Abstract
Purpose
To compare pain incidence and changes in pain scores with fentanyl versus placebo as pre-emptive treatment during turning and 30 min post-turning in mechanically ventilated critically ill patients.
Methods
We performed a randomized, double-blind, parallel-group, placebo-controlled clinical trial in the intensive care unit of a university hospital. Seventy-five mechanically ventilated patients were randomized to an intervention group (fentanyl) or a control group (placebo). Patients in the intervention group received 1 µg/kg (medical patients) or 1.5 µg/kg (surgical patients) of fentanyl 10 min before turning. Pain indicators were assessed using the behavioral pain scale. Safety was assessed by determining the frequency and severity of pre-defined adverse events. Pain was evaluated at rest (T0), at turn start and end (T1 and T2) and at 5, 15 and 30 min post-turning (T3, T4 and T5).
Results
The two groups had similar baseline characteristics. The area under the curve for BPS values was significantly smaller in the fentanyl group than in the control group [median and interquartile range (IQR): 132 (108–150) vs. 147 (125–180); p = 0.016, respectively]. Nineteen non-serious adverse events were recorded in 14 patients, with no significant between-group differences (23 % fentanyl group vs. 14 % control group; p = 0.381).
Conclusions
These results suggest an intravenous bolus of fentanyl of 1 µg/kg for medical patients or 1.5 µg/kg for surgical patients reduces the incidence of turning-associated pain in critically ill patients on mechanical ventilation.
ClinicalTrials.gov: NCT 01950000.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Almost 70 % of adult critical care patients experience pain during their stay in the intensive care unit (ICU) [1, 2]. Several factors can account for this high incidence rate, including the underlying disease requiring ICU admission, the inability of mechanically ventilated patients to report pain, and common nursing procedures that can induce pain such as turning, tracheal suctioning, wound care, and line insertion.
Most ICU patients (90–95 %) receive opioid analgesics for background pain, typically administered via continuous intravenous (IV) infusion [3–5]. However, opioid doses administered when patients are at rest are often insufficient to prevent pain associated with the routine, standard procedure of turning [6, 7]. Nevertheless, administration of pre-emptive analgesia before nursing procedures is relatively uncommon [3–9]. Although current guidelines support the use of pre-emptive analgesia before chest tube removal [1], evidence on the effectiveness of this approach is limited [8, 10–14].
To our knowledge, despite the high frequency of turning in ICU patients, no previous clinical trials have studied the efficacy of pre-emptive opioid analgesia to reduce pain in ICU patients on mechanical ventilation (MV) [9, 15–18]. We hypothesized that pre-emptive analgesia with a single fentanyl bolus would reduce pain during routine ICU interventions without increasing the risk of opioid-related adverse events. The primary aim of the study was to compare the two groups in terms of pain incidence rates and changes in pain scores from baseline to 30 min post-turning. A secondary aim was to evaluate the safety of IV fentanyl.
Patients and methods
This was a randomized, double-blind, parallel-group, placebo-controlled clinical trial. Patients were recruited between May 2011 and April 2012 in a 34-bed general ICU of a university hospital with an average annual admission rate of 500 in Barcelona, Spain. The study was approved by the hospital’s ethics committee (IRS-FEN-2010-01). Consent was signed by one patient only and by the legal guardian in all other cases. The study was registered in the clinical trials database (ClinicalTrials.gov: NCT 01950000; http://www.clinicaltrials.gov/).
Study population
Inclusion criteria were: age ≥18 years; receiving MV for over 24 h; hemodynamic stability (according to the treating physician’s criteria); ability to understand Spanish; and presence of a legal guardian authorized to consent to the patient’s participation in the study if the patient was unable to do so.
Exclusion criteria were: known hypersensitivity to fentanyl; administration of supplementary IV opioid drugs in the 4 h before the study; treatment with neuromuscular blocking agents; cognitive impairment as determined from medical records or information provided by the patient’s family; neurocritical illness; brain death or vegetative state; treatment with monoamine oxidase inhibitors; or pregnancy.
Measures
All pain behaviors were assessed by the same investigator (GR) using the behavioral pain scale (BPS) [16]. The BPS has three subscales that include behaviors related to facial expressions, upper limb movements, and compliance with ventilation. Subscale scores range from 1 to 4 and the total score ranges from 3 (no pain) to 12 (maximum pain). A score of >3 reflects pain, whereas a score of >5 indicates significant pain [1]. The BPS is considered one of the most valid and reliable scales available to evaluate pain in nonverbal ICU patients (Cronbach α 0.63–0.72, Kappa coefficients 0.67–0.83) [1, 19].
Fentanyl safety was evaluated by the frequency of adverse events (presence or absence) at pre-defined time intervals [from the start of turning (T1) to 30 min post-turning (T5)]. Adverse events were classified as serious when they led to prolonged hospitalization or persistent or significant disability, put the patient’s life at risk, or resulted in death. All other adverse events that could be considered related to fentanyl use during the procedure were classified as non-serious. The adverse events considered were: respiratory depression (respiratory rate ≤8 rpm and/or switch to apnoea ventilation mode in patients with ventilator support pressure mode); bradycardia (heart rate <40 bpm); and hypotension (blood pressure drop >20 % over baseline for ≥4 min). Other potential adverse events included muscle stiffness, hypersensitivity reactions (bronchospasm, pruritus, urticarial), and gastrointestinal events (nausea or vomiting, delayed tolerance to enteral feeding).
The primary outcome was area under the curve (AUC) for BPS scores between T1 and T5. Secondary outcomes were the frequency of adverse events.
Protocol
After informed consent was obtained, 75 patients were randomized to a single intravenous bolus of pre-emptive fentanyl or placebo using balanced randomization in blocks of 10 patients. The person responsible for creating this list (IG) kept one copy and sent another copy to the pharmacist who prepared the study medication. The pharmacist was the only person not blinded to the randomization outcome.
Prior to pain assessment, demographic and clinical data were collected from the patients’ medical records. During the turning procedure and up to 30 min after completing the turning, the principal investigator (G.R.) assessed and recorded all pain-related information using the BPS, vital signs, and adverse events. Data were collected at six time points: with the patient at rest before the procedure (baseline, T0); after the first turn (T1); after the second turn (T2); and at 5 min (T3), 15 min (T4), and 30 min (T5) after the second turn. We performed two turns (T1 and T2) as in routine care procedures. No other procedures were performed for at least 30 min before T0 was measured or during pain assessment. The median time from T0 until T1 was 45 min [interquartile range (IQR), 34–60 min]. Each turning procedure took a median time of 3 min (IQR 2–4 min). Patients were followed until ICU discharge. The total number of days on MV, length of stay, and mortality rates were recorded.
When a patient entered the study, the principal investigator requested the medication from the pharmacist, supplied as a single, specifically identified opaque vial. The vials contained 2 mL of a 100-µg fentanyl solution (50 µg/ml) or 2 mL placebo (saline solution) and were identical in appearance. Each vial had a numerical code on the label and only the pharmacist was aware of the vial contents. The 2-ml vial containing the study medication was diluted in 8 ml of saline solution and administered by an ICU nurse according to pre-established instructions approximately 10 min before the turning procedure. The predetermined volume, calculated according to the patient’s weight and diagnosis (medical or surgical), was slowly injected and the IV was then flushed with 10 mL of saline solution. The total fentanyl dose administered was 1 µg/kg for medical patients and 1.5 µg/kg for surgical and trauma patients. The dose calculation was based either on the ideal or actual weight of the patient, whichever was lowest. No patient received more than 100 µg of fentanyl. During the study, patients receiving continuous infusions of morphine, midazolam, and propofol as part of their regular care continued to receive these infusions as usual.
Analgesia and sedation of MV patients was performed according to the nursing protocol followed in our ICU since 2011. The analgesic most commonly used in our ICU is continuous morphine infusion. The nurse–patient ratio was 1:2.
Statistical analysis
Sample size was calculated assuming a pain incidence of 95 % during turning of control patients, according to previous observations [9]. To achieve a 20 % relative decrease in the number of patients whose turning-related pain was reduced through fentanyl use and assuming a loss of 15 % of patients, we calculated that 75 patients were needed to achieve a power of 80 (error type II), and a type I error rate of 0.05.
The Kolmogorov–Smirnov test was used to check data distribution. Dichotomous variables were expressed as number of cases and percentages. These variables were compared using contingency tables and the Chi square test or Fisher’s exact test. Quantitative variables were expressed as medians with interquartile range (IQR) and compared using the non-parametric Wilcoxon rank-sum for independent data. Pain and vital signs during turning (Tmov) were evaluated by the arithmetic mean of T1 (end of first turn) and T2 (end of second turn). Pain incidence and significant pain incidence at each time point (T0, T1, T2, T3, T4 and T5) was evaluated according to the following formula: pain incidence = number of patients with BPS >3/total number of patients evaluated × 100; and significant pain incidence = number of patients with BPS >5/total number of patients evaluated × 100.
To evaluate changes in the magnitude of pain behavior over time and to avoid multiple comparisons, we used the area under curve (AUC). The effect of fentanyl on pain behavior was also measured by calculating the relative risk (RR) and the number needed to treat (NNT).
All analyses were carried out on an intention-to-treat basis and all patients were included in the same group to which they were originally randomized. There were no missing data. For all analyses, statistical significance was set at 5 % (alpha = 0.05) and testing was 2-tailed. All data were recorded in a Microsoft Excel spreadsheet and imported into IBM-SPSS (v.21.0) for further analysis.
Results
Demographic characteristics
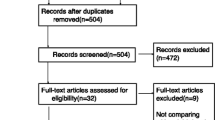
Figure 1 shows the study flow chart of 75 patients who were eligible for participation: 39 were randomized to the fentanyl group and 36 to the control group. There were no significant between-group differences in baseline characteristics (Table 1).
Baseline pain
At rest (T0), 57 % (95 % confidence interval (CI), 45–69 %] of patients had baseline pain (BPS >3), with a median score of 4 (3–5). There were no significant differences in the prevalence of baseline pain between the fentanyl and control groups [54 % (95 % CI, 37–70 %)] versus 61 % [95 % CI, 44–77 %]; p = 0.641]. A total of 17 % of patients (95 % CI, 10–28 %) had significant baseline pain (BPS >5), without significant between-group differences at baseline [13 % (95 % CI, 4–27 %) for the fentanyl group versus 22 % (95 % CI, 10–39 %) for the control group (p = 0.365)].
Procedural pain
The median dose of fentanyl used in the fentanyl group was 70 µg (58–100). The median BPS score was 5 [4–7] for the fentanyl group versus 6 [5–7] for the control group during the turning time (Tmov), a non-significant difference (p = 0.139).
The pain incidence rate (i.e. BPS >3) during turning for all patients was 84 % (95 % CI, 74–91 %), with a median score of 6 [5–7]. However, the incidence rate during turning was significantly lower in the fentanyl group [74 % (95 % CI, 58–87 %) vs. 94 % (95 % CI, 81–99 %); p = 0.026], with a relative risk of 0.79 (95 % CI, 0.64–0.96; p = 0.039). In other words, administration of fentanyl prior to the procedure was associated with a significant 21 % reduction in the risk of pain during turning. Pain during turning was thus prevented in one of every 5 patients receiving fentanyl (NNT = 5; 95 % CI, 3–22) (Fig. 2).
Incidence of pain scores, during turning (Tmov) according to the behavioral pain scale (BPS). Tmov = (T1 = end of first turn, T2 = end of second turn). Tmov were evaluated by the arithmetic mean of T1 and T2. Pain incidence = number of patients with BPS >3/total number of patients evaluated × 100 Significant pain incidence = number of patients with BPS >5/total number of patients evaluated × 100; p < 0.05
Regarding the overall incidence of significant pain (BPS >5), 56 % of all patients (95 % CI, 44–67 %) had significant pain during turning, without significant between-group differences (fentanyl vs. control, respectively) in the incidence of significant pain during turning [49 % (95 % CI, 33–65 %) vs. 64 % (95 % CI, 48–80 %); p = 0.246], with a relative risk of 0.76 (95 % CI, 0.21–1.36; p = 0.130). In other words, administration of fentanyl was associated with a non-significant 24 % reduction in the risk of significant pain during turning (Fig. 2).
The results of behavior scores and the incidence of behaviors in T3, T4 and T5 are presented in the supplementary material (see Electronic Supplement 1 for pain behavior scores and the incidence of behaviors).
Magnitude of pain (AUC)
The fentanyl group had a lower magnitude of pain (AUC) than the control group [132 (IQR 108–150) vs. 147 (IQR 125–180); p = 0.016] during the turning procedure and in the 30 min after the procedure (from T1 to T5) (Fig. 3).
Area under the curve (AUC) of the fentanyl group and control group for the behavioral pain scale (BPS) scores from T1 to T5. Tmov (T1 = end of first turn, T2 = end of second turn), T3 = 5 min after T2, T4 = 15 min after T2, and T5 = 30 min after T2. Time from T1 to T2 was 3 min, from T2 to T3 it was 5 min, from T3 to T4 it was 10 min, and from T4 to T5 it was 15 min. The total time AUC (T1 to T5) was 33 min. AUC fentanyl group: 132 (IQR 108–150) (95 %CI 1.65–34); AUC control group: 147 (IQR 125–180) (95 %CI 1.64–34); Wilcoxon rank-sum test
Adverse events
No serious adverse events were registered. A total of 19 non-serious adverse events in 14 patients were recorded during T1 to T5 (respiratory depression, four events; transient hypotension, 14; and vomiting, 1), with no significant differences between the fentanyl and control groups (23 vs. 14 %, respectively; p = 0.381). The four episodes of respiratory depression occurred in patients receiving fentanyl, with one requiring a switch from pressure-support to assist-control ventilation mode. Nine of the 14 episodes of transient hypotension occurred in the fentanyl group and five in the control group, and three patients required increased doses of norepinephrine (two in the fentanyl group and one in the control group). The single case of vomiting occurred in the control group.
Vital signs
All vital signs evaluated increased during turning but decreased over time to baseline levels. We observed no differences between the two groups in vital sign parameters over time (Table 2).
Discussion
To our knowledge, this is the first study to show a pre-emptive IV fentanyl bolus reduces pain associated with turning in non-communicative MV patients. In this study, we tested the magnitude of pain behaviors to evaluate the efficacy of pre-emptive IV fentanyl.
The magnitude of pain over the entire time measured by AUC was significantly smaller for patients who received fentanyl. These patients had a significantly lower incidence of pain (more than 20 % lower than in patients who received placebo) during turning. Similarly, at 30 min after turning, the incidence of pain in the fentanyl group was 39 versus 64 % in the control group. These findings support the benefits of pre-emptive analgesia with IV fentanyl in a high percentage of patients.
Despite these positive findings, it is important to note that pre-emptive fentanyl analgesia did not relieve turning-related pain in a sizeable percentage of patients: three-quarters of patients treated with fentanyl showed pain during turning (Tmov), and nearly 50 % showed significant pain. We thus conclude that pain during turning was not well controlled in these critically ill patients. These results were not fully satisfactory but we believe they are relevant given the frequent use of a painful procedure such as turning in ICU patients. In addition, we consider that our results reinforce the concept of dynamic analgesia, an approach that merits further investigation.
This high incidence rate of pain could be attributable to various causes. First, even though we followed the recommended dose and time interval between administration and turning, these may not have been optimal in these patients. During MV, the dose of sufentanil needed to avoid turning-related pain in 90 % of patients was 0.15 µg/kg according to a prospective dose–response study [20]. Considering equivalencies among various opioids (morphine 1, fentanyl 100, sufentanil 1000) for a 70-kg patient, our mean dose of fentanyl was 25 % less than the previously reported effective dose of sufentanil (70 µg instead the theoretical 105 µg of fentanyl). Second, in addition to pain caused by turning, other associated components, such as anxiety and fear, may also have contributed to the increase in pain [21]. And third, a high percentage of patients had pain at rest. Baseline pain has been independently associated with greater pain during turning [7]. Similarly, other authors have reported that when pain at rest is well-controlled, procedural-related pain is lower [22].
Despite following a protocol for analgesia and sedation, and even though 90 % of our patients received a continuous infusion of morphine, nearly half the patients had pain at rest (significant pain in 25 %). These findings confirm previous reports and reflect the importance of this issue [23]. The causes are varied and could include increased sensitivity to analgesic, sedation, low use of validated scales to detect pain, variability in implementing these scales, and fear of side effects of analgesics. Pain at rest could also be secondary to various nursing procedures that patients undergo every day in a phenomenon called central sensitization [24].
While continuous opioid infusions are often used to minimize background or resting pain [3, 5], a more “dynamic” and “individualized” approach to analgesia may be needed during a procedure to address ‘pain upon pain” and each patient’s particular needs. Such an approach could reduce the overall pain incidence and side effects of cumulative opioid doses, while potentially decreasing long-term pain sequelae [25]. With the exception of suctioning, nursing procedures are not generally urgent, so planning pre-emptive analgesia should be a viable option to reduce pain associated with such interventions.
Although the study may be underpowered to assess adverse effects, our results suggest that preventive treatment with fentanyl was well tolerated. We observed no serious adverse events and found an incidence of non-serious adverse events similar to other authors [26]. De Jong et al. [26] showed that tramadol (an opioid analgesic) could reduce turning-related pain without causing serious adverse effects and with a low rate of non-serious adverse effects (17 %), similar to the 23 % observed in our fentanyl group [26]. Despite these findings regarding adverse events, the four episodes of respiratory depression in our study cannot be overlooked, and the risk of such events underscores the need to exercise caution when administering opioid analgesics. Nevertheless, ours is not the first study to report this adverse event. Casey et al. [27] reported similar findings (two episodes of respiratory depression in 30 cardiac surgery patients), although they used a different opioid analgesia (remifentanil) in their study.
Our study has several limitations. First, as a single-center study of mechanically ventilated patients, we cannot ensure that the lower pain incidence observed for single doses of fentanyl can be extrapolated to mechanically ventilated patients in other ICUs or to non-mechanically ventilated patients. The administration of pre-emptive fentanyl should be considered with caution in non-intubated patients because respiratory depression or apnea is a major safety problem. However, many ICU patients who require MV may benefit from pre-emptive analgesia. Second, we did not control for baseline pain rating, cumulative doses of analgesia received or chronic conditions that could have influenced BPS observations. However, double-blind randomization and covariance analysis using baseline pain and the amount of sedatives and opioids received ensured that the two groups were comparable. Third, since we only studied a single nursing procedure, we cannot necessarily extrapolate our results to other procedures. Nevertheless, our findings are valuable given that turning is one of the most frequently performed procedures for ICU patients [7]. And fourth, because this study focused on the effect of a single dose of fentanyl, we cannot extrapolate our results to the efficacy and safety of repeated doses. Despite these limitations, our findings are strengthened by the randomized, double-blind design and the fact that no patients were lost to follow-up. Additionally, as pain was assessed by a single investigator, inter-rater differences were avoided.
Conclusions
Our results suggest that a pre-emptive fentanyl bolus of 1 µg/kg fentanyl for medical patients or 1.5 µg/kg for surgical patients reduces pain associated with turning in critically ill MV patients. However, a higher dose of fentanyl might be needed to decrease “significant” pain. Further research on pre-emptive analgesics is warranted to identify variables that influence the incidence of turning-related pain and to improve the degree and duration of pain relief.
References
Barr J, Fraser GL, Puntillo KA, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R (2013) Clinical practice guidelines for the management of pain, agitation, and delirium in adult ICU patients. Crit Care Med 41:263–306
Celis-Rodríguez E, Birchenall C, de la Cal MÁ, Castorena Arellano G, Hernández A, Ceraso D, Díaz Cortés JC, Dueñas Castell C, Jimenez EJ, Meza JC, Muñoz Martínez T, Sosa García JO, Pacheco Tovar C, Pálizas F, Pardo Oviedo JM, Pinilla DI, Raffán-Sanabria F, Raimondi N, Righy Shinotsuka C, Suárez M, Ugarte S, Rubiano S (2013) Clinical practice guidelines for evidence-based management of sedoanalgesia in critically ill adult patients. Med Intensiva 37:519–574
Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, Binhas M, Genty C, Rolland C, Bosson JL (2007) Current practices in sedation and analgesia for MV critically ill patients: a prospective multicenter patient-based study. Anesthesiology 106:687–695 (quiz 891–2)
Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J, Investigators DOLOREA (2009) Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post hoc analysis of the DOLOREA Study. Anesthesiology 111:1308–1316
Martin J, Franck M, Fischer M, Spies C (2006) Sedation and analgesia in German intensive care units: how is it done in reality? Results of a patient-based survey of analgesia and sedation. Intensive Care Med 32:1137–1142
Puntillo KA, Wild LR, Morris AB, Stanik-Hutt J, Thompson CL, White C (2002) Practices and predictors of analgesic interventions for adults undergoing painful procedures. Am J Crit Care 11:415–429 (quiz 430–1)
Puntillo KA, Max A, Timsit JF, Vignoud L, Chanques G, Robleda G, Roche-Campo F, Mancebo J, Divatia JV, Soares M, Ionescu DC, Grintescu IM, Vasiliu IL, Maggiore SM, Rusinova K, Owczuk R, Egerod I, Papathanassoglou ED, Kyranou M, Joynt GM, Burghi G, Freebairn RC, Ho KM, Kaarlola A, Gerritsen RT, Kesecioglu J, Sulaj MM, Norrenberg M, Benoit DD, Seha MS, Hennein A, Periera FJ, Benbenishty JS, Abroug F, Aquilina A, Monte JR, An Y, Azoulay E (2014) Determinants of procedural pain intensity in the intensive care unit. The Europain® study. Am J Respir Crit Care Med 189:39–47
Arroyo-Novoa CM, Figueroa-Ramos MI, Puntillo KA, Stanik-Hutt J, Thompson CL, Wild LR (2008) Pain related to tracheal suctioning in awake acutely and critically ill adults: a descriptive study. Intensive Crit Care Nurs 24:20–27
Robleda G, Roche-Campo F, Membrilla-Martinez L, Fernández-Lucio A, Villamor-Vázquez M, Merten A, Gich I, Mancebo J, Català-Puigbó E, Baños J-E (2015) Evaluation of pain during mobilization and endotracheal aspiration in critical patients. Med Intensiva. doi:10.1016/j.medin.2015.03.004
Fulton TR, Peet GI, McGrath MA, Hilton JD, Smith RE, Sigurdsson AF, Forrest QG (2000) Effects of 3 analgesic regimens on the perception of pain after removal of femoral artery sheaths. Am J Crit Care 9:125–129
Puntillo KA, Stannard D, Miaskowski C, Kehrle K, Gleeson S (2002) Use of a pain assessment and intervention notation (P.A.I.N.) tool in critical care nursing practice: nurses’ evaluations. Heart Lung 31:303–314
Brocas E, Dupont H, Paugam-Burtz C, Servin F, Mantz J, Desmonts JM (2002) Bispectral index variations during tracheal suction in MV critically ill patients: effect of an alfentanil bolus. Intensive Care Med 28:211–213
Yiannakopoulos CKA, Kanellopoulos AD (2004) Innoxious removal of suction drains. Orthopedics 27:412–414
Haynes JM (2015) Randomized controlled trial of cryoanalgesia (Ice Bag) to reduce pain associated with arterial puncture. Respir Care 60:1–5
Puntillo KA, White C, Morris AB, Perdue ST, Stanik-Hutt J, Thompson CL, Wild LR (2001) Patients’ perceptions and responses to procedural pain: results from thunder project II. Am J Crit Care 10:238–251
Payen JF, Bru O, Bosson JL, Lagrasta A, Novel E, Deschaux I, Lavagne P, Jacquot C (2001) Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 29:2258–2263
Young J, Siffleet J, Nikoletti S, Shaw T (2006) Use of a behavioural pain scale to assess pain in ventilated, unconscious and/or sedated patients. Intensive Crit Care Nurs 22:32–39
Vázquez M, Pardavila MI, Lucia M, Aguado Y, Margall MÁ, Asiain MC (2011) Pain assessment in turning procedures for patients with invasive mechanical ventilation. Nurs Crit Care 16:178–185
Gelinas C, Puntillo KA, Joffe AM (2013) A validated approach to evaluating psychometric properties of pain assessment tools for use in nonverbal critically ill adults. Semin Respir Crit Care Med 34:153–168
Chaveron D, Silva S, Sanchez-Verlaan P, Conil JM, Sommet A, Geeraerts T, Génestal M, Minville V, Fourcade O (2012) The 90 % effective dose of a sufentanil bolus for the management of painful positioning in intubated patients in the ICU. Eur J Anaesthesiol 29:280–285
Gélinas C, Chanques G, Puntillo K (2014) In pursuit of pain: recent advances and future directions in pain assessment in the ICU. Intensive Care Med 40:1009–1014
Ahlers SJ, van Gulik L, van Dongen EP, Bruins P, van de Garde EM, van Boven WJ, Tibboel D, Knibbe CA (2012) Efficacy of an intravenous bolus of morphine 2.5 versus morphine 7.5 mg for procedural pain relief in postoperative cardiothoracic patients in the intensive care unit: a randomised double-blind controlled trial. Anaesth Intensive Care 40:417–426
Chanques G, Sebbane M, Barbotte E, Viel E, Eledjam JJ, Jaber S (2007) A prospective study of pain at rest: incidence and characteristics of an unrecognized symptom in surgical and trauma versus medical intensive care unit patients. Anesthesiology 107:858–860
Woolf CJ, Chong MS (1993) Preemptive analgesia-treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 77:362–379
Granja C, Gomes E, Amaro A, Ribeiro O, Jones C, Carneiro A, Costa-Pereira A; JMIP Study Group (2008) Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med 36:2801–2809
de Jong A, Molinari N, de Lattre S, Gniadek C, Carr J, Conseil M, Susbielles MP, Jung B, Jaber S, Chanques G (2013) Decreasing severe pain and serious adverse events while moving intensive care unit patients: a prospective interventional study (the NURSE-DO project). Crit Care 17:R74
Casey E, Lane A, Kuriakose D, McGeary S, Hayes N, Phelan D, Buggy D (2010) Bolus remifentanil for chest drain removal in ICU: a randomized double-blind comparison of three modes of analgesia in post-cardiac surgical patients. Intensive Care Med 36:1380–1385
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Take-home message: Turning of critically ill patients is a common but painful procedure. Pre-emptive analgesia with an intravenous fentanyl bolus before turning minimizes pain and is safe.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Robleda, G., Roche-Campo, F., Sendra, MÀ. et al. Fentanyl as pre-emptive treatment of pain associated with turning mechanically ventilated patients: a randomized controlled feasibility study. Intensive Care Med 42, 183–191 (2016). https://doi.org/10.1007/s00134-015-4112-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-4112-7