Abstract
Purpose
To compare cardiac output (CO) and blood volumes measured by COstatus® (Transonic Systems Inc., NY, USA) versus PiCCO (Philips IntelliVue MP40 with PiCCO-technology module M3012A#10, Netherlands) in adult ICU patients.
Methods
This was a prospective single-center study. Each of the 30 patients studied received a 5-Fr Pulsiocath femoral arterial and a standard central venous catheter. Twenty ml of iced 5% dextrose solution was injected for PiCCO measurements. For COstatus measurements, an extracorporeal arteriovenous loop, with two sensors placed on it, was connected between the Pulsiocath femoral arterial and central venous catheters. Blood was circulated through this loop at 12 ml/min for 5–8 min using a pump. Twenty ml of warm saline was injected into the venous side for measurements. For each method, three injections were averaged for comparison.
Results
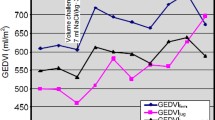
A good agreement for measured CO (range 3.65–16.3 l/min) with a percentage error of 20% was observed, with r = 0.95, bias = −0.037 l/min. PiCCO’s global end-diastolic volume (GEDV) was 2.5 times larger than the analogous COstatus’s total end-diastolic volume (TEDV) [TEDV = 0.28 × GEDV + 176 ml]. PiCCO’s intrathoracic blood volume (ITBV) was larger than the analogous COstatus’s central blood volume (CBV) [CBV = 0.73 × (ITBV) +78 ml].
Conclusions
CO measured by COstatus was found to be equivalent and hence interchangeable with PiCCO in this study population. COstatus blood volumes were found to be within the expected physiological range whilst PiCCO blood volumes were significantly higher, which was also observed in other studies. Future studies using 3D echo/MRI are required to validate these blood volumes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assessment of cardiac output (CO) and blood volumes is fundamental to the management of critically ill patients [1–4] as CO without blood volumes or cardiac filling pressures may not indicate the complete hemodynamic state of the critically ill. Transpulmonary thermodilution (TT) technology (Philips IntelliVue MP40 monitor with PiCCO-technology module M3012A#10) has been explored to measure CO (COTT) and preload parameters such as global end-diastolic volume (GEDV, sum of all end-diastolic volumes of the atria and ventricles) and intrathoracic blood volume (ITBV, sum of GEDV and pulmonary blood volume) [5].
A novel minimally invasive ultrasound dilution (UD) technology (COstatus) was recently introduced to routinely measure CO (COUD) and blood volumes in ICU patients by using an extracorporeal arteriovenous (AV) loop approach [6]. According to the manufacturer, COstatus can be used with patients of any age and weight as it works off existing standard arterial (radial, ulnar, femoral, or pedal) and central venous catheters in the patients and uses isotonic saline as an indicator.
COstatus measures total end-diastolic blood volume (TEDV, blood volume in the four chambers of the heart at the end of diastole) and central blood volume (CBV, blood volume in the heart, lungs, and major vessels such as aorta, pulmonary artery, and vein) [6]. A detailed description of the procedure for using COstatus, and the theory of ultrasound dilution and methodology for calculation of different volumes are presented in an in vitro study [6] published by the manufacturers. The purpose of our study was to compare CO and blood volumes (TEDV and CBV) measured by COstatus with CO and analogous blood volumes (GEDV and ITBV) measured by PiCCO in adult ICU patients.
Materials and methods
Patients
After obtaining approval from the local ethics committee and informed consent from each patient, 30 patients from the hematology ICU were evaluated prospectively. The inclusion criteria were ICU patients with existing PiCCO arterial catheter and a central venous catheter. The exclusion criteria were contraindication to femoral artery catheterization, atrial fibrillation, and allergy to heparin. All the patients recruited had a triple-lumen central venous catheter (Certofix Trio; BBraun Melsungen AG, Germany) either in the internal jugular or the subclavian vein. Patients were mechanically ventilated in a volume-controlled mode and ventilation parameters were adjusted as per blood gas monitoring.
All patients were sedated with midazolam (6 mg/h) and only those with severe respiratory failure were relaxed with atracurium besylate (50–100 mg) with proper sedation to control ventilator asynchrony, for a short duration. Seventeen patients received dopamine (6–15 μg kg−1 min−1) and 14 patients additionally received phenylephrine (0.5–3 μg kg−1 min−1). Infusion rates of catecholamines were adapted according to clinical requirements. Table 1 in “Electronic supplementary material” lists the clinical characteristics and patient demographics.
PiCCO measurements
A 5-F arterial catheter (Pulsiocath PV2015L20) was placed in the descending aorta via the femoral artery by using the Seldinger technique. The catheters were connected to an IntelliVue MP40 monitor equipped with PiCCO-technology module M3012A#10 (Phillips Medical Systems). Measurements were obtained by injecting 20 ml of iced 5% dextrose solution into the central venous catheter.
COstatus measurements
An extracorporeal AV loop was primed (priming volume 5 ml) with heparinized saline and connected between the Pulsiocath arterial catheter and the distal lumen of the central venous catheter. Two reusable sensors that measure changes in blood ultrasound velocity and blood flow in the AV loop were clamped onto the arterial and venous limbs of the loop. To obtain measurements, a roller pump was turned on to circulate blood from the artery to the vein through the AV loop at a rate of 10–12 ml/min for 5–7 min. During this process, 25 ml of isotonic saline, pre-warmed to body temperature in a bag warmer (HFW 1000, Transonic Systems Inc., NY, USA), was injected into the venous side of the AV loop. The indicator is first sensed by the venous sensor which records the time and volume of injection. The indicator then travels through the cardiopulmonary system and after passing through the arterial catheter is recorded by the arterial sensor on the AV loop. The arterial sensor measures the change in concentration of saline in blood as dilution and also records the indicator travel time through the cardiopulmonary system. From the venous and arterial sensor measurements, the COstatus monitor, using standard equations [6], automatically calculates and displays CO and blood volumes on the screen. At the conclusion of a measurement session, the blood in the AV loop was returned to the patient and the AV loop was filled with heparinized saline to be ready for the next measurement session.
For each method, three injections were performed randomly over the respiratory cycle and the measurements were averaged for comparison. An additional injection was performed if a “repeat/error” message was displayed.
Data analysis
CO and blood volume parameters were compared by using the linear regression analysis. Bias was calculated as per the Bland–Altman approach [7] and the percentage error was calculated as two times the standard deviation (2SD) of the paired differences divided by mean COTT. As per Critchley and Critchley [8], a percentage error of less than 30% was defined as the criterion for clinical acceptability.
Results
Table 1 summarizes the comparison between CO and blood volumes measured by PiCCO and COstatus. Bland–Altman analysis between COUD and COTT revealed a small bias of −0.037 l/min with limits of agreement (LOA = bias ± 1.96SD) of −1.72 and +1.65 l/min (Fig. 1). The percentage error was 20%, which is below the clinically acceptable 30% threshold [8]. Table 1 shows that GEDV was 2.5 times greater than TEDV (r = 0.75, bias = 860 ml, LOA = 290 and 1,490 ml) and ITBV was also greater than CBV (r = 0.91, bias = 397 ml, LOA = −34 and 829 ml).
Discussion
UD technology was introduced in 1995 and since then it has been widely used in hemodialysis [9, 10] for measuring shunt flow, recirculation, and CO. This technology was then adapted for use in critical care settings with COstatus [6]. COstatus CO measurements were compared with pulmonary artery thermodilution measurements in adult ICU patients [11] and in patients undergoing abdominal surgery under anesthesia [12] with clinically acceptable results.
The current study is the first clinical comparison between COstatus and PiCCO and is of special interest because it compares CO as well as anatomically same blood volumes measured by two different dilution techniques. The results show good agreement between CO measurements, but not between blood volumes (Table 1). The good agreement between CO measurements could be due to the following factors: (1) both methods are based on indicator dilution principles; and (2) both use the Stewart Hamilton equation for calculation of CO, which is independent of whether the indicator is diffusible or not. The lack of agreement between blood volumes could be due to the following two factors. The first factor is that COstatus uses a non-diffusible indicator (saline) that travels only through the intravascular space [9, 10, 13], whilst PiCCO uses a diffusible indicator (thermal) that travels through both intravascular and extravascular space, during the first pass [5]. Since it is the time taken by the indicator to travel only through intravascular space that is used to estimate blood volumes [14], special assumptions and adjustments will have to be made when using a diffusible indicator [5]. The second factor is the difference in the assumptions and mathematical models for the calculation of blood volumes which lead to using different formulae by the two methods (see “Theory” section in the “Electronic supplementary material”).
To further understand the significant differences in blood volume calculations, we performed a literature review. In [15–17] authors suggest that GEDV and ITBV measured by PiCCO could overestimate the physiological values and also those determined by different MRI methods. Brivet et al. [15] speculate that GEDV does not correspond to the largest blood volume contained in the four heart chambers, but to the end-diastolic blood volume of the heart plus aortic blood volume.
We did not find any reference data on the total blood volume in all four heart chambers at the end of diastole. Since there is a considerable amount of published data on left ventricular end-diastolic volume (LVEDV), we hypothetically assumed that the total heart blood volume is four times the LVEDV. We found a mean of 227–379 ml/m2 in healthy volunteers and in patients with different pathologies (over 400 patients from six publications, see Table 2 in the “Electronic supplementary material”). This suggests that COstatus blood volumes were within the expected physiological range whilst PiCCO blood volumes were significantly higher, which was also observed in other studies [15–17]. However, this hypothetical approach is not useful for validating the heart blood volume, nor is it conclusive in nature, but is presented here only to give an approximate physiological range. Further studies using 3D echo/MRI would be useful to evaluate the accuracy of these blood volumes.
The major advantage of the COstatus system is that it does not require a dedicated catheter, which allows for the measurement of CO and blood volumes in patients of any age and size with in situ regular arterial and central venous catheters. This observation was confirmed in prior validation studies performed in small animal models [18, 19] and in pediatrics [20]. The limitations of this technology are: it requires the operation of a pump for 5–8 min during which the arterial pressure signal is not available; and it does not provide continuous CO.
In summary, we found a close, clinically acceptable agreement between CO measurements by COstatus and PiCCO in our ICU population, suggesting that they are interchangeable in this population. COstatus blood volumes were within the expected physiological range whilst PiCCO blood volumes were significantly higher. Further studies using 3D Echo/MRI would be useful to evaluate the accuracy of these blood volumes. Also, studies are needed in which changes in both CO and preload status are measured with COstatus and an accepted comparator.
References
Shippy CR, Appel RL, Shoemaker WC (1984) Reliability of clinical monitoring to assess blood volume in critically ill patients. Crit Care Med 12:107–112
Spiess BD, Patel MA, Soltow LO, Wright IH (2001) Comparison of bioimpedance versus thermodilution cardiac output during cardiac surgery: evaluation of a second-generation bioimpedance device. J Cardiothorac Vasc Anesth 15:567–573
Godje O, Peyerl M, Seebauer T, Lamm P, Mair H, Reichart B (1998) Central venous pressure, pulmonary capillary wedge pressure and intrathoracic blood volumes as preload indicators in cardiac surgery patients. Eur J Cardiothorac Surg 13:533–540
Hoeper MM, Maier R, Tongers J, Niedermeyer J, Hohlfeld JM, Hamm M, Fabel H (1999) Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am J Respir Crit Care Med 160:535–541
Pulsion PiCCOplus operator’s manual (2002) Pulsion Medical Systems, Munich, Germany
Krivitski NM, Kislukhin VV, Thuramalla NV (2008) Theory and in vitro validation of a new extracorporeal approach for hemodynamic assessment in pediatric and neonatal ICU patients. Pediatr Crit Care Med 9:423–428
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Critchley LA, Critchley JA (1999) A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 15:85–91
Krivitski N (1995) Novel method to measure access flow during hemodialysis by ultrasound dilution technique. ASAIO J 41:M741–M745
Krivitski N, Depner T (1999) Cardiac output and central blood volume during hemodialysis: methodology. Adv Ren Replace Ther 6:225–232
Eremenko AA, Safarov PN (2007) Validation of new cardiac output ultrasound dilution method in cardiac ICU patients. Intensive Care Med 33:S232
Tsutsui M, Matsuoka N, Ikeda T, Sanjo Y, Kazama T (2009) Comparison of a new cardiac output ultrasound dilution method with thermodilution technique in adult patients under general anesthesia. J Cardiothorac Vasc Anesth 23:835–840
Moser M, Kenner T (1982) Distribution spaces of intravenously injected saline, plasma and red cell concentrate. Pflugers Arch 394S:R48
Zierler KL (1962) Circulation times and the theory of indicator-dilution methods for determining blood flow and volume. In: Hamilton WF, Dow P (eds) Handbook of physiology, section 2: circulation, vol 1. American Physiological Society, Washington, pp 585–615
Brivet FG, Jacobs F, Colin P (2004) Calculated global end-diastolic volume does not correspond to the largest heart blood volume: a bias for cardiac function index? Intensive Care Med 30:2133–2134
Joshi D, Sizer E, Bernal W, Wendon J, Auzinger G (2009) High values of intrathoracic blood volume in hepatopulmonary syndrome. Crit Care Resusc 11:129–131
Nowak R, Zwadlo C, Piepenbrock S, Winterhalter M (2009) Overestimation of intrathoracic blood volume in a patient with atrial fibrillation and subsequent severely reduced atrial blood-flow. Br J Anaesth 102:284–285
Gleed RD, Smith T, Callahan M, Darling E, Searles B, Kislukhin V, Thuramalla NV, Krivitski NM (2006) Validation of novel ultrasound dilution cardiac output method for pediatric and neonatal patients. Intensive Care Med 32:659
de Boode WP, van Heijst AF, Hopman JC, Tanke RB, van der Hoeven HG, Liem KD (2009) Cardiac output measurement using an ultrasound dilution method: a validation study in ventilated piglets. Pediatr Crit Care Med 11:103–108
Crittendon I, Dreyer W, Kim J (2009) Validation of ultrasound dilution cardiac output measurements in pediatric patients. Crit Care Med 37:A90
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Galstyan, G., Bychinin, M., Alexanyan, M. et al. Comparison of cardiac output and blood volumes in intrathoracic compartments measured by ultrasound dilution and transpulmonary thermodilution methods. Intensive Care Med 36, 2140–2144 (2010). https://doi.org/10.1007/s00134-010-2003-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-2003-5