Abstract
To study agreement in cardiac index (CI), systemic vascular resistance index (Systemic VRI) and stroke volume variation (SV variation) between the FloTrac/Vigileo at radial and femoral arterial cannulation sites, and pulmonary artery catheter (PAC) thermodilution, in patients undergoing orthotopic liver transplantation. A prospective observational study of 25 adult patients with liver failure. Radial and femoral arteries were cannulated with standardised FloTrac/Vigileo arterial transducer kits and a PAC was inserted. CI, SV variation and Systemic VRI were measured four times (30 min after induction of anesthesia, 30 min after portal vein clamping, 30 min after graft reperfusion, 30 min after commencement of bile duct anastomosis). The bias, precision, limits of agreement (LOA) and percentage errors were calculated using Bland–Altman statistics to compare measurements from radial and femoral arterial cannulation sites and PAC thermodilution. Neither radial nor femoral CI achieved acceptable agreement with PAC CI [radial to PAC bias (SD) 1.17 (1.49) L/min/m2, percentage error 64.40 %], [femoral to PAC bias (SD) −0.71 (1.81) L/min/m2, percentage error 74.20 %]. Agreement between radial and femoral sites for CI [mean difference (SD) −0.43 (1.51) L/min/m2, percentage error 70.40 %] and Systemic VRI [mean difference (SD) 0.03 (4.17) LOA ±8.17 mmHg min m2/L] were also unacceptable. Agreement in SV variation between radial and femoral measurement sites approached a clinically acceptable threshold [mean difference (SD) 0.68 (2.44) %), LOA ±4.78 %]. FloTrac/Vigileo CI cannot substitute for PAC thermodilution CI, regardless of measurement site. SV variation measurements may be interchangeable between radial and femoral sites for determining fluid responsiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The maintenance of hemodynamic stability and organ perfusion is a vital component of anesthetic care during orthotopic liver transplantation (OLTx). This challenge is increased in patients with the associated complex intercurrent illness and circulatory derangement that accompany liver failure. Very wide ranges in all haemodynamic variables occur frequently and CIs ranging from 2 to 12 L/min/m2 have been observed. The pulmonary artery catheter has commonly been used for the intraoperative measurement of CI to guide clinical decision-making. Interest in less-invasive methods of CI measurement has led to a variety of technologies and devices becoming available. One such device is the FloTrac/Vigileo (Edwards Lifesciences, USA), which uses the arterial pulse pressure waveform from an arterial cannula to estimate stroke volume, along with stroke volume variation (SV variation) as an index of volume status and fluid responsiveness. In common with other pulse-pressure derived measurement devices, it delivers a stroke volume and CI measurement on a beat-to-beat basis. However, unlike some of these other devices it does not require an initial calibration manoeuvre and does not stipulate the required site of the arterial line placement, thereby permitting measurement from either radial or femoral arteries.
In a recent study, we reported that poor agreement in pulse pressure measurements was observed from arterial lines placed at the radial and femoral arteries in patients with liver failure undergoing liver transplantation surgery [1]. Given that FloTrac/Vigileo estimates CI from the arterial pulse pressure waveform, we hypothesised that poor agreement between radial and femoral pulse pressures would lead to poor agreement in SV and CI estimates. This has important implications for anesthetic practice, as the inaccurate estimation of CI could lead to inappropriate fluid and vasoactive drug administration.
In this prospective, observational study, we recorded arterial pressure derived measurements by the FloTrac/Vigileo at radial and femoral arteries to measure any clinically significant differences and to establish whether SV and CI measurement at the two sites can be used interchangeably. Comparison of CI from both sites was also made with pulmonary artery catheter bolus thermodilution CI as a reference standard. Agreement in SV variation between the two measurement sites was also investigated.
2 Methods
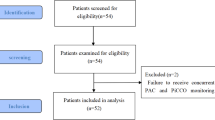
The study was approved by the local Human Research Ethics Unit (approval number H2012/04546). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The severity of the liver disease making the transplant necessary was assessed by the Model for End-Stage Liver Disease (MELD) score, United Network for Organ Sharing (UNOS) modification [2]. Inclusion criteria were adult patients (age >18 years), undergoing orthotopic liver transplantation for liver failure. Exclusion criteria were any contraindication or technical barriers to successful radial and femoral arterial cannulation or pulmonary arterial catheter insertion, liver transplantation for indications other than liver failure (MELD score <10), and the need for veno-venous bypass. The study was conducted between 22nd March 2012 and 30th September 2013.
Induction of anesthesia consisted of a balanced technique combining intravenous midazolam 0.02–0.03 mg/kg, propofol 1–3 mg/kg, fentanyl 1–2 mcg/kg and a neuromuscular blocker. Anesthesia was maintained with isoflurane at inspired concentrations of 0.5–1.5 MAC in a 50 % oxygen-air balance and an infusion of fentanyl 2–5 mcg/kg/h. Ventilation was via a low flow circle breathing system with the ventilator set to provide a tidal volume of 7–8 ml/kg using volume control mode without positive end expiratory pressure. The end tidal partial pressure of carbon dioxide measured by capnography was maintained between 30 and 40 mmHg. Routine monitoring included continuous electrocardiography, pulse oximetry and capnography. In accordance with routine practice in our centre, invasive radial artery and femoral artery pressure, central venous pressure, pulmonary artery pressure, mixed venous oximetry, urine output and core body temperature monitoring were established after induction of anesthesia. A vasopressor infusion of intravenous noradrenaline was administered according to routine practice at a rate determined by the attending anesthesiologist on the basis of patient hemodynamics (arterial pressure, cardiac output and ventricular preload).
Radial and femoral arteries were cannulated with identical 18gauge, 18 cm long arterial catheterization sets (Leader-Cath, Laboratoires Pharmaceutiques, Vygon, Ecouen, France). The two catheters were connected to identical FloTrac transducers with identical connecting tubing of a standard 180 cm length. The FloTrac transducers were connected to separate EV1000 Clinical Platforms (Edwards Lifesciences) using the 3rd generation Vigileo software, the most current software version at the time. Patient data (age, sex, body weight and height) was entered into each clinical platform and the systems calibrated to atmospheric pressure according to standard practice (“zeroed”). Transducers for each system were mounted, side-by-side, on a manifold at the mid-axillary line level. The square wave test was performed after initial setup and at each subsequent measurement time point to confirm that the damping and dynamic responses of the two pressure measurement systems were similar.
A pulmonary artery catheter was inserted (CCOmbo, Edwards Lifesciences, Irvine, CA, USA). CI was measured by bolus thermodilution (PAC CI) by averaging the results of three consecutive thermodilution measurements within a 3-min time interval. Thermodilution was performed using 10 mL room temperature saline injected in <4 s. The measurements were accepted if the thermodilution curve showed a typical morphology without indication of artefact.
CI obtained from the radial and femoral sites (R CI and F CI), and SV variation and Systemic VRI at each site were recorded during clinical steady state conditions (where mean arterial pressure varied by <10 % over the preceding 3 min). This was done at four time points: 30 min after skin incision (Time point 1), 30 min after portal vein clamping (Time point 2), 30 min after liver graft reperfusion (Time point 3) and 30 min after commencement of bile duct anastomosis (Time point 4). Arterial pressure waveform CI was updated every 20 s and was averaged over the steady state period corresponding with the PAC CI measurements.
The primary endpoint was the difference between R CI and F CI for all measurements across the four time points (“overall”). Bias, limits of agreement (LOA) and percentage errors were calculated using the Bland and Altman method with correction for multiple measurements. Bias represents the systematic difference between both methods and was calculated as the mean difference between measurements. Precision represents the random error or variability in agreement between the two techniques and was calculated as the standard deviation (SD) of the difference. The LOA represent the range in which 95 % of the differences between methods are expected to lie as was calculated as the bias ± 1.96SD. The percentage error was calculated as LOA divided by the mean [3, 4]. In accordance with the standard of Critchley and Critchley, CI measurements were considered interchangeable if the percentage error was <30 % [5]. Concordance in CI measurements was also examined, where the concordance rate is the proportion of data points that are in two of the four quadrants of agreement (upper right and lower left) with a central exclusion zone of equal or <15 % change in measurement from both devices or cannulation sites. Good concordance was considered to be a concordance rate of ≥92 % [6].
The secondary endpoints were the differences between radial and femoral CI and PAC CI obtained from thermodilution, and the differences between radial and femoral SV variation and Systemic VRI. We also examined the relationships between MELD score as an index of disease severity and agreement between these variables. Student’s t test was used to determine the statistical significance of differences in normally distributed continuous variables (CI, SV variation, Systemic VRI and MELD score), and the Wilcoxon sign rank test was used for non-normal data. A probability (p) value ≤0.05 was considered statistically significant, with Bonferroni correction for multiple measurements. Statistical analysis was conducted on Microsoft Excel 2008 and Stata (Statacorp). Bland and Altman comparisons were conducted and computed with LabVIEW (National Instruments, Austin, Texas, USA).
3 Results
25 eligible patients were enrolled in our study. 9 were female and 16 were male. The mean (SD) age was 46 (11.8) years, weight was 81 (19.8) kg, height was 172 (7.5) cm, and body surface area was 1.96 (0.3) m2. Indications for OLTx were hepatitis C cirrhosis (n = 10), alcoholic cirrhosis (n = 3), non-alcoholic steatohepatitis (n = 3), autoimmune hepatitis (n = 2), primary sclerosing cholangitis (n = 2), cryptogenic cirrhosis (n = 2), hepatitis B cirrhosis (n = 1), liver failure from chronic graft rejection (n = 1), and hepatic abscesses with septic shock (n = 1). The average MELD score was 24/40 (7.2). 7 right radial arterial lines and 10 right femoral arterial lines were inserted. All other arterial lines were left sided. All patients received a noradrenaline infusion [mean 4.6 (5.0) mcg/min]. The mean (SD) and ranges of noradrenaline infusion rates were as follows. At Time point 1, 0.23 (0.44) mcg/min, range 0–1.5 mcg/min. At Time point 2, 4.31 (4.45) mcg/min, range 0.5–16mcg/min. At Time point 3, 7.80 (5.14) mcg/min, range 0–20 mcg/min. At Time point 4, 6.12 (4.91) mcg/min, range 0–22mcg/min. No patients were administered adrenaline or phenylephrine.
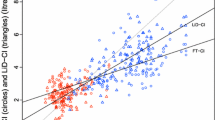
R CI values ranged from 1.60 to 10.60 L/min/m2 [mean 3.95, (1.19) L/min/m2]. F CI values ranged from 2.00 to 14.50 L/min/m2 [mean 4.49, (1.75) L/min/m2]. PAC CI ranged from 2.00 to 8.20 L/min/m2 [mean 5.12 (1.58) L/min/m2]. The bias between R CI and F CI was −0.43 L/min/m2 and 95 % LOA were ±2.96 L/min/m2 (Fig. 1). The percentage error was 70.4 % (Table 1).
Bland–Altman plots showing cardiac index difference in paired measurements from radial and femoral artery FloTrac/Vigileo, and pulmonary artery catheter sites plotted against the average of the paired measurements. CI cardiac index, PAC pulmonary artery catheter, SV variation stroke volume variation, Systemic VRI systemic vascular resistance index, LOA limits of agreement (1.96 SDs)
Neither R CI nor F CI achieved a clinically acceptable agreement with PAC CI. The bias between R CI and PAC CI was −1.17 L/min/m2 and 95 % LOA were ±2.92 L/min/m2 (Fig. 1). The percentage error was 64.40 % (Table 1). The bias between F CI and PAC CI was −0.71 L/min/m2 and 95 % LOA were ±3.55 L/min/m2. The percentage error was 74. 20 %. Acceptable concordance between radial, femoral and pulmonary artery catheter measurements sites was not achieved. A total of 73 paired measurements were obtained for each comparison. The concordance rate between delta R CI and delta F CI was 48 % (30/63). The concordance rate between delta R CI and delta PAC CI was 63 % (44/70). The concordance rate between delta femoral CI and delta PAC CI was 57 % (39/69). The data is displayed graphically on four quadrant plots (Fig. 2).
Four quadrant concordance plots for cardiac index change using paired measurements from radial and femoral artery FloTrac/Vigileo, and pulmonary artery catheter sites. Data was excluded if the delta CI from both measurement devices/cannulation sites was ≤15 %. The exclusion zone is indicated by the grey square along with the best fit regression line and the square of the correlation coefficient R
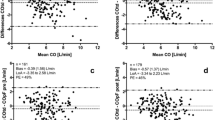
Radial SV variation values ranged from 1 to 27 % [mean 8.56 (4.79) %]. Femoral SV variation values ranged from 2 to 21 % [mean 7.91, (4.54) %]. The bias between radial and femoral SV variation was 0.68 and 95 % LOA were ±4.78 % (Fig. 3 and Table 2).
Radial systemic VRI values ranged from 4.55 to 31.70 mmHg min m2/L [mean 11.87 (5.24) mmHg min m2/L]. Femoral Systemic VRI values ranged from 5.06 to 31.96 mmHg min m2/L [mean 12.93 (5.52) mmHg min m2/L]. The bias between radial and femoral Systemic VRI was 0.03 mmHg min m2/L and 95 % LOA were ± 8.17 mmHg min m2/L (Fig. 4 and Table 3).
There was no correlation between F CI to PAC CI difference and femoral Systemic VRI (r = 0.08). There was moderate correlation between R CI difference to PAC CI difference and radial Systemic VRI (r = 0.58). There was no correlation between Systemic VRI and MELD score (for the femoral site, r = 0.02; for the radial site, r = 0.01).
4 Discussion
In this prospective observational study, our findings were consistent with the hypothesis that there is unacceptably poor agreement between radial and femoral sites in the measurement of CI using the FloTrac/Vigileo pulse pressure waveform analysis system in adults undergoing liver transplantation. In addition, agreement of either radial or femoral measurement of CI with bolus thermodilution via a PAC was equally poor. The percentage errors in all cases greatly exceeded the ±30 % criterion for acceptability suggested by Critchley and Critchley [5]. The observed percentage errors were also much wider than those found for a variety of technologies in a recent meta-analysis of the precision of minimally invasive devices for cardiac output measurement, which ranged between ±40 and 45 % when compared with bolus thermodilution [7, 8].
The poor precision of agreement between F CI and R CI relates to the wide and unpredictable difference in both systolic and pulse pressure measured at the two cannulation sites we previously reported in this patient sample [1]. Similar to other techniques based on pulse pressure analysis, the FloTrac/Vigileo system uses the area under the pulse pressure curve to estimate changes in stroke volume. Unlike some other systems, such as PiCCO™, a calibration manoeuvre is not required for the FloTrac/Vigileo. The manufacturer’s proprietary algorithm uses the skewness and kurtosis of the pulse pressure waveform among other variables to estimate baseline stroke volume. Previous reviews of the accuracy and precision of the device have included little data from patients with vasodilated circulatory derangement, and reliable estimation of stroke volume and CI has proven particularly difficult in this patient group when clinical reference standards such as thermodilution are used as the comparator [8, 9]. In particular, there is conflicting evidence on the accuracy of FloTrac/Vigileo in the setting of liver transplantation surgery [10–14], cardiac surgery [15–18], and critically ill patients on vasoactive drugs [19–21]. Our study, by collecting data simultaneously at two measurement sites, demonstrates that the measurement of stroke volume and CI by FloTrac/Vigileo is prone to internal inconsistency as well as poor agreement with an external reference method. This reflects differences in the amplitude and shape of the arterial pressure waveform at different cannulation sites, and the ability of the algorithm employed by FloTrac/Vigileo to impute stroke volume from these in a consistent fashion. This is important, as the manufacturer of the FloTrac/Vigileo does not stipulate a particular arterial cannulation site for use of the device.
The difference between radial and femoral pulse pressure measurements that accompanied these measurements of CI and stroke volume by the FloTrac/Vigileo may involve factors such as arterial diameter, elastance and resistance, and different microcirculation characteristics in the upper and lower limbs. The underlying mechanisms were outside the scope of our study and remain a topic for further investigation. However, it is important to note that these differences were unpredictable as well as substantial, with a mean bias (standard deviation) of −13.5 (25.5) mmHg [1]. While radial pulse pressure measurements tended to underestimate those from the femoral site, the wide LOA (±50 mmHg) made it impossible to reliably predict femoral from radial pulse pressure or systolic pressure. This is reflected in the wide LOA between F CI by R CI in the current study (Table 1). Similarly, prediction of PAC CI from either F CI or R CI is not possible in any given patient (Tables 2 and 3). There was little indication that severity of liver disease, as measured by MELD score, predicted the degree of lack of agreement between the various measurements sites. Since the completion of our study, Edwards Lifesciences has released the 4th generation FloTrac software, which is marketed as rapidly adjusting to changes in vascular tone in a studied cohort that includes moderate to high-risk surgical patients, and this warrants further validation in vasodilated states in a similar fashion to the current study.
In contrast, despite the known differences in waveform characteristics between peripheral and central arteries in a variety of settings [22–25], our study found that agreement in SV variation between radial and femoral measurement sites was better than for CI and lies closer to a clinically acceptable threshold. Because of its derivation from the pulse pressure waveform, SV variation from the FloTrac/Vigileo is closely correlated to pulse pressure variation [26, 27]. For SV variation, an acceptable LOA may be considered to be ±4 %, based upon a 9–13 % “grey zone” described for pulse pressure variation by Cannesson et al. [28]. These authors showed that pulse pressure variation lying between these limits did not discriminate fluid responders from non-responders in a large sample of ventilated patients. The LOA in our study were ±4.78 %, meaning that the imperfect agreement between radial and femoral SV variation would result in approximately 6 % of measurements misclassifying a patient as fluid responsive when they are fluid unresponsive. This error is relatively small and would not preclude FloTrac/Vigileo from being used to determine patient fluid responsiveness. Agreement in SV variation measurements from radial and femoral arteries in patients undergoing liver transplantation has not been extensively studied and the results have been conflicting. A study by Kim et al. [29] demonstrate high correlation (r = 0.961) between radial and femoral SV variation but was limited by averaging SV variation over a period of 3 min, which would have reduced variability. Conversely, Thiele and colleagues found systolic pressure variation and pulse pressure variation to be relatively dependent on the site at which they were measured, in contrast to frequency domain-based estimates of respiratory variation [30]. The smaller degree of disagreement between sites in SV variation, as opposed to CI measurement, reflects the fact that SV variation is calculated from relative change in pulse pressure induced by ventilation, and this is likely to be less affected by discrepancies in absolute pulse pressure and in the shape of the arterial waveform measured between the two sites.
In conclusion, in adults undergoing liver transplantation, FloTrac/Vigileo arterial pulse pressure derived CI cannot be substituted for bolus thermodilution CI, regardless of measurement site. In addition, poor agreement in CI measurements between radial and femoral cannulation sites, which arises from underlying poor agreement in arterial pulse pressure, means that the two measurement sites are not interchangeable for CI. However, SV variation measurement between the two sites may be interchangeable.
References
Lee M, Weinberg L, Pearce B, Scurrah N, Story DA, McCall PR, McNicol LP, Peyton PJ. Agreement between radial and femoral arterial blood pressure measurements during orthotopic liver transplantation. Crit Care Resusc. 2015;17:101–7.
Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:797–805.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–82.
Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15:85–91.
Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth. 2011;25:536–46.
Peyton PJ, Chong SW. Minimally invasive measurement of cardiac output during surgery and critical care: a meta-analysis of accuracy and precision. Anesthesiology. 2010;113:1220–35.
Peyton PJ, Chong SW. Bias and precision statistics: should we still adhere to the 30% benchmark for cardiac output monitor validation studies? Anesthesiology. 2011;114:1245–6.
Mayer J, Boldt J, Poland R, Peterson A, Manecke GR Jr. Continuous arterial pressure waveform-based cardiac output using the FloTrac/Vigileo: a review and meta-analysis. J Cardiothorac Vasc Anesth. 2009;23:401–6.
Krejci V, Vannucci A, Abbas A, Chapman W, Kangrga IM. Comparisons of calibrated and uncalibrated arterial pressure-based cardiac output monitors during orthotopic liver transplantation. Liver Transpl. 2010;16:773–82.
Biancofiore G, Critchley L, Lee A, Yang XX, Bindi LM, Esposito M, Meacci L, Mozzo R, Filipponi F. Evaluation of a new software version of the FloTrac/Vigileo (Version 3.02) and a comparison with previous data in cirrhotic patients undergoing liver transplant surgery. Anesth Analg. 2011;113:515–22.
Biancofiore G, Critchley L, Lee A, Bindi L, Bisa M, Esposito M, Meacci L, Mozzo R, DeSimone P, Urbani L, Filipponi F. Evaluation of an uncalibrated arterial pulse contour cardiac output monitoring system in cirrhotic patients undergoing liver surgery. Br J Anaesth. 2009;102(1):47–54.
Biais M, Souette-Gaulain K, Cottonceau V, Vallet A, Cochard JF, Revel P, Sztark F. Cardiac output measurement in patients undergoing liver transplantation: pulmonary artery catheter versus uncalibrated arterial pressure waveform analysis. Anesth Analg. 2008;106:1480–6.
Della Rocca G, Costa MG, Chiarandini P, Bertossi G, Lugano M, Pompei L, Coccia C, Sainz-Barriga M, Pietropaoli P. Arterial pulse cardiac output agreement with thermodilution in patients in hyperdynamic conditions. J Cardiothorac Vasc Anesth. 2008;22:681–7.
Staier K, Wiesenack C, Guenkel L, Keyl C. Cardiac output determination by thermodilution and arterial pulse waveform analysis in patients undergoing aortic valve replacement. Can J Anesth. 2008;55:22–8.
Vasdev S, Chauhan S, Choudhury M, Hote MP, Malik M, Kiran U. Arterial pressure waveform derived cardiac output FloTrac/Vigileo system (third generation software): comparison of two monitoring sites with the thermodilution cardiac output. J Clin Monit Comput. 2012;26:115–20.
Mehta Y, Chand RK, Sawhney R, Bhise M, Singh A, Trehan N. Cardiac output monitoring: comparison of a new arterial pressure waveform analysis to the bolus thermodilution technique in patients undergoing off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2008;22:394–9.
Schramm S, Albrecht E, Frascarolo P, Chassot P-G, Spahn DR. Validity of an arterial pressure waveform analysis device: does the puncture site play a role in the agreement with intermittent pulmonary artery catheter thermodilution measurements? J Cardiothorac Vasc Anesth. 2010;24:250–6.
De Backer D, Marx G, Tan A, Junker C, Can Nuffelen M, Hueter L, Ching W, Michard F, Vincent JL. Arterial pressure-based cardiac output monitoring: a multicentre validation of the third-generation software in septic patients. Intensive Care Med. 2011;37:233–40.
Compton FD, Zukunft B, Hoffmann C, Zidek W, Schaefer JH. Performance of a minimally invasive uncalibrated cardiac output monitoring system (FloTrac/Vigileo) in haemodynamically unstable patients. Br J Anaesth. 2008;100:451–6.
Metzelder S, Coburn M, Fries M, Reinges M, Reich S, Rossaint R, Marx G, Rex S. Performance of cardiac output measurement derived from arterial pressure waveform analysis in patients requiring high-dose vasopressor therapy. Br J Anaesth. 2011;106:776–84.
Kanazawa M, Fukuyama H, Kinefuchi Y, Takiguchi M, Suzuki T. Relationship between Aortic-to-radial arterial pressure gradient after cardiopulmonary bypass and changes in arterial elasticity. Anesthesiology. 2003;99:48–53.
Manecke GR, Parimucha M, Stratmann G, Wilson WC, Roth DM, Auger WR, Kerr KM, Jamieson SW, Kapelanski DP, Mitchel MM. Deep hypothermic circulatory arrest and the femoral-to-radial arterial pressure gradient. J Cardiothorac Vasc Anesth. 2004;18:175–9.
Dorman T, Breslow MJ, Lipsett PA. Radial artery pressure monitoring underestimates central arterial pressure during vasopressor therapy in critically ill surgical patients. Crit Care Med. 1998;26:1646–9.
Kim WY, Jun JH, Huh JW. Radial to femoral arterial blood pressure differences in septic shock patients receiving high-dose norepinephrine therapy. Shock. 2013;40:527–31.
Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by FloTrac/Vigileo and automated pulse pressure variation. Eur J Anaesthesiol. 2012;29:64–9.
Le Manach Y, Hofer CK, Lehot JJ, Vallet B, Goarin JP, Tavernier B, Cannesson M. Can changes in arterial pressure be used to detect changes in cardiac output during volume expansion in the perioperative period? Anesthesiology. 2012;117:1165–74.
Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot JJ, Vallet B, Tavernier B. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology. 2011;115:231–41.
Kim YK, Shin WJ, Song JG, Jun IG, Kim HY, Seong SH, Hwang GS. Comparison of stroke volume variation derived from radial and femoral arterial pressure waveforms during liver transplantation. Transplant Proc. 2009;41:1220–4228.
Thiele RH, Colquhoun DA, Tucker-Schwartz JM, Gillies GT, Durieux ME. Radial-femoral concordance in time and frequency domain-based estimates of systemic arterial respiratory variation. J Clin Monit Comput. 2012;26:393–400.
Acknowledgements
This work was funded by departmental resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lee, M., Weinberg, L., Pearce, B. et al. Agreement in hemodynamic monitoring during orthotopic liver transplantation: a comparison of FloTrac/Vigileo at two monitoring sites with pulmonary artery catheter thermodilution. J Clin Monit Comput 31, 343–351 (2017). https://doi.org/10.1007/s10877-016-9840-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9840-x