Abstract
Objectives
This study aimed to assess the incidence of amikacin plasma peak concentration (Cmax) below 60 mg·L−1 in critically ill children receiving an amikacin dosing regimen of 30 mg kg−1·day−1. Secondary objectives were to identify factors associated with low Cmax and to assess the incidence of acute kidney injury (AKI).
Methods
A retrospective observational study was performed in two French pediatric intensive care units. All admitted children who received 30 mg·kg−1 amikacin and had a Cmax measurement were eligible. Clinical and biological data, amikacin dose, and concentrations were collected.
Results
In total, 30 patients were included, aged from 3 weeks to 7 years. They received a median amikacin dosage of 30 mg kg−1·day−1 (range 29–33) based on admission body weight (BW), corresponding to 27 mg kg−1·day−1 (range 24–30) based on actual BW. Cmax was < 60 mg·L−1 in 21 (70%) children and none had a Cmax ≥ 80 mg·L−1. Among the 15 patients with a measured minimum inhibitory concentration (MIC), 13 (87%) had a Cmax/MIC ratio > 8. Univariate analysis showed that factors associated with Cmax < 60 mg·L−1 were high estimated glomerular filtration rate (p = 0.015) and low blood urea concentration (p = 0.001). AKI progression or occurrence was observed after amikacin administration in two (7%) and six (21%) patients, respectively.
Conclusions
Despite the administration of the maximal recommended amikacin dose, Cmax was below the pharmacokinetic target in 70% of our pediatric population. Further studies are needed to develop a pharmacokinetic model in a population of critically ill children to optimize target attainment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite the administration of the maximal recommended amikacin dose of 30 mg·kg−1, plasma peak concentration was below the 60 mg·L−1 pharmacokinetic target in 70% of our critically ill pediatric patients and none had a plasma peak concentration ≥ 80 mg·L−1. |
Among the patients with a documented infection and a measurement of minimum inhibitory concentration (MIC), 13 (83%) had a peak/MIC ratio > 8. |
Peak above the 60 mg·L−1 pharmacokinetic target was associated with low blood urea concentration and high estimated glomerular filtration rate in univariate analysis. |
1 Introduction
Appropriate and early antibiotic administration reduces mortality linked to septic shock [1]. The aminoglycoside amikacin has high bactericidal activity, especially against Gram-negative bacilli, and even in cases of extended spectrum beta-lactamase-producing Enterobacteriaceae [2]. This antibiotic is thus particularly useful in combination therapy to broaden the spectrum in cases of nosocomial sepsis [3]. However, this must be balanced against the fact that administration of aminoglycosides is a well-known risk factor for acute kidney injury (AKI) [4, 5].
French national guidelines recommend amikacin plasma concentration monitoring in critically ill children, especially for those with sepsis [6]. Amikacin efficacy is associated with reaching a peak plasma concentration (Cmax) between 60 and 80 mg·L−1, corresponding to eight to ten times the minimum inhibitory concentration (MIC) breakpoint for sensitive strains [7, 8]. A dose of 25–30 mg·kg−1 is recommended to reach this optimal Cmax for patients with an increased volume of distribution (Vd) [6]. In previous adult studies, reaching this target was associated with reduced intensive care unit mortality without an increased risk of AKI [9, 10].
In one of these studies, of the 110 critically ill adults receiving an amikacin dose of 30 mg·kg−1, 40% achieved the target Cmax and another 40% had a Cmax > 80 mg·L−1 [9]. Other adult studies have shown that a positive 24-h fluid balance, a low serum sodium concentration, and a body mass index (BMI) < 25 kg·m−2 were predictive factors of Cmax < 60 mg·L−1 [11,12,13]. This suggests that a modification of amikacin pharmacokinetic parameters can contribute to inter- and intrasubject variability in critically ill patients [10, 14].
Reports in the literature describing amikacin plasma concentrations in critically ill children are scarce. One study showed that pediatric patients with burn wound sepsis required an initial amikacin dose ≥ 25 mg·kg−1 to reach the pharmacokinetic target [15]. Another study compared this cohort with a cohort of pediatric patients with cancer and febrile neutropenia and found that the pharmacokinetics of amikacin were impacted differently by the underlying disease [15]. In addition, a study conducted in 50 critically ill children showed that a high gentamicin dose of 8 mg·kg−1 was required to achieve the pharmacokinetic target [17]. Despite the recommendations, to our knowledge, high-dose amikacin has never been evaluated in critically ill children.
This retrospective study aimed to assess the incidence of Cmax < 60 mg·L−1 in critically ill children receiving an initial amikacin dose of 30 mg·kg−1. Secondary objectives were to identify factors associated with low Cmax and to assess the incidence of AKI.
2 Materials and Methods
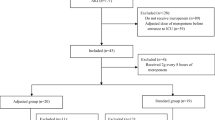
We conducted a retrospective observational study in all consecutive patients treated at two pediatric intensive care units (PICUs)—Necker Enfants Malades and Robert Debré Hospitals (Paris, France)—from November 2017 to June 2018. We included all patients aged < 18 years who received a once-daily intravenous amikacin dose of 30 mg·kg−1 calculated according to admission or actual body weight (BW) and with an amikacin Cmax measurement.
The exclusion criteria were inappropriate dosing (> 33 mg·kg−1 or < 27 mg·kg−1), inappropriate duration of amikacin infusion, or inappropriate sampling time (30 ± 15 min).
2.1 Amikacin Administration, Monitoring and Pharmacokinetic Analysis
Patients received an intravenous dose of amikacin (Mylan™, Saint Priest, France) 30 mg kg−1·day−1, in combination with another broad-spectrum antibiotic, in cases of suspected or proven bacterial infection requiring amikacin [6]. The infusion duration was 30 min and was performed using a programmable electric syringe through a central venous catheter (CVC) or peripheral venous access.
Cmax was measured 30 min after the end of the infusion via a peripheral blood sample or a CVC other than that used for amikacin whenever possible [6]. For each patient, amikacin pharmacokinetic monitoring was performed after the first or second injection. Trough level (Cmin) needed to be below 2.5 mg·L−1 to allow subsequent amikacin administration and was measured 24 h following the infusion. According to national recommendations, Cmax was targeted between 60 and 80 mg·L−1 and Cmin was targeted to be less than 2.5 mg·L−1 [6]. Treatment duration was based on the clinician’s decision and was usually limited to 3 days according to national recommendations [6].
2.2 Amikacin and Creatinine Assays
Amikacin plasma concentrations were analyzed using Architect immunoassay (Abbott, IL, USA) (lower limit of quantification at 2.0 µg·mL−1). Serum creatinine concentrations were measured using an enzymatic method that was traceable and standardizable with respect to the reference mass spectrometry method with isotope dilution (Architect C16000, Abbott Diagnostics, Lake Forest, IL, USA).
2.3 Data Collection
The following data were collected at the time of amikacin administration: age; sex; height; admission and actual BW; Z score for BMI; medical history including main diagnosis and comorbidities; pediatric logistic organ dysfunction version 2 (PELOD-2) at PICU admission; number of organ dysfunctions; need for vasopressors and/or inotropes; need for invasive mechanical ventilation, extracorporeal membrane oxygenation (ECMO), or continuous renal replacement therapy (CRRT) [18, 19]; reason for PICU admission; and condition treated with amikacin. Renal function data were also reported for blood urea level, serum creatinine level, urine output, fluid balance over the 24 h prior to amikacin administration, and coadministration of nephrotoxic drugs (tacrolimus, ciclosporin, furosemide, acetazolamide, acyclovir, vancomycin, and sulfonamide). Microbiological data included site of infection, pathogen identification and sensitivity (MIC), white blood cell count, procalcitonin, and C-reactive protein.
Pharmacokinetic parameters collected were amikacin dosage and Cmin and Cmax concentrations.
2.4 Definitions
Actual BW was defined as the weight measured on the day of amikacin administration. Hydric balance was the difference between fluid intake and output (urine output, drain, gastric aspirates, and insensible water loss) over the 24 h prior to amikacin administration [20]. Z-scores for BMI were calculated for each patient using the World Health Organization BMI for age growth curves [21]. Septic shock and sepsis-associated organ dysfunction in children were defined according to the International Sepsis Consensus Conference [22]. The severity of each case was assessed using the PELOD-2, the predictive death rate, and the number of organ dysfunctions [18, 19]. AKI was defined and staged using Kidney Disease Improving Global Outcomes criteria [23]. Estimated glomerular filtration rate (eGFR) was determined using the original Schwartz formula [24]. Renal function was evaluated from the first day of amikacin infusion to the 7th day after treatment start. The MIC of amikacin was measured or determined to classify isolates as susceptible or resistant to amikacin according to the EUCAST (European Committee on Antimicrobial Susceptibility Testing) breakpoint [25].
2.5 Statistical Analysis
Continuous variables were expressed as median (minimum–maximum). Binary and categorical data were expressed as counts and frequency (%). Factors associated with Cmax < 60 mg·L−1 were entered into univariate analyses. Differences between groups were assessed using Fisher’s exact test and the Mann–Whitney U test as appropriate. Correlation was analyzed using Pearson’s correlation. A p value <0.05 was considered significant. Statistical analyses were performed using R version 2.3.3 (The R Foundation for Statistical Computing, Vienna, Austria).
2.6 Ethics
The French Pediatric Society Ethics Committee (Comité d’éthique de la recherche de la Société Française de Pédiatrie) approved this observational study and waived the need for written informed consent (n° CERSFP_2018_091-2).
3 Results
A total of 56 patients received amikacin and had a peak measured. Of these, 26 (46%) were excluded: ten (18%) because of inappropriate sampling time and 16 (29%) because of insufficient dosing. Finally, 30 patients were analyzed. In total, 21 patients had a BW measurement at the time of the amikacin infusion; the median actual BW was increased by 10% compared with the admission BW. Patient characteristics are shown in Table 1.
3.1 Primary Outcome
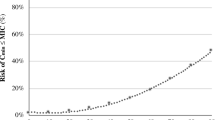
The 30 patients in our cohort received a median amikacin dose of 30 (range 29–33) mg·kg−1 based on admission BW, corresponding to 27 (range 24–30) mg·kg−1 based on actual BW. Cmax was < 60 mg·L−1 for 21 patients (70%) and was never above 80 mg·L−1 (Fig. 1). Median Cmax was 50 (range 31.3–79.6) mg·L−1. Of the 15 patients with measured Cmin, six (29%) had a Cmin ≥ 2.5 mg·L−1.
The median duration of treatment was 2 days (range 1–3), with a single patient receiving three amikacin infusions.
The univariate analysis to identify factors associated with a Cmax < 60 mg·L−1 is reported in Table 2. Blood urea (p = 0.001) and eGFR (p = 0.015) were significantly associated with a low Cmax. None of the other parameters evaluated were significantly associated.
3.2 Infections, Pathogens, and Pharmacodynamics
In total, 29 pathogens were identified in 20 (67%) patients: Klebsiella pneumoniae (n = 8), Enterobacter aerogenes or Enterobacter cloacae (n = 5), Escherichia coli (n = 3), Haemophilus influenzae (n = 3), Staphylococcus aureus or Staphylococcus epidermidis (n = 3), Moraxella catarrhalis (n = 2), Acinetobacter baumannii (n = 1), Enterococcus faecalis (n = 1), Pseudomonas aeruginosa (n = 1), Stenotrophomonas maltophilia (n = 1), and Streptococcus gallolyticus (n = 1).
Pathogens were identified in 24 sites: tracheal samples (n = 9), CVC blood cultures (n = 6), urinary tract (n = 3), peripheral blood cultures (n = 3), per surgical samples (n = 2, one peritoneal and one mediastinal), and cerebrospinal fluid (n = 1). Median MIC for amikacin was 2 mg·L−1 (range 1.5–16), and MIC was ≤ 8 mg·L−1 in 14 of the 15 patients (93%) with a pathogen and a measured or estimated MIC (Table 3). A total of 13 (87%) patients with a measured MIC reached a Cmax/MIC ratio > 8.
3.3 Acute Kidney Injury
Data for AKI were missing for one patient. Among the other 29 patients, ten (34%) had AKI at the onset of amikacin therapy. Two patients were on CRRT prior to amikacin administration. AKI was observed in eight (28%) patients, two (7%) with worsening and six (21%) with initial occurrence, with a median delay of 2 days (range 0–7) following amikacin administration. All patients with AKI after amikacin administration received at least one other concomitant nephrotoxic drug. Three patients had an overdose of a concomitant nephrotoxic drug, two with tacrolimus and one with vancomycin. There was no significant difference between the median Cmax of patients with or without AKI progression or occurrence (53.8 mg·L−1 [range 33–79.6] vs. 50 mg·L−1 [range 30.9–75.8], respectively). The number of patients with a measured Cmin and AKI was too low to study the association between these factors. There was no correlation between Cmin and Cmax (r < 0.1).
4 Discussion
To our knowledge, this is the first study to assess the Cmax resulting from an amikacin dose of 30 mg·kg−1 in critically ill children.
In the present study, 70% of the patients had a peak concentration under 60 mg·L−1. The proportion of patients in our cohort who achieved the recommended Cmax was similar to that in previous simulations conducted in a pediatric cohort with burn wound sepsis [16]. In addition, even though the same dosing regimen was used in all patients, it still resulted in a wide distribution of Cmax in our population, likely explained by a high intra- and interindividual variability in this critically ill population, with rapid changes of pharmacokinetic parameters over time [14, 26]. This makes it difficult to predict target attainment and advocates for daily therapeutic drug monitoring of both Cmax and Cmin.
Difficulty in achieving a Cmax ≥ 60 mg·L−1 is explained by altered pharmacokinetic parameters in critically ill patients [14]. This is in accordance with previous studies that showed increased amikacin Vd in this population, partly explained by fluid overload and the use of ECMO and CRRT [27, 28]. Two hypotheses may explain the high proportion of patients with low Cmax and the absence of patients with high Cmax in our population compared with critically ill adults. First, the Vd is theoretically higher for hydrophilic drugs in children because of the higher proportion of water in their body compared with adults. Second, in adult studies, BMI > 25 kg·m−2 was associated with reaching the target. However, unlike adult populations, BMIs were low in our population, with a negative median Z-score. This may have contributed to a higher proportion of patients with low Cmax [9, 11]. Moreover, the dose administered was based on admission BW and not on actual BW, leading to the risk of low Cmax since the actual BW increases with the fluid management of sepsis; in our study, the admission BW was 10% lower than the actual BW. Using admission BW in critically ill children underestimates amikacin Vd and may lead to underexposure. These observations highlight the importance of selecting the most accurate BW (admission or actual BW) to calculate dosing.
When focusing on measured MIC for susceptible strains (MIC ≤ 8 mg·L−1), nearly all our patients (87%) reached the recommended Cmax/MIC ratio. In our cohort, most of the isolated strains had low MIC and, in these cases, low Cmax was sufficient to obtain a Cmax/MIC > 8. This explains the difference in the rate of target attainment between recommended Cmax and actual Cmax/MIC. However, MIC is usually unknown at the time of amikacin infusion, and some typical pathogens such as P. aeruginosa may have an MIC > 8 mg·L−1, justifying a target between 60 and 80 mg·L−1 [6, 25]. Clinical data confirmed this target, with a reduced intensive care unit mortality rate in adults [9].
In our study, low blood urea concentrations were significantly associated with low Cmax, and eGFR was significantly higher for patients with Cmax < 60 mg·L−1. Similar results were previously described in two adult studies [13, 29]. Similar to these studies, our eGFR calculation uses serum creatinine, an imperfect surrogate of renal function, as it is also a surrogate of hemodilution [24]. Blood urea concentration also depends on both renal function and hemodilution. The most probable explanation is that low levels of those biomarkers could be correlated with an increased Vd, leading to a low Cmax. A less probable assumption is that Cmax is lowered by increased renal clearance since the elimination process begins during the distribution phenomenon [29].
The incidence of AKI progression or occurrence after amikacin administration was 28%, which was similar to the previously published incidence for AKI in PICUs [30]. Although none of these episodes could be exclusively related to amikacin use, we should remain cautious in this vulnerable population, especially when other nephrotoxic drugs are administered. Clinicians should select the indications where the expected benefit of amikacin treatment outweighs the risk. We did not identify an association between Cmax and AKI, as shown in a previous study [28]. Moreover, Cmax was not correlated with Cmin. These results reinforce that a high Cmax can be reached safely if Cmin is monitored systematically to prevent further amikacin administration in case of high concentrations [31].
This study has a number of limitations. The number of included patients was too low to perform a multivariate analysis, and the number of samples per patient was too low to develop a population pharmacokinetic approach. Thus, we were unable to provide precise guidance to optimize the pharmacokinetic target attainment. Also, because of the small number of patients with a measured MIC, we could not study the covariates for this specific population. Finally, we were unable to associate the Cmax and clinical outcome.
5 Conclusions
This retrospective evaluation in a French cohort showed that, despite the use of the maximal recommended amikacin dose of 30 mg·kg−1, Cmax was below the pharmacokinetic target in 70% of our critically ill children. These results suggest the need to optimize amikacin use in critically ill children by (1) using the maximal recommended dose of 30 mg·kg−1 based on the actual BW and monitoring the Cmax to ensure efficiency and (2) monitoring the Cmin to prevent renal toxicity. Further studies are needed to develop a population pharmacokinetic model for critically ill children to optimize amikacin exposure.
References
Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–55.
Rapports ONERBA—Onerba. Available from: http://onerba.org/publications/rapports-onerba/.
Gauzit R. Actualités en antibiothérapie—aminosides toujours et encore: bon usage et suivi thérapeutiqueLatest developments in antibiotherapy—aminoglycosides, again and again: correct use and therapeutic monitoring. Réanimation. 2011;20:290–8.
Slater MB, Gruneir A, Rochon PA, Howard AW, Koren G, Parshuram CS. Risk factors of acute kidney injury in critically ill children. Pediatr Crit Care Med. 2016;17:e391-398.
Slater MB, Gruneir A, Rochon PA, Howard AW, Koren G, Parshuram CS. Identifying high-risk medications associated with acute kidney injury in critically ill patients: a pharmacoepidemiologic evaluation. Pediatr Drugs. 2017;19:59–67.
Update on good use of injectable aminoglycosides, gentamycin, tobramycin, netilmycin, amikacin. Pharmacological properties, indications, dosage, and mode of administration, treatment monitoring. Med Mal Infect. 2012;42:301–8.
Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–9.
Ruiz J, Ramirez P, Company MJ, Gordon M, Villarreal E, Concha P, et al. Impact of amikacin pharmacokinetic/pharmacodynamic index on treatment response in critically ill patients. J Glob Antimicrob Resist. 2017;12:90–5.
Allou N, Bouteau A, Allyn J, Snauwaert A, Valance D, Jabot J, et al. Impact of a high loading dose of amikacin in patients with severe sepsis or septic shock. Ann Intensive Care. 2016;6:106.
Rea RS, Capitano B. Optimizing use of aminoglycosides in the critically ill. Semin Respir Crit Care Med. 2007;28:596–603.
de Montmollin E, Bouadma L, Gault N, Mourvillier B, Mariotte E, Chemam S, et al. Predictors of insufficient amikacin peak concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med. 2014;40:998–1005.
Botha FJ, van der Bijl P, Seifart HI, Parkin DP. Fluctuation of the volume of distribution of amikacin and its effect on once-daily dosage and clearance in a seriously ill patient. Intensive Care Med. 1996;22:443–6.
De Winter S, Wauters J, Meersseman W, Verhaegen J, Van Wijngaerden E, Peetermans W, et al. Higher versus standard amikacin single dose in emergency department patients with severe sepsis and septic shock: a randomised controlled trial. Int J Antimicrob Agents. 2018;51:562–70.
Lugo G, Castañeda-Hernández G. Relationship between hemodynamic and vital support measures and pharmacokinetic variability of amikacin in critically ill patients with sepsis. Crit Care Med. 1997;25:806–11.
Yu T, Stockmann C, Healy DP, Olson J, Wead S, Neely AN, et al. Determination of optimal amikacin dosing regimens for pediatric patients with burn wound sepsis. J Burn Care Res. 2015;36:e244–52.
Liu X, Smits A, Wang Y, Renard M, Wead S, Kagan RJ, et al. Impact of disease on amikacin pharmacokinetics and dosing in children. Ther Drug Monit. 2019;41:44–52.
Lopez SA, Mulla H, Durward A, Tibby SM. Extended-interval gentamicin: population pharmacokinetics in pediatric critical illness. Pediatr Crit Care Med. 2010;11:267–74.
Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F, et al. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013;41:1761–73.
Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2.
Bontant T, Matrot B, Abdoul H, Aizenfisz S, Naudin J, Jones P, et al. Assessing fluid balance in critically ill pediatric patients. Eur J Pediatr. 2015;174:133–7.
WHO | BMI-for-age. WHO. World Health Organization; Available from: https://www.who.int/childgrowth/standards/bmi_for_age/en/.
Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21:e52-106.
Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204.
Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–63.
EUCAST: Clinical breakpoints and dosing of antibiotics. Available from: https://eucast.org/clinical_breakpoints/.
Thakkar N, Salerno S, Hornik CP, Gonzalez D. Clinical pharmacology studies in critically ill children. Pharm Res. 2017;34:7–24.
Marsot A, Guilhaumou R, Riff C, Blin O. Amikacin in critically ill patients: a review of population pharmacokinetic studies. Clin Pharmacokinet. 2017;56:127–38.
Gálvez R, Luengo C, Cornejo R, Kosche J, Romero C, Tobar E, et al. Higher than recommended amikacin loading doses achieve pharmacokinetic targets without associated toxicity. Int J Antimicrob Agents. 2011;38:146–51.
Carrié C, Delzor F, Roure S, Dubuisson V, Petit L, Molimard M, et al. Population pharmacokinetic study of the suitability of standard dosing regimens of amikacin in critically ill patients with open-abdomen and negative-pressure wound therapy. Antimicrob Agents Chemother. 2020;64:e02098-e2119.
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20.
Roger C, Nucci B, Louart B, Friggeri A, Knani H, Evrard A, et al. Impact of 30 mg/kg amikacin and 8 mg/kg gentamicin on serum concentrations in critically ill patients with severe sepsis. J Antimicrob Chemother. 2016;71:208–12.
Acknowledgements
The authors thank Evelyne Jacqz-Aigrain MD, PhD, for her advice and Sarah MacKenzie, PhD, for medical writing assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was secured for this study.
Conflict of interest
Rym Medjebeur Hanna, Michael Levy, Emmanuelle Bille, Carole Hennequin, Fabrice Lesage, Jérôme Naudin, Florence Moulin, Marie Blanquer, Agathe Béranger, Sylvain Renolleau, Mehdi Oualha, and Mathieu Genuini have no conflicts of interest that are directly relevant to the content of this article.
Availability of data and material
The datasets generated and analyzed during the current study are not publicly available because of privacy restrictions but are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author contributions
RMH collected data, carried out data analyses, drafted the initial manuscript, and reviewed and revised the manuscript. MG conceptualized and designed the study, coordinated and supervised data collection and analysis, and reviewed and revised the manuscript. MO contributed to the conceptualization and design of the study and critically reviewed the manuscript for important intellectual content. FM, FL, and SR contributed to the conceptualization and design of the study. AB, EB, JN, ML, CH, and MB contributed to the conceptualization of the study and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Ethics approval
The French Pediatric Society Ethics Committee (Comité d’éthique de la recherche de la Société Française de Pédiatrie) approved this observational study and waived the need for written informed consent (n° CERSFP_2018_091-2).
Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Medjebeur Hanna, R., Levy, M., Bille, E. et al. Assessment of the Effects of a High Amikacin Dose on Plasma Peak Concentration in Critically Ill Children. Pediatr Drugs 23, 395–401 (2021). https://doi.org/10.1007/s40272-021-00456-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-021-00456-0