Abstract
Purpose
Amikacin requires pharmacodynamic targets of peak serum concentration (C max) of 8–10 times the minimal inhibitory concentration, corresponding to a target C max of 60–80 mg/L for the less susceptible bacteria. Even with new dosing regimens of 25 mg/kg, 30 % of patients do not meet the pharmacodynamic target. We aimed to identify predictive factors for insufficient C max in a population of critically ill patients.
Methods
Prospective observational monocentric study of patients admitted to a general ICU and requiring a loading dose of amikacin. Amikacin was administered intravenously at the dose of 25 mg/kg of total body weight. Independent determinants of C max < 60 mg/L were identified by mixed model multivariate analysis.
Results
Over a 1-year period, 181 episodes in 146 patients (SAPS 2 = 51 [41–68]) were included. At inclusion, the SOFA score was 8 [6–12], 119 (66 %) episodes required vasopressors, 150 (83 %) mechanical ventilation, and 81 (45 %) renal replacement therapy. The amikacin C max was 69 [54.9–84.4] mg/L. Overall, 60 (33 %) episodes had a C max < 60 mg/L. The risk of C max < 60 mg/L associated with BMI < 25 kg/m2 varied across quarters of inclusion. Independent risk factors for C max < 60 mg/L were a BMI < 25 kg/m2 over the first quarter (odds ratio (OR) 15.95, 95 % confidence interval (CI) [3.68–69.20], p < 0.001) and positive 24-h fluid balance (OR per 250-mL increment 1.06, 95 % [CI 1.01–1.11], p = 0.018).
Conclusions
Despite an amikacin dose of 25 mg/kg of total body weight, 33 % of patients still had an amikacin C max < 60 mg/L. Positive 24-h fluid balance was identified as a predictive factor of C max < 60 mg/L. When total body weight is used, low BMI tended to be associated with amikacin underdosing. These results suggest the need for higher doses in patients with a positive 24-h fluid balance in order to reach adequate therapeutic targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The management of severe infections in the ICU represents a major challenge for clinicians, and prompt initiation of effective antibiotic therapy is a critical intervention to improve patients’ survival [1, 2]. Although recent guidelines [1] do not specifically recommend combination antimicrobial therapy, a recent meta-analysis showed a survival improvement with combination therapy over monotherapy in patients with septic shock [3]. Empirical combination antibiotic therapy aims to cover Gram-negative bacilli, and usually includes a β-lactam in combination with an aminoglycoside or a fluoroquinolone, data suggesting that aminoglycosides could offer broader coverage [4]. Amikacin is the aminoglycoside of choice given its good bactericidal activity on Pseudomonas aeruginosa and the low resistance rate observed with other Gram-negative bacilli [5].
The antibacterial effect of amikacin on Gram-negative bacilli is determined by the ratio of peak serum concentration (C max) over the minimal inhibitory concentration (MIC) of the targeted pathogen (C max/MIC ratio). Optimal antibacterial activity and clinical response rate are achieved with a C max/MIC ratio of between 8 and 10 [6, 7]. In critically ill patients with severe infections, antimicrobial therapy should target difficult-to-treat pathogens such as P. aeruginosa and enterobacteriaceae, with MIC as high as 8 mg/L for amikacin [8]. The target C max for amikacin should therefore be 64–80 mg/L. A target C max of ≥60 mg/L is considered as clinically relevant, and has been recommended by the latest French guidelines on aminoglycoside use [9].
It has been well demonstrated that critically ill patients have increased distribution volumes for aminoglycosides [10, 11] and recent studies suggest that, in such patients, loading doses of 25–30 mg/kg are necessary to achieve the pharmacodynamic target of C max ≥ 64 mg/L [12, 13]. However, in a previous study, an increased loading dose of 25 mg/kg of total body weight (TBW) failed to achieve an amikacin C max ≥ 64 mg/L in 30 % of patients, while 36 % had a C max over the pharmacodynamic objective of 80 mg/L [12]. Indeed, there is considerable inter- and intra-individual variability in the distribution volume of amikacin, which is a hydrophilic molecule with very low serum protein binding [14].
Although factors increasing amikacin distribution volume have been identified in critically ill patients [15], to date no study has identified the predictive factors of insufficient amikacin C max in critically ill patients, and the relationship between C max and outcome is not known when a high initial dosing regimen is used. We therefore undertook a prospective study to identify the predictive factors of insufficient amikacin C max concentrations and their potential impact on patient outcomes [16].
Materials and methods
Study design and patients
We conducted a prospective observational study in the 25-bed general ICU of a university hospital from December 2011 to January 2013. Inclusion criteria were age ≥18 years and a suspected Gram-negative infection requiring an antimicrobial treatment, including a loading dose of intravenous amikacin. Exclusion criteria were (1) administration of any dose of amikacin within the previous 7 days; (2) incorrect amikacin regimen (<22.5 or >27.5 mg/kg); (3) incorrect time of amikacin infusion (±5 min); (4) incorrect time of C max measurement (±15 min); or (5) the absence of C max measurement. A patient could be included more than once, provided that the last amikacin dose was given more than 7 days before inclusion. The study protocol was approved by the local hospital ethics committee and an information letter was given to the patient or to the family whenever possible.
Study endpoints
Amikacin C max ≥ 60 mg/L after the first injection was considered the target concentration and corresponded to the primary endpoint. This threshold is less stringent than 64 mg/L, as clinically relevant, and is recommended by the latest French guidelines on aminoglycoside use [9]. Subsequent amikacin injections were not recorded. All patients were followed until hospital discharge or death. Secondary endpoints were (1) hospital and ICU mortality; (2) hospital and ICU length of stay; (3) duration of supportive therapies (catecholamines, mechanical ventilation, and renal replacement therapy). Acute kidney injury (AKI) in patients alive at ICU discharge was monitored in patients with amikacin overdosage, as defined by a C max > 80 mg/L.
Amikacin administration and serum concentration dosage
Amikacin was administered according to the standardized protocol of our ICU: 25 mg/kg of TBW (weight of the day, using a weighing bed), diluted in 50 ml of NaCl 0.9 % and continuously infused over a 30-min duration. Peak amikacin concentration was measured 30 min after the end of infusion (C max), and trough serum concentration 24 h after the end of infusion (C min). The toxicology laboratory performed amikacin measurements as a routine procedure available 24 h a day, 7 days a week, using a fluorescence polarization immunoassay (FPIA) [17].
Data collection
Data were prospectively collected for the study using a standardized form, including demographics, comorbidities, and reason for admission. Disease severity was characterized by the Simplified Acute Physiology Score 2 (SAPS 2) [18] and chronic health status by the Knaus chronic health status score [19].
At inclusion, i.e. on the day of amikacin administration, we recorded clinical and biological parameters. Organ dysfunction was assessed using the Sequential Organ Failure Assessment (SOFA) score [20]. In patients discharged alive from ICU, AKI was defined as a RIFLE score “R” or “I” or “F” [21]. Glomerular filtration rate (GFR) was based on urinary creatinine excretion. Fluid intake was defined by the addition of volume of saline, colloids, and transfusions. Saline used for intravenous medication was not taken into account. Fluid output was defined by the addition of volume of urine, ultrafiltration, and drains. Fluid balance was defined as the difference between fluid intake and fluid output. Proteinemia and hematocrit were recorded on inclusion and 24 h before inclusion and the delta was calculated as (X h0 − X h−24)/(X h0 + X h−24)/2. Pharmacodynamic/pharmacokinetic parameters, including patients’ weight, were recorded at inclusion. Microbiologic variables, including site of infection and identified pathogen, were recorded at inclusion and at ICU discharge or death. Associated nephrotoxic treatments were defined as the use of vancomycin, colimycin, or iodinated contrast material at any time during the patients’ ICU stay.
Statistical analysis
To perform a multivariate analysis with six variables [22, 23], we considered that 60 patients were needed in the C max < 60 mg/L group. On the basis of a study using the same amikacin regimen and showing 30 % of patients with C max < 64 mg/L [12], we calculated that 180 patients had to be included for a minimum of 60 patients in the C max < 60 mg/L group.
Categorical variables were expressed as numbers and percentages, and continuous variables as median and interquartile range. The statistical unit of analysis was the episode of amikacin administration.
For the primary objective, demographics and clinical and biological factors associated with a C max < 60 mg/L were identified using univariate mixed models, introducing the patient as a random effect, in order to take into account multiple episodes. Factors associated with a C max < 60 mg/L in the univariate analysis (p < 0.10), or clinically relevant, were included in a multivariate mixed model. Colinearity between independent factors was investigated. In the case of colinearity between factors, the most clinically relevant factor was chosen to construct the multivariate model. Unadjusted and adjusted odds ratios were obtained, with their 95 % confidence interval [OR (95 % CI)].
The variable “24-h fluid balance” could not be collected in all patients, because fluid intake and output in the past 24 h could not be monitored in patients included on the day of ICU admission, resulting in missing data. Missing data were handled according to a recently published algorithm using multiple imputations [24, 25]. This analysis constituted a sensitivity analysis.
Statistical analyses were performed using R version 2.15.2 (©2012 The R Foundation for Statistical Computing). A p value less than 0.05 was considered significant. Mixed models were computed using the “lme4” package, and the “mi” package was used for multiple imputations.
Results
Patients
From December 2011 to January 2013, 248 episodes of amikacin initiation in patients with suspected Gram-negative infections were screened for eligibility, of which 67 were excluded (Fig. 1). Therefore, 181 episodes in 146 patients were included in the final analysis (Fig. 1). Patient characteristics at admission and at inclusion are reported in Table 1. Main reasons for ICU admission were acute respiratory failure and severe sepsis/septic shock. Patients were severely ill, as reflected by high admission SAPS 2 and SOFA scores. BMI was 24.6 [21.7–29.4] kg/m2.
At inclusion, the SOFA score was 8 [6–12], 119 (66 %) patients being treated with catecholamines, 150 (83 %) were under mechanical ventilation, and 81 (45 %) under renal replacement therapy (Table 1). Of the 81 patients undergoing renal replacement at inclusion, 70 (86.4 %) were under intermittent hemodialysis and 11 (13.6 %) were under continuous veno-venous hemodialysis. Extracorporeal membrane oxygenation (ECMO) support was used in 28 (15 %) patients. Fluid balance in the 24 h preceding inclusion was 0 [−1,200 to 1,200] mL, fluid balance in the 6 h preceding inclusion was 300 [−150 to 1,000] mL.
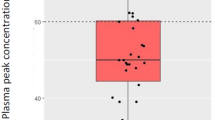
Amikacin pharmacokinetic parameters are reported in Table 2. The C max was 69 [54.9–84.4] mg/L. The pharmacodynamic target of C max ≥ 60 mg/L was not reached in 60 (33 %) episodes. C min was recorded in 147 episodes (81.2 %), and was above the toxicity threshold of 5 mg/L in 96 (65.3 %) episodes. Of the 70 patients undergoing intermittent hemodialysis, 51 (73 %) had their renal replacement therapy session between the C max and the C min. The distribution of C max among the 181 episodes is represented in Fig. 2.
Detailed sites of infection and microorganisms are reported in the supplementary material (Table 1).
ICU and hospital mortality rates were 45 and 51 %, respectively. Durations of stay in the ICU and in hospital were 18 [7–42] days and 40 [20–73] days, respectively. Median supportive treatment duration was 7 days for catecholamines, 13 days for mechanical ventilation, and 2 days for renal replacement therapy. Of the 100 patients alive at ICU discharge, 39 (39 %) had AKI, and 30 (30 %) had had associated nephrotoxic treatment.
Primary outcome: predictive factors for C max < 60 mg/L
Complete univariate analysis is reported in Table 2 of the supplementary material. Identified predictive factors were BMI < 25 kg/m2 at admission (22.8 [20.3–26.5] kg/m2 for C max < 60 versus 25.1 [23.1–30.1] for C max ≥ 60, OR = 2.8 [1.4–5.6]); cirrhosis (8 (13.3 %) versus 4 (3.3 %), OR = 5.3 [1.4–19.9]); ascites (8 (13.3 %) versus 6 (5 %), OR = 3.2 [1.0–10.3]); 24-h fluid balance (550 [−450 to 2,450] mL versus −500 [−1,600 to 750], OR = 1.7 [1.0–1.1] per 250-mL increment]; and 24-h fluid intake (1,550 [500–3,060] mL versus 950 [75–2,000], OR = 1.1 [1.0–1.1] per 250-mL increment). In contrast, SAPS 2 at admission, SOFA score at inclusion, use of ECMO, use of catecholamine or mechanical ventilation, 6-h fluid balance, 24-h hematocrit and proteinemia delta, reason for antibiotherapy initiation and site of infection were not different between groups. Figure 3 represents the distribution of 24-h fluid balance according to amikacin C max.
Analysis of amikacin C max distribution over time showed a statistically significant difference between periods (p < 0.001), with C max < 60 mg/L more frequent in the first quarter, as compared to the three others (66.6 versus 31.5 %, OR = 0.2 [0.1–0.5]). Moreover, we showed that the risk of C max < 60 mg/L associated with BMI < 25 kg/m2 varied across quarters, corresponding to a significant interaction between inclusion period and BMI. Therefore, this interaction was introduced into the multivariate model. Supplementary Fig. 1 represents identified risk factors for amikacin C max < 60 mg/L according to the inclusion period. No other interaction was identified.
Multivariate analysis of factors associated with amikacin C max < 60 mg/L (Table 3) retained BMI < 25 kg/m2 over the first period (OR 15.95, 95 % CI [3.68–69.20], p < 0.001 in first period, but OR 2.04, 95 % CI [0.70–5.89], p = 0.19 in second period] and 24-h fluid balance (OR per 250-mL increment 1.06, 95 % CI [1.01–1.11], p = 0.018). Cirrhosis was not significantly associated with C max < 60 mg/L (OR = 4.15, [0.92–18.76], p = 0.065). Sensitivity analysis after multiple imputations did not show major differences with the first model (Table 3).
Secondary outcomes
Univariate analysis of outcomes at ICU and hospital discharge or death according to C max < 60 mg/L are reported in Table 3 of the supplementary material. Amikacin C max < 60 mg/L was associated with a significant increased ICU mortality at day 14 (27 versus 13 %, p = 0.03) in univariate analysis, but did not remain significant after adjustment with the SOFA score at inclusion. Among ICU survivors, durations of mechanical ventilation, renal replacement therapy, and catecholamine treatment were not influenced by an amikacin C max < 60 mg/L. ICU discharge rate at day 14 was equivalent in both groups.
In patients discharged alive from ICU, there was no statistical difference in the incidence of AKI between the group of patients with C max > 80 mg/L (n = 13, 46.4 %) and the group of patients with C max < 80 mg/L (n = 26, 44.1 %) (p = 0.82).
Discussion
In this prospective study performed in a large cohort of critically ill patients and receiving an amikacin loading dose of 25 mg/kg of TBW, we identified a positive 24-h fluid balance as being an independent risk factor for an amikacin C max < 60 mg/L. BMI tended to be identified as an independent risk factor, but its effect varied across periods. Our study evaluated amikacin pharmacokinetics in a mixed medical/surgical population of ICU patients with high severity scores at admission and inclusion, including patients with shock requiring mechanical circulatory support (ECMO).
The only previous study with a similar design included 74 general ICU patients with severe sepsis/septic shock and had failed to detect any correlation between amikacin C max and clinical or hemodynamic variables, partly owing to the important interindividual variability of amikacin distribution volume in this specific population [12].
Our results are in accordance with previous studies that showed increased amikacin distribution volume in critically ill patients [11, 23, 24] and stress the need for even higher doses and/or a tailored regimen adapted to each patient individually. We used TBW for dose calculation because it has been shown that use of ideal body weight could lead to underdosing in critically ill patients [12]. Indeed, use of TBW takes into account patients with both capillary leakage and large amounts of water retention, which significantly increases amikacin distribution volume. The target used in the present study, namely a C max < 60 mg/L, was slightly different from that of C max < 64 mg/L, dictated by pharmacodynamic data as strictly eight times the MIC of the less susceptible pathogens potentially encountered. We chose this threshold because it is recommended by the latest French guidelines on aminoglycoside use [9], and has been used in recently published studies on amikacin loading doses [13].
Using a TBW dose of 25 mg/kg, we found that BMI < 25 kg/m2 tended to be independently associated with amikacin underdosing. Those results are concordant with previously published data [12] and are at least partly explained by the use of TBW versus ideal body weight (IBW) or adjusted body weight (ABW). Indeed, as amikacin is a hydrophilic molecule, use of TBW for dose calculation overestimates amikacin distribution volume in patients with high BMI and increased fat compartment. Use of ABW for dose calculation is proposed to avoid this problem, but does not take into account fluid retention that can occur in critically ill patients. However, use of ABW is currently recommended in obese patients, regarding the use of hydrophilic molecules [26]. Cirrhosis tended to be associated with amikacin underdosing, but this was not statistically significant because of the small number of patients per analyzed group. This finding is consistent with the fact that cirrhotic patients with sepsis have increased amikacin distribution volume [27]. Finally, our multivariate analysis identified 24-h fluid balance as a predictive factor for C max < 60 mg/L. Fluid balance had not been identified in previous studies as a risk factor for amikacin underdosing. We believe this may be due to an insufficient number of patients included in previous studies and to the use in our study of a less strict cutoff value for defining amikacin underdosing.
Severity scores at admission and at inclusion were not predictive of amikacin C max, nor were the use of catecholamines, mechanical ventilation, or renal replacement therapy. Hemodilution parameters such as 24-h proteinemia and hematocrit delta were not associated with amikacin 60 mg/L. Failure to identify clinical and biological variables associated with amikacin underdosage has been attributed to an important interindividual variability of amikacin pharmacokinetics [28]. These results support amikacin management by tight serum monitoring in critically ill patients [29]. Data on ECMO and aminoglycosides pharmacokinetics are scarce. Pediatric studies suggest that ECMO in neonates increases gentamicin distribution volume, but generalization to adults is not possible because the volume of blood contained in an ECMO circuit is superior to that in a neonate [30, 31]. In our study, the use of ECMO was not associated with a lower amikacin C max. Therefore, our data do not support amikacin dose adjustment in this subset of patients.
Using the definition of AKI established by international consensus [21], we found that C max > 80 mg/L was not associated with an increase of acute kidney injury at ICU discharge. However a high C max is associated with an increase of the duration with trough concentration above the nephrotoxic threshold of 5 mg/L. The interpretation of these results is difficult, as AKI is multifactorial in ICU, and 30 % of our patients had received other nephrotoxic drugs during their stay. Furthermore, these results, in accordance with those of a previous study that reported no correlations between amikacin C max and renal function at ICU discharge [13], should encourage higher amikacin loading doses in order to achieve the pharmacodynamic target in a higher percentage of patients. However, we did not analyze subsequent trough and peak concentrations after the first 24 h of the study.
This study has several limitations. First, it is a single-center study in the general ICU of a tertiary university hospital with 19 % of patients admitted for postoperative care of cardiac surgery, and our results may not be generalizable to other ICUs. Second, 22 % of screened episodes were not included because of an error in the protocol of amikacin administration or C max measurement. However, the majority of protocol errors concerned C max measurements, and amikacin was incorrectly administered in only 19 (7.7 %) episodes. These rates are explained by strict quality criteria that could not always be met in emergency situations at the bedside. Third, considering the correct time of C max measurement, the chosen interval of ±15 min as an exclusion criteria could be too wide, considering the short half-life of amikacin, and could have impacted the accuracy of C max measurement. Fourth, although patients were included consecutively over the 1-year inclusion period, amikacin C max distribution varied with the period of inclusion, and patients were more frequently underdosed in the first quarter of inclusion. We could not identify any cause for this effect, and despite statistical adjustment in the multivariate model, we cannot be certain that these factors did not impact on our final results. A population pharmacokinetic analysis approach could have overcome some of our study limitations; however, this possibility was not taken into account at study initiation, and the collected data were not sufficient to carry it out post hoc.
Conclusion
Despite a loading dose of 25 mg/kg of TBW of amikacin, 33 % of critically ill patients did not reach the target of C max ≥ 60 mg/L. A positive 24-h fluid balance was identified as a risk factor for amikacin C max < 60 mg/L. On the basis of the literature where regimens of 30 mg/kg TBW have been previously used in critically ill patients without increased nephrotoxicity [13], our results suggest the use of a 30 mg/kg dose in patients with a positive 24-h fluid balance, in association with a tight therapeutic drug monitoring. When TBW is used, patients with low BMI tend to have lower amikacin C max, but this needs to be confirmed in further studies. Whether these regimens are associated with improved outcomes is unknown. A prospective randomized controlled study in critically ill patients is warranted to assess the effects of higher loading doses of amikacin on C max, infection control and survival, and its impact on renal and hearing functions.
References
Dellinger RP, Levy MM, Rhodes A et al (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228. doi:10.1007/s00134-012-2769-8
Ferrer R, Artigas A, Suarez D et al (2009) Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med 180:861–866. doi:10.1164/rccm.200812-1912OC
Kumar A, Safdar N, Kethireddy S, Chateau D (2010) A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med 38:1651–1664. doi:10.1097/CCM.0b013e3181e96b91
Micek ST, Welch EC, Khan J et al (2010) Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother 54:1742–1748. doi:10.1128/AAC.01365-09
Observatoire National de l’Epidémiologie de la Resistance Bactérienne aux Antibiotiques (ONERBA) (2011) Rapport d’activité 2009-10/Annual report 2009-10. www.onerba.org, Vivactis Plus. Accessed 20 Mar 2014
Moore RD, Lietman PS, Smith CR (1987) Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 155:93–99
Zelenitsky SA, Harding GKM, Sun S et al (2003) Treatment and outcome of Pseudomonas aeruginosa bacteraemia: an antibiotic pharmacodynamic analysis. J Antimicrob Chemother 52:668–674. doi:10.1093/jac/dkg403
EUCAST (2013) Aminoglycosides: EUCAST clinical MIC breakpoints. http://www.eucast.org/clinical_breakpoints/. Accessed 20 Mar 2014
ANSM (2011) Bon usage des aminosides administrés par voie injectable: gentamicine, tobramycine, netilmicine, amikacine—Mise au point. http://ansm.sante.fr/Mediatheque/Publications/Recommandations-Medicaments. Accessed 20 Mar 2014
Beckhouse MJ, Whyte IM, Byth PL et al (1988) Altered aminoglycoside pharmacokinetics in the critically ill. Anaesth Intensive Care 16:418–422
Marik PE, Havlik I, Monteagudo FS, Lipman J (1991) The pharmacokinetic of amikacin in critically ill adult and paediatric patients: comparison of once- versus twice-daily dosing regimens. J Antimicrob Chemother 27 (Suppl C):81–89
Taccone FS, Laterre P-F, Spapen H et al (2010) Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care 14:R53. doi:10.1186/cc8945
Gálvez R, Luengo C, Cornejo R et al (2011) Higher than recommended amikacin loading doses achieve pharmacokinetic targets without associated toxicity. Int J Antimicrob Agents 38:146–151. doi:10.1016/j.ijantimicag.2011.03.022
Craig W (2010) Amikacin. In: Kucer’s the use of antibiotics, 6th edn. Edward Arnold, London, pp 712–726
Lugo G, Castañeda-Hernández G (1997) Relationship between hemodynamic and vital support measures and pharmacokinetic variability of amikacin in critically ill patients with sepsis. Crit Care Med 25:806–811
de Montmollin E, Gault N, Bouadma L et al (2013) Risk factors for insufficient serum amikacin peak concentrations in critically ill adults. 53rd ICAAC, Denver, Colorado. Category A, 1035
Blaser J, König C, Fatio R et al (1995) Multicenter quality control study of amikacin assay for monitoring once-daily dosing regimens. International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. Ther Drug Monit 17:133–136
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Knaus WA, Zimmerman JE, Wagner DP et al (1981) APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med 9:591–597
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Bellomo R, Ronco C, Kellum JA et al (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi:10.1186/cc2872
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387. doi:10.1002/(SICI)1097-0258(19960229)15:4<361:AID-SIM168>3.0.CO;2-4
Peduzzi P, Concato J, Kemper E et al (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49:1373–1379
Vesin A, Azoulay E, Ruckly S et al (2013) Reporting and handling missing values in clinical studies in intensive care units. Intensive Care Med 39:1396–1404. doi:10.1007/s00134-013-2949-1
Rubin DB, Schenker N (1991) Multiple imputation in health-care databases: an overview and some applications. Stat Med 10:585–598
Pai MP, Bearden DT (2007) Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 27:1081–1091. doi:10.1592/phco.27.8.1081
Lugo G, Castañeda-Hernández G (1997) Amikacin Bayesian forecasting in critically ill patients with sepsis and cirrhosis. Ther Drug Monit 19:271–276
Pea F, Viale P, Furlanut M (2005) Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin Pharmacokinet 44:1009–1034
Nicolau DP (2003) Optimizing outcomes with antimicrobial therapy through pharmacodynamic profiling. J Infect Chemother 9:292–296. doi:10.1007/s10156-003-0279-x
Cohen P, Collart L, Prober CG et al (1990) Gentamicin pharmacokinetics in neonates undergoing extracorporal membrane oxygenation. Pediatr Infect Dis J 9:562–566
Southgate WM, DiPiro JT, Robertson AF (1989) Pharmacokinetics of gentamicin in neonates on extracorporeal membrane oxygenation. Antimicrob Agents Chemother 33:817–819
Acknowledgments
The authors would like to thank Marina Esposito-Farese for statistical advice. The authors would like to thank Jan Devenish for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take home message: An amikacin loading dose of 25 mg/kg is insufficient to reach the pharmacodynamic target in 33 % of critically ill patients, in particular in patients with a positive 24-h fluid balance. When total body weight is used, BMI < 25 kg/m2 tended to be associated with amikacin underdosing. This study suggests the need for tight amikacin serum monitoring in every ICU patient, and higher loading doses in this particular subset of patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Montmollin, E., Bouadma, L., Gault, N. et al. Predictors of insufficient amikacin peak concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med 40, 998–1005 (2014). https://doi.org/10.1007/s00134-014-3276-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3276-x