignancy. Assessment of linearity of continuous predictors is detailed in the eMethods section.
Sensitivity analyses
To assess the robustness of our findings, we performed a series of sensitivity analyses. We repeated our primary analysis using a cause-specific proportional hazards model which treated death before ICU admission as a censoring variable instead of a competing event. We also performed multiple imputation for missing laboratory values using the Markov chain Monte Carlo (MCMC) methods creating five imputed datasets (eMethods).
Assessment of secondary outcomes
In the restricted cohort of those patients that were admitted to the ICU, we reported the receipt of invasive mechanical ventilation, dialysis and tracheostomy as well as ICU and hospital mortality. To assess for changes in hospital mortality over time among critically ill patients, we fitted a generalized estimating equations model that accounted for clustering at the institution of diagnosis and was adjusted for potential confounders (eMethods).
For all analyses we considered a p value < 0.05 for statistical significance. All analyses were performed in SAS Enterprise version 7.15 at ICES. Additional Figures were created using RStudio version 1.4.
Results
Baseline characteristics
During the 11-year study period, 87,965 patients were diagnosed with a new hematologic malignancy. Baseline characteristics of study participants are detailed in Table 1. Mean age was 67 years (SD 15.7) and 39,075 (44.4%) were female. The most frequent type of hematologic malignancy was aggressive non-Hodgkin lymphoma (n = 21,211 [24.1%]), followed by multiple myeloma (n = 12,303 [14%]), chronic lymphocytic leukemia (n = 12,081 [13.7%]) and indolent lymphoma (n = 11,623 [13.2%]). Acute myeloid leukemia was diagnosed in 7149 (8.1%) of the patients. Cardiovascular disease (n = 19,364 [22.3%]) and chronic obstructive pulmonary disease (n = 13,002 [14.8%]) were frequent comorbidities. During the first year after diagnosis, chemotherapy was administered to 42,779 (48.6%) patients and 4076 (4.6%) underwent hematopoietic stem cell transplantation (n = 3155 [77.5%] autologous transplants and n = 921 [22.5%] allogeneic transplants, eTables 3 and 4, in the Supplement).
Cumulative incidence of ICU admission within 1 year
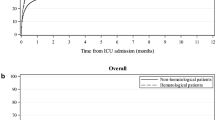
The cumulative incidence of ICU admission during the year after diagnosis of hematologic malignancy is shown in Figs. 1 and 2. Overall, 12,247 (13.9%, 95% CI 13.7–14.2) patients were admitted to ICU during the first year after diagnosis. The highest incidence of ICU admission was among those with acute myeloid leukemia (n = 1607 [22.5%, 95% CI 21.5–23.4]), followed by aggressive non-Hodgkin lymphoma (n = 3745 [17.7%, 95% CI 17.1–18.2]) and acute lymphoblastic leukemia (n = 272 [17.5%, 95% CI 15.6–19.4]) (Fig. 2). Intensive care unit admission occurred within 30 days of hematologic malignancy diagnosis for half (n = 5887 [48.1%, 95% CI 47.2–49]) of the patients, and the median time from diagnosis to ICU admission was 35 days (IQR 3–132, eFigure 2). Intensive care unit subtypes are described in eTable 5.
Intensive care unit admission and mortality at 1 year in patients with hematologic malignancy. ICU, intensive care unit. 1This describes the 1-year mortality for patients admitted to the ICU considering the diagnosis of hematologic malignancy as the index date. It includes those patients who died during hospital stay (n = 2441 [19.9%] in the ICU and n = 3790 [31.0%] in-hospital). The 1-year mortality for those patients that were discharged alive was 36.4% (3076/8457) which considers hospital discharge as the index date. Created with BioRender.com
Cumulative incidence, time to ICU admission and related conditions and procedures. A The plot represents the cumulative incidence function curves for each type of hematologic malignancy, ordered by frequency as detailed in the table beside the plot. B Description of time to ICU admission using kernel density plots, frequent conditions and procedures of critically ill patients. The procedure “mechanical ventilation” includes both invasive and noninvasive ventilation. ICU intensive care unit, CI confidence interval, NHL non-Hodgkin lymphoma, CLL chronic lymphocytic leukemia, HL Hodgkin lymphoma, MPN myeloproliferative neoplasm, MM multiple myeloma, MDS myelodysplastic syndrome, ALL acute lymphoblastic leukemia, AML acute myeloid leukemia
Factors associated with increased risk of ICU admission
The factors associated with increased risk of ICU admission within a year of diagnosis of a hematologic malignancy are shown in Fig. 3A. In the multivariable analysis, female sex and a higher neighborhood income quintile were associated with a lower risk of ICU admission, whereas age cohorts of 50–69 (compared to 18–29) and presence of comorbidities were associated with a higher risk of ICU admission. Compared to indolent lymphoma, all other subtypes of hematologic malignancy had a higher risk of ICU admission except chronic lymphocytic leukemia; the highest risk was among patients with acute myeloid leukemia (sHR, 3.09; 95% CI 2.84–3.35), aggressive lymphoma (sHR, 2.47; 95% CI 2.31–2.65) and acute lymphoblastic leukemia (sHR, 2.46; 95% CI 2.15–2.8). Treatment with hematopoietic cell transplant also increased the risk of ICU admission (sHR, 2.79; 95% CI 2.48–3.15). We did not observe an association between the year of diagnosis (sHR, 1; 95% CI 0.99–1.01, per year increase) and ICU admission.
Association of patients’ characteristics and ICU admission within 1 year. The plots show the subdistribution hazard ratio for time to ICU admission for each predictor. A All variables included in the model are included in the plot. Of note, the place of residence and the year of diagnosis of hematologic malignancy were not associated with ICU admission. B The plot shows the subdistribution hazard ratio for time to ICU admission for each blood work parameter. This model is restricted to patients with a diagnosis of hematologic malignancy in the year 2012 and onward. This model has been adjusted by the following variables: sex, age group, income category, place of residence, year of diagnosis, type of hematologic malignancy, time varying hematopoietic stem cell transplant and baseline comorbidities included in A. We observed interaction between certain malignancy subtypes and the presence of anemia (eTable 10 in the supplement). ICU intensive care unit, CI confidence interval, NHL non-Hodgkin lymphoma, CLL chronic lymphocytic leukemia, HL Hodgkin lymphoma, MPN myeloproliferative neoplasm, MM multiple myeloma, MDS myelodysplastic syndrome, ALL acute lymphoblastic leukemia, AML acute myeloid leukemia, HCT hematopoietic stem cell transplantation, COPD chronic obstructive pulmonary disease
In the analysis restricted to 2012 and onward, we observed that the presence of baseline anemia (sHR, 1.31; 95% CI 1.21–1.41, hemoglobin < 100 g/L), low platelets (sHR, 1.13; 95% CI 1.04–1.24 [platelet count 50–100 × 109/L versus > 100 × 109]; sHR, 1.30; 95% CI 1.12–1.45 [platelet count < 50 × 109/L versus > 100 × 109]) and high creatinine at baseline (sHR, 1.13, 95% CI 1.05–1.22 [creatinine 100–200 μmol/L versus < 100 μmol/L]; sHR, 1.36; 95% CI 1.22–1.50 [creatinine > 200 μmol/L versus < 100 μmol/L]) were also associated with a higher risk of ICU admission (Fig. 3B). The results of our sensitivity analyses using cause-specific hazard models and multiple imputation yielded similar results (eFigures 3, 4, in the Supplement).
ICU diagnosis, procedures and mortality
Across the cohort admitted to the ICU, 5896 (48.1%) patients had acute respiratory failure, 4597 (37.5%) acute kidney injury and 3707 (30.3%) sepsis (Fig. 2). One-third (n = 4490 [36.7%]) of patients received invasive mechanical ventilation, 741 (6.0%) hemodialysis and 414 (3.4%) received a tracheostomy. The ICU procedures and outcomes across each subtype of hematologic malignancy, age subgroup and year of ICU admission are available in eTables 6, 7 and 8. A higher proportion of patients with acute myeloid leukemia had a diagnosis of acute respiratory failure and required invasive mechanical ventilation compared to other hematologic malignancy subgroups whereas a higher proportion of patients with multiple myeloma required dialysis in the ICU compared to other subgroups (eTable 6). The frequency of use invasive measures such as mechanical ventilation and dialysis decreased over the age of 80 (eTable 7).
Among patients admitted to the ICU, 2441 (19.9%, 95% CI 19.2–20.6) died in the ICU and 3790 died (31%, 30.1–31.8) during hospital stay. Across the entire cohort admitted to the ICU, 6343 (51.8%, 95% CI 50.9–52.7) died up until 1 year following diagnosis of hematologic malignancy. Out of the 8457 patients discharged from the hospital alive after critical illness, 3076 (36.4%, 95% CI 35.4–37.4) died within 1 year of hospital discharge. Considering the entire cohort of 87,965 patients diagnosed with hematologic malignancy (including those admitted to the ICU), 21,666 (24.6%, 96% CI 24.4–24.9) died within 1 year (Fig. 1).
ICU admission and in-hospital mortality over time
The incidence of ICU admission for each of the 11-year period ranged from 14% (95% CI 13.2–15) in 2006 to 12.6% (95% CI 12.0–13.3) in 2016 (eTable 9, in the Supplement). Figure 4 depicts the cumulative incidence of invasive mechanical ventilation, ICU mortality and hospital mortality by every year of ICU admission. The crude hospital mortality ranged from 32.3% (95% CI 28.7–36) in 2006 to 31.5% (95% CI 29–34.2) in 2016. After adjusting for age, sex, comorbidities and use of invasive mechanical ventilation we observed a decrease in the odds of mortality with time (odds ratio 0.97, 95% CI 0.96–0.98, per year increase in ICU admission: eFigure 5 in the Supplement).
Incidence of invasive ventilation and mortality over time. The plot describes the cumulative incidence of crude mortality among the critically ill patients for each year of ICU admission. Each point represents a proportion with its corresponding 95% confidence interval. Incidence of mechanical ventilation per year has been modeled using a local polynomial regression (LOESS) with year as the only independent variable and invasive ventilation as the dependent variable. IMV invasive mechanical ventilation, ICU intensive care unit
Discussion
In this population-based cohort study of 87,965 adult patients with an incident diagnosis of a hematologic malignancy, 13.9% required ICU admission within 1 year. However, the incidence of ICU admission was variable and ranged from 7.3% for patients with indolent lymphoma to 22.5% in patients with acute myeloid leukemia. Several patient-related and treatment factors increased the risk of ICU admission, including sex, baseline comorbidities, and hematopoietic cell transplant. Overall hospital mortality for patients admitted to an ICU was 31%, although this incidence decreased over time.
Our study is the largest to date to evaluate a contemporary cohort of newly diagnosed patients with hematologic malignancy and highlights the variable incidence of critical illness after diagnosis across different subtypes. Our data are population level, derived from an entire province, improving generalizability and allowing for comprehensive population-based assessment of risk rather than being derived solely from high volume academic centers [22]. Importantly, our findings are a reflection of a public health care system where health insurance and access are less likely to influence care and candidacy for ICU admission. Indeed, thresholds for ICU admission likely vary based on multiple factors [32, 33], and some units tend to admit patients with cancer to the ICU primarily for monitoring. In Ontario, ICU admission is largely restricted to those patients requiring advanced respiratory support, vasopressors or close hemodynamic or neurologic monitoring. Almost one in four patients with acute myeloid leukemia in our cohort required ICU admission, similar to findings from a study by Halpern and colleagues [13]. In our cohort, acute lymphoblastic leukemia and aggressive types of non-Hodgkin lymphoma were other subtypes with a high risk of ICU admission. These findings suggest that disease subtypes that are more aggressive—or which require more intensive treatment regimens—are likely to impact on risk of critical illness [9, 12, 13, 21, 34]. In our cohort, more than 25% of the ICU cohort was admitted very early in the disease trajectory, highlighting the central role of the ICU in the peri-diagnostic period.
Understanding the frequency and timing of critical illness after a new diagnosis of hematologic malignancy can help inform treatment recommendations and policy planning. Knowledge that a patient has a high risk of requiring admission to an ICU during treatment can provide important context in the consent process for treatment. For example, advance care planning would be particularly useful across patients who are anticipated to be poor candidates for ICU admission based upon disease characteristics, comorbidities or frailty status [35, 36]. Our results are important for policy makers and health planners, as they demonstrate the high likelihood of expensive ICU treatments after diagnosis—but also highlight the improved hospital mortality rates (31%) for these patients when compared to historic controls who had a mortality that often exceeded 50% [7, 16, 37, 38]. Despite the improved mortality over time, when compared to a general ICU population, the mortality still remains high [39]. Furthermore, our study highlights that among all deaths within the first year of patients with hematologic malignancy, 30% occur in an ICU.
This study has several limitations. Although we aimed to describe the occurrence of critical illness in patients with hematologic malignancies, we used admission to the ICU as a surrogate of critical illness. Most patients who developed critical illness and need organ support were admitted to the ICU; however, patient preferences (i.e., desire to not be admitted to an ICU) were not captured by this population-based cohort—and therefore our study may underestimate the true risk [40, 41]. We were not able to capture all variables that might impact on the risk of admission to an ICU and subsequent mortality, as our study relied only on data available across multiple administrative health databases. For example, we were not able to assess the proportion of patients that required vasopressors or hemodynamic support, and therefore, we could not accurately compare the severity of disease between this and previous cohorts. Moreover, the exact date of chemotherapy administration could not be ascertained from this data, and thus we did not include this variable in our multivariable model. However, we were able to estimate the incidence of ICU admission for different types of hematologic malignancy, a variable that is easily extrapolated to different settings. Furthermore, our secondary analysis considering laboratory results obtained near the time of diagnosis offers additional insights that have not been available from other population-based studies. Our study was not designed to assess a causal association between the predictors and the risk of ICU admission, and many of the variables associated with ICU admission are not modifiable. However, these findings are still clinically important to raise awareness of which patients might benefit for a closer follow-up after diagnosis, to help inform the consent process for patients contemplating treatments (e.g., hematopoietic stem cell transplant), and to help plan resource allocation after diagnosis of hematologic malignancy. Finally, although we examined a contemporary cohort in the era of novel cancer therapies, most patients were diagnosed with hematologic malignancy prior to the incorporation of chimeric antigen receptor T cell (CAR-T) therapy as a therapeutic option for certain types of hematologic malignancy (e.g., acute lymphoblastic leukemia, diffuse large B cell lymphoma); CAR-T may further increase the risk of critical illness [42,43,44,45].
In summary, our study confirms that the risk of critical illness is high among patients with a new diagnosis of hematologic malignancy, though this risk varies according to baseline patient characteristics and across different subtypes of hematologic malignancy. While mortality rates have improved markedly compared to historic reports, in-hospital mortality rates remain high.
Data availability
The study was conducted using relevant provincial administrative databases available at ICES in Toronto, Canada. This data is not publicly available and only researchers with ICES credentials can access this data.
Code availability
The codes used for this study will be available upon reasonable request to the corresponding author.
References
Jurcic JG (2017) Highlights in hematologic malignancy treatments: leukemia, myelodysplastic syndromes, and allotransplant-new drugs on the horizon for acute myeloid leukemia. JAMA Oncol 3:299–300. https://doi.org/10.1001/jamaoncol.2016.3928
Pulte D, Jansen L, Castro FA, Brenner H (2016) Changes in the survival of older patients with hematologic malignancies in the early 21st century. Cancer 122:2031–2040. https://doi.org/10.1002/cncr.30003
Ma H, Mallampati S, An G, Wang J (2015) Targeted therapy in hematological malignancies: from basic research to clinical practice. Biomed Res Int 2015:157570. https://doi.org/10.1155/2015/157570
Craddock C, Friedberg JW (2021) Immunotherapy for hematologic malignancies. J Clin Oncol 39:343–345. https://doi.org/10.1200/JCO.20.03106
Sant M, Minicozzi P, Mounier M, Anderson LA, Brenner H, Holleczek B, Marcos-Gragera R, Maynadie M, Monnereau A, Osca-Gelis G, Visser O, De Angelis R, Group E-W (2014) Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol 15:931–942. https://doi.org/10.1016/S1470-2045(14)70282-7
Pulte D, Jansen L, Brenner H (2020) Changes in long term survival after diagnosis with common hematologic malignancies in the early 21st century. Blood Cancer J 10:56. https://doi.org/10.1038/s41408-020-0323-4
van Vliet M, Verburg IW, van den Boogaard M, de Keizer NF, Peek N, Blijlevens NM, Pickkers P (2014) Trends in admission prevalence, illness severity and survival of haematological patients treated in Dutch intensive care units. Intensive Care Med 40:1275–1284. https://doi.org/10.1007/s00134-014-3373-x
Azoulay E, Schellongowski P, Darmon M, Bauer PR, Benoit D, Depuydt P, Divatia JV, Lemiale V, van Vliet M, Meert A-P, Mokart D, Pastores SM, Perner A, Pène F, Pickkers P, Puxty KA, Vincent F, Salluh J, Soubani AO, Antonelli M, Staudinger T, von Bergwelt-Baildon M, Soares M (2017) The Intensive Care Medicine research agenda on critically ill oncology and hematology patients. Intensive Care Med 43:1366–1382. https://doi.org/10.1007/s00134-017-4884-z
Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, Vincent F, Nyunga M, Bruneel F, Laisne L-M, Rabbat A, Lebert C, Perez P, Chaize M, Renault A, Meert A-P, Benoit D, Hamidfar R, Jourdain M, Darmon M, Schlemmer B, Chevret S, Lemiale V (2013) Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from france and belgium—a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol 31:2810–2818. https://doi.org/10.1200/JCO.2012.47.2365
Azoulay E, Pène F, Darmon M, Lengliné E, Benoit D, Soares M, Vincent F, Bruneel F, Perez P, Lemiale V, Mokart D, de Recherche Respiratoire en Réanimation Onco-Hématologique Grrr-OH G (2015) Managing critically ill hematology patients: time to think differently. Blood Rev 29:359–367. https://doi.org/10.1016/j.blre.2015.04.002
Azoulay E, Soares M, Benoit D (2016) Focus on immunocompromised patients. Intensive Care Med 42:463–465. https://doi.org/10.1007/s00134-016-4224-8
Grgić Medić M, Gornik I, Gašparović V (2015) Hematologic malignancies in the medical intensive care unit–outcomes and prognostic factors. Hematology 20:247–253. https://doi.org/10.1179/1607845414Y.0000000206
Halpern AB, Culakova E, Walter RB, Lyman GH (2017) Association of risk factors, mortality, and care costs of adults with acute myeloid leukemia with admission to the intensive care unit. JAMA Oncol 3:374–378. https://doi.org/10.1001/jamaoncol.2016.4858
Ferreyro BL, Munshi L, Detsky ME, Minden MD, Cheung A, Burry L, Lee C (2018) Acute promyelocytic leukemia in the intensive care unit: a retrospective analysis. Leuk Res 73:41–43. https://doi.org/10.1016/j.leukres.2018.08.004
Mokart D, Salluh JIF, Staudinger T (2014) Has survival increased in cancer patients admitted to the ICU? Yes. Intensive Care Med 40:1573–1575. https://doi.org/10.1007/s00134-014-3433-2
Darmon M, Bourmaud A, Georges Q, Soares M, Jeon K, Oeyen S, Rhee CK, Gruber P, Ostermann M, Hill QA, Depuydt P, Ferra C, Toffart AC, Schellongowski P, Muller A, Lemiale V, Mokart D, Azoulay E (2019) Changes in critically ill cancer patients’ short-term outcome over the last decades: results of systematic review with meta-analysis on individual data. Intensive Care Med. https://doi.org/10.1007/s00134-019-05653-7
Zampieri FG, Romano TG, Salluh JIF, Taniguchi LU, Mendes PV, Nassar AP Jr, Costa R, Viana WN, Maia MO, Lima MFA, Cappi SB, Carvalho AGR, De Marco FVC, Santino MS, Perecmanis E, Miranda FG, Ramos GV, Silva AR, Hoff PM, Bozza FA, Soares M (2021) Trends in clinical profiles, organ support use and outcomes of patients with cancer requiring unplanned ICU admission: a multicenter cohort study. Intensive Care Med 47:170–179. https://doi.org/10.1007/s00134-020-06184-2
Lueck C, Stadler M, Koenecke C, Hoeper MM, Dammann E, Schneider A, Kielstein JT, Ganser A, Eder M, Beutel G (2018) Improved short- and long-term outcome of allogeneic stem cell recipients admitted to the intensive care unit: a retrospective longitudinal analysis of 942 patients. Intensive Care Med 44:1483–1492. https://doi.org/10.1007/s00134-018-5347-x
Biard L, Darmon M, Lemiale V, Mokart D, Chevret S, Azoulay E, Resche-Rigon M (2019) Center effects in hospital mortality of critically ill patients with hematologic malignancies. Crit Care Med 47:809–816. https://doi.org/10.1097/CCM.0000000000003717
Pène F, Percheron S, Lemiale V, Viallon V, Claessens Y-E, Marqué S, Charpentier J, Angus DC, Cariou A, Chiche J-D, Mira J-P (2008) Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit Care Med 36:690–696. https://doi.org/10.1097/CCM.0B013E318165314B
Vijenthira A, Chiu N, Jacobson D, Freedman Z, Cheung MC, Goddard S, Fowler R, Buckstein R (2020) Predictors of intensive care unit admission in patients with hematologic malignancy. Sci Rep 10:21145. https://doi.org/10.1038/s41598-020-78114-7
Thygesen LC, Ersboll AK (2014) When the entire population is the sample: strengths and limitations in register-based epidemiology. Eur J Epidemiol 29:551–558. https://doi.org/10.1007/s10654-013-9873-0
Gavrielov-Yusim N, Friger M (2014) Use of administrative medical databases in population-based research. J Epidemiol Community Health 68:283–287. https://doi.org/10.1136/jech-2013-202744
Wunsch H, Harrison DA, Rowan K (2005) Health services research in critical care using administrative data. J Crit Care 20:264–269. https://doi.org/10.1016/j.jcrc.2005.08.002
Izquierdo JN, Schoenbach VJ (2000) The potential and limitations of data from population-based state cancer registries. Am J Public Health 90:695–698. https://doi.org/10.2105/ajph.90.5.695
Robles SC, Marrett LD, Clarke EA et al (1988) An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol 14.41(5):495–501
Clarke E, Marrett L, Kreiger N (1991) Cancer registration in Ontario: a computer approach. Cancer registration principlesand methods. IARC Publications, Lyon, pp 246–257
Garland A, Yogendran M, Olafson K, Scales DC, McGowan K-L, Fransoo R (2012) The accuracy of administrative data for identifying the presence and timing of admission to intensive care units in a Canadian province. Med Care 50:e1-6. https://doi.org/10.1097/MLR.0b013e318245a754
Scales DC, Guan J, Martin CM, Redelmeier DA (2006) Administrative data accurately identified intensive care unit admissions in Ontario. J Clin Epidemiol 59:802–807. https://doi.org/10.1016/j.jclinepi.2005.11.015
Austin PC (2009) Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38:1228–1234. https://doi.org/10.1080/03610910902859574
Austin PC, Fine JP (2017) Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 36:4391–4400. https://doi.org/10.1002/sim.7501
Hill AD, Stukel TA, Fu L, Scales DC, Laupacis A, Rubenfeld GD, Wunsch H, Downar J, Rockwood K, Heyland DK, Sinha SK, Zimmermann C, Gandhi S, Myers J, Ross HJ, Kozak JF, Berry S, Dev SP, La Delfa I, Fowler RA (2019) Trends in site of death and health care utilization at the end of life: a population-based cohort study. CMAJ Open 7:E306–E315. https://doi.org/10.9778/cmajo.20180097
Yarnell CJ, Fu L, Manuel D, Tanuseputro P, Stukel T, Pinto R, Scales DC, Laupacis A, Fowler RA (2017) Association between immigrant status and end-of-life care in Ontario, Canada. JAMA 318:1479–1488. https://doi.org/10.1001/jama.2017.14418
Staudinger T, Stoiser B, Müllner M, Locker GJ, Laczika K, Knapp S, Burgmann H, Wilfing A, Kofler J, Thalhammer F, Frass M (2000) Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit Care Med 28:1322–1328
Spring J, McKinlay J, Puxty K, Metaxa V, Detsky M, Mehta S, Thyagu S, McGuigan K, Munshi L (2021) Perspectives on advance care planning for patients with hematologic malignancies: an international clinician questionnaire. Ann Am Thorac Soc. https://doi.org/10.1513/AnnalsATS.202006-678OC
Carr D, Luth EA (2017) Advance care planning: contemporary issues and future directions. Innov Aging 1:igx012. https://doi.org/10.1093/geroni/igx012
Azoulay E, Schellongowski P, Darmon M, Bauer PR, Benoit D, Depuydt P, Divatia JV, Lemiale V, Vliet M, Meert A-P, Mokart D, Pastores SM, Perner A, Pène F, Pickkers P, Puxty KA, Vincent F, Salluh J, Soubani AO, Antonelli M, Staudinger T, Bergwelt-Baildon M, Soares M (2017) The Intensive Care Medicine research agenda on critically ill oncology and hematology patients. Intensive Care Med 43:1–17. https://doi.org/10.1007/s00134-017-4884-z
Mokart D, Pastores SM, Darmon M (2014) Has survival increased in cancer patients admitted to the ICU? Yes. Intensive Care Med 40:1570–1572. https://doi.org/10.1007/s00134-014-3433-2
Hill AD, Fowler RA, Pinto R, Herridge MS, Cuthbertson BH, Scales DC (2016) Long-term outcomes and healthcare utilization following critical illness–a population-based study. Crit Care 20:76. https://doi.org/10.1186/s13054-016-1248-y
Fried TR, Bradley EH, Towle VR, Allore H (2002) Understanding the treatment preferences of seriously ill patients. N Engl J Med 346:1061–1066. https://doi.org/10.1056/NEJMsa012528
Garrouste-Orgeas M, Montuclard L, Timsit JF, Reignier J, Desmettre T, Karoubi P, Moreau D, Montesino L, Duguet A, Boussat S, Ede C, Monseau Y, Paule T, Misset B, Carlet J, French ASG (2005) Predictors of intensive care unit refusal in French intensive care units: a multiple-center study. Crit Care Med 33:750–755. https://doi.org/10.1097/01.ccm.0000157752.26180.f1
Azoulay E, Darmon M, Valade S (2020) Acute life-threatening toxicity from CAR T-cell therapy. Intensive Care Med 46:1723–1726. https://doi.org/10.1007/s00134-020-06193-1
Komanduri KV (2021) Chimeric antigen receptor T-cell therapy in the management of relapsed non-Hodgkin lymphoma. J Clin Oncol 39:476–486. https://doi.org/10.1200/JCO.20.01749
Reagan PM, Neelapu SS (2021) How I manage: pathophysiology and management of toxicity of chimeric antigen receptor T-cell therapies. J Clin Oncol 39:456–466. https://doi.org/10.1200/JCO.20.01616
Azoulay E, Castro P, Maamar A, Metaxa V, de Moraes AG, Voigt L, Wallet F, Klouche K, Picard M, Moreau AS, Van De Louw A, Seguin A, Mokart D, Chawla S, Leroy J, Boll B, Issa N, Levy B, Hemelaar P, Fernandez S, Munshi L, Bauer P, Schellongowski P, Joannidis M, Moreno-Gonzalez G, Galstian G, Darmon M, Valade S, Ii N (2021) Outcomes in patients treated with chimeric antigen receptor T-cell therapy who were admitted to intensive care (CARTTAS): an international, multicentre, observational cohort study. Lancet Haematol 8:e355–e364. https://doi.org/10.1016/S2352-3026(21)00060-0
Acknowledgements
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). Parts of this material are based on data and information compiled and provided by Cancer Care Ontario (CCO). The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. The authors would also like to thank Dr. Fernando Binder for his help with Fig. 4.
Funding
BF was supported by a Vanier Canada Graduate Scholarship. DS holds operating grants from the Canadian Institute for Health Research. HW is supported by a Canada Research Chair [Tier 2] in Critical Care Organization and Outcomes.
Author information
Authors and Affiliations
Contributions
BLF had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: BLF, DCS, HW, RS, LM. Acquisition, analysis and interpretation of data: all authors. Drafting of the manuscript: BLF, DCS, LM. Critical revision of the manuscript for important intellectual content and approval of the final draft: all authors. Statistical analysis: BLF, RS.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest relevant to the contents of this manuscript.
Ethical approval
ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health-care and demographic data, without consent, for health system evaluation and improvement. The use of the data in this project is authorized under section 45 of Ontario’s Personal Health Information Protection Act (PHIPA) and does not require review by a Research Ethics Board.
Consent to participate
Not applicable (see “Ethical approval”).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferreyro, B.L., Scales, D.C., Wunsch, H. et al. Critical illness in patients with hematologic malignancy: a population-based cohort study. Intensive Care Med 47, 1104–1114 (2021). https://doi.org/10.1007/s00134-021-06502-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-021-06502-2