Abstract
The aim of this study is to evaluate the effects of the effluent of Elazig Municipality Wastewater Treatment Plant on the oxidative defense capacity of aquatic plants (Lemna minor L. and Lemna gibba L.). For this purpose, malondialdehyde (MDA), glutathione (GSH), oxidized glutathione (GSSG), vitamin A (retinol), vitamin E (α-tocopherol), and vitamin C (Ascorbic acid) levels were determined by the HPLC (high performance liquid chromatography) in the control groups and the groups adapting to reactors fed with discharge water. The depletion of vitamins (A, E, and C), decrease of GSH/GSSG ratio, and increase of MDA that reflect a precarious state of the cell in L. minor L. and L. gibba L. were observed after exposure to wastewater. It can be suggested that the selected biomarkers are useful in understanding the biochemical mechanisms of the secondary effluents from wastewater treatment plant in L. minor L. and L. gibba L. as early warning indicators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Exposure to the treated municipal effluents is considered to cause a variety of stress-related changes in the aquatic organism health (Gagne et al. 2008). Chemical analysis is not usually sufficient to evaluate water quality as such polluting substances are present in enormous numbers in the surface waters (Radic et al. 2011). In general, biomarkers has been receiving increasing attention during the last few years, since they constitute an attractive alternative to the chemical determinations and to can be carried out as a supplementary evaluation of the environmental impact of wastes (Petala et al. 2009). Duckweed is a small, floating aquatic plant within the family of Lemnaceae (Bergmann et al. 2000). Meanwhile Lemna minor L. and Lemna gibba L. are the most common species selected as the model plants to evaluate the ecological risks exposed by contaminants for environmental, and human health (Appenroth et al. 2010).
Oxidative stress parameters as non-specific end-points are used to assess the effects of the single chemicals and complex mixtures of aquatic environmental contaminants (Nunesa et al. 2008). Within the antioxidant system, reduced glutathione (GSH) is known to be one of the first lines of the defense against the reactive oxygen species (ROS) (Cnubben et al. 2001; Dickinson and Forman 2002). Some nonenzymatic antioxidants such as vitamins A and E are expended under the impact of xenobiotics and their levels may fall below the normal range (Barim and Karatepe 2010). It has been proposed that lipid peroxidation is a sensitive measure of oxidative damage and is considered as a useful biomarker for the oxidative stress (Chen et al. 2005). Lipid peroxidation was assumed indirectly in vitro as the formation of malondialdehyde (MDA), a by-product of lipid peroxidation that reacts with thiobarbituric acid (Ortega-Villasante et al. 2005).
In the present study, the antioxidant responses to the secondary effluent exposure in L. minor L. and L. gibba L., popularly known as duckweed that is an aquatic plant recommended for use as a reference organism for phytotoxicity assessments, was investigated.
Materials and Methods
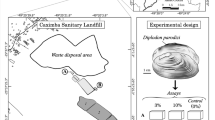
The wastewater of secondary clarifier was taken from Wastewater Treatment Plant of Elazığ, Turkey, while the natural water is obtained from Antalya, Turkey. Physiochemical characteristics of wastewater and naturel water are shown in Table 1. L. gibba L. and L. minor L. used in this study were collected from Botanical Garden of Istanbul University.
Control group consists of the plants exposed to the natural water, while adapted group consists of the plants exposed to the wastewater. The plants were first grown in natural water for 1 month in laboratory conditions accepted as a control group and then exposed to wastewater in the four of four conical reactors including 75 L of reactor solution (upper diameters as 50 cm, bottom diameters as 40 cm, height of water column as 12 cm) separately accepted as adapted group. Approximately 200 g of each plant was placed into the reactors. Two reactors containing L. gibba L., two reactors containing L. minor L. were used. The reactors were placed outside for providing natural day light at approximately 30°C. Plants were placed in these reactors were operated in a continuous regime of about 0.02 L/min of effluent wastewater. The samples of L. minor L. and L. gibba L. plants were sampled daily during 7 day with the water in their environment in the control and adapted groups. Plant samples collected from the reactors daily were stored in the freezer at −70°C until analyzed. All studies were conducted (three replicates) using a total of 48 plants.
Physicochemical characteristics [temperature, pH, electrical conductivity (EC), biochemical oxygen demand (BOD5), chemical oxygen demand (COD), nitrite nitrogen (NO2 −-N), nitrate nitrogen (NO3 −-N), phosphate phosphorus (PO4 −3-P), ammonium nitrogen (NH4 +-N), Al, As, Cd, Cr, Ni] of wastewater in the secondary clarifier and the natural water (obtained from Antalya) were conducted. Metal analysis were conducted by using inductively coupled plasma mass spectroscopy (ICP/MS—Perkin-Elmer ELAN 9000) technique at ACME Analytical Laboratories Ltd., Canada.
Dissolved oxygen and temperature were measured in the field with WTW Oxi 330 dissolved oxygen meter; pH and Conductivity were measured by Hach Lange 30d pH and Electrical Conductivity Meter in the field. BOD5 was determined according to standard methods by using AQUA LYTIC, BOD-System AL606 (APHA 2005). The chemical oxygen demand (COD) was analyzed using with Nova60 spectroquant instrument spectrophotometer with closed reflux Method (5220D). O-PO4 −3 measurement was performed with Nova60 spectroquant instrument with the use of SM 4500-P (APHA 2005). NH4 +-N levels according to 10023 Test N Tube LR salicylate method, NO2 −-N levels according to 10019 Test N Tube vials diazotization method and NO3 −-N levels 10020 Spectrophotometer Test N Tube chromatic acid method, were determined with Hach Lange DR3800 spectrophotometer.
Analyses of the biomarkers [vitamins (A, E, C), GSH, GSSG, and MDA] were carried out with a high-performance liquid chromatographic system (Shimadzu) consisting of LC-10 ADVP pumps and SIL-10 ADVP, along with a degasser unit DGU-14A and Class VP software (Shimadzu, Kyoto Japan).
First, 0.5 g of homogenized plant samples were taken into the tubes and 0.5 mL of 0.5 M HClO4 was added. The proteins were precipitated and the solution was vortexed for 3 min. Then 2.5 mL of purified water was added and vortexed for 3 more minutes. The tubes were kept in the ultrasonic bath for 15 min and then centrifuged for 15 min at 4000 rpm. At the end, the separated phase was collected in vials and analyzed for vitamin C, GSH, GSSG, and MDA by the HPLC apparatus (Tavazzi et al. 1992; Cerhata et al. 1994).
Meanwhile, 0.5 g of homogenized plant samples were taken into the tubes and 5 mL of 60% ethyl alcohol was added to them and the proteins were precipitated. Similar to the former tubes, after vortexing them for 3 min, they were kept in ultrasonic water bath for 15 min. 0.5 mL of n-hexane were added to each tube then centrifuged at 4000 rpm for 15 min. The separated hexane-phase was taken to glass crest and extraction of hexane for each plant sample was repeated twice. Thus, all of the vitamin E in the plant was taken to the hexane phase. After the hexane phase was evaporated under nitrogen gas, the samples were dissolved again in 0.5 mL of methanol and the methanol in the tube was taken up in vials and analyzed for vitamins A and E by the HPLC instrument (Miller et al. 1984).
Analysis of variance (ANOVA) and independent sample t test were used in the SPSS 15.0 package program in comparing the differences between the treatment groups and the control groups in the statistical evaluation of the obtained data. Descriptive statistics are expressed for the results as arithmetic mean ± standard error.
Results and Discussion
Physico-chemical characteristics of wastewater in the secondary clarifier and the reference water were shown in Table 1. Control group consists of the plants exposed to the natural water, while adapted group consists of the plants exposed to the wastewater.
MDA levels are shown in Figs. 1 and 2; GSH and GSSG levels and GSH/GSSG ratios are shown in Figs. 3 and 4; vitamin A, E, and C levels are shown in Figs. 5 and 6, in both control and adapted L. minor L. and L. gibba L. fronds. Consequently it has been found that the amount of the MDA in the fronds of L. minor and L. gibba exposed to the effluent are increased compared to the control (p < 0.05) (Figs. 1, 2). The highest MDA level in L. minor L. and L. gibba L. is observed in the fourth day. The level of vitamin A in the L. minor L. varies depending on the exposure time. Vitamin E levels decreased in L. minor L. exposed to the effluent compared to the control. However vitamin C levels in L. minor L. significantly increased compared to control (p < 0.05). Furthermore vitamin A and C levels decreased, but vitamin E levels increased in L. gibba L. exposed to the effluent compared to the control. GSH and GSSG quantities as well as GSH/GSSG ratios measured in the fronds of L. minor L. and L. gibba L. are shown in Fig. 5 and are given in Fig. 6. The GSH levels in L. minor L. were found high in adapted group compared to the control group (p < 0.05). GSSG levels in adapted group were found higher than the control in L. minor L. (p < 0.05) but not in L. gibba L. The ratio of GSH/GSSG decreased in L. minor L. exposed to the effluent but increased in L. gibba L. when compared with the control (Figs. 5, 6). A decrease in the amount of GSH and an increase in the amount of GSSG are observed in L. gibba L. frond, particularly after the fourth day (Fig. 6).
This study assessed biochemical response in L. gibba L. and L. minor L. exposed to the secondary effluents from municipal wastewater treatment plant of Elazığ. Oxidative defense potentials that were evaluated by using the levels of MDA, GSH, GSSG, and vitamins A, E, and C in L. gibba L. and L. minor L.
COD is an index for organic pollutants which can induce lipid peroxidation and toxicity to submersed plants (Wang et al. 2002). Qiu-jin et al. (2007) founded that wastewater with high COD concentration can cause a substantial damage to submersed plant. BOD is a measure of the dissolved oxygen consumed by microorganisms during the oxidation of reduced substances in waters and wastes. The discharge of wastes with high levels of BOD can cause water quality problems such as severe dissolved oxygen depletion and fish kills in receiving water bodies (Penn). The levels of BOD and COD were found to be higher in wastewater suggesting the presence of high levels of organic and inorganic pollutants (Table 1).
Many environmental pollutants are capable of inducing oxidative stress in aquatic organisms. As shown Table 1 As, Al, Cd, Cr, Ni were determined in wastewater. Exposure of plants to excess concentrations of heavy metals results in accumulation of the metal in plants and cause an increase in reactive oxidative species (ROS). The first response to elevated amounts of ROS is increased levels of enzymatic and non-enzymatic antioxidants that reduce oxidative stress (Yamamoto et al. 1997; Razinger et al. 2007). Depending on concentration, plant species and exposure time, heavy metals cause changes in antioxidant enzyme activities and MDA contents in aquatic plants (Prasad et al. 2001; Doganlar 2013). In present study, different concentration of heavy metals were found in the secondary clarifier (Table 1).
The level of MDA has been known as an indicator of oxidative stress (Hou et al. 2007) In current research, It has been found that the amounts of MDA in the fronds of L. minor L. and L. gibba L. exposed to the effluent increased compared to the control (p < 0.05). The increase in MDA levels suggests that the heavy metals in wastewater causes oxidative stress in the plants. Zhao et al. (2008) suggested that the amount of MDA increased in the leaves of the plants exposed to water-stress, which also agrees with our findings. Karatas and Öbek Kamıslı (2009) investigated the variation of MDA in plants placed in the wastewater and grown in the natural water. Chemicals can increase the levels of MDA by increasing the formation of free radicals or by reducing the defense capacity against cellular peroxidation reactions (Lackner 1998). They found that the amount of MDA for the plants placed in the wastewater takes a maximum value at the second day and then decreases insignificantly until the fourth day, which again agrees with our findings. In our study, the level of MDA increases until the fourth day and then decreases insignificantly until the seventh day. Duckweed was observed to adapt to adverse environmental conditions within the first 4 days of exposure to wastewater and then continue to survive under oxidative stress conditions by elevating some vitamin levels.
Vitamins are organic compounds that must be taken by plants such as duckweed and are cofactors for various enzymes. In addition, vitamins A, E, and C play antioxidant roles against oxidative damage (Stryer 1995). Vitamins A, C, and E and element Se are some of the major non-enzymatic antioxidants in the organisms (Halliwell 1994). Depletion of a vitamin may be due to its utilization in the metabolic processes. Alterations in the amount of vitamins may be also due to the high energy demand arising from the stress conditions caused by xenobiotics. The decreases in the levels of antioxidant vitamins can result in their consumption for preventing oxidative stresses taking place during the treatment (Karatas and Öbek Kamıslı 2009). In the present study, vitamin A and C levels decreased in L. gibba L. and also vitamin E levels decreased in L. minor L. exposed to the effluent when compared to the control. The depletion of vitamin E shows enhanced sensitivity to the stress in the precarious state of cell since vitamin E prevents lipid peroxidation by trapping the free radicals (Karatas and Öbek Kamıslı 2009). Vitamins A and C also have effects on stopping other oxygen radicals because of the property of scavenging singled oxygen (Hercberg et al. 1998).
ROS can be eliminated by several functionally interrelated antioxidant systems that are crucial in adaptation and ultimate survival of plants during oxidative stress periods (Mittler 2002). Glutathione is involved as a co-substrate in conjugation reactions and is an important antioxidant in plants (Rijstenbil et al. 1994; Hamoutene et al. 1995). Xenobiotics can change quantities of reduced GSH (Rama Devi and Prasad 1998). Indeed, the induction of GSH shows an adaptation to pollutants and has been proven to play a critical role in maintaining cellular homeostasis (Doyotte et al. 1997). The changes in concentrations of GSH and GSSG are expressed as the ratio of GSSG/GSH that is one of the bioindicators of oxidative stress (Morel and Barouki 1999). In our study, the GSH and GSSG levels in L. minor L. were found high in adapted group compared to the control group (p < 0.05). The ratio of GSH/GSSG was decreased in L. minor L. exposed to the effluent when compared to control (Figs. 5, 6). Zhang et al. (2005) showed that increment in the amounts of GSH and GSSG might be due to the result of oxidative stress. In the present study, the decrease in the amount of GSH and the increase in the amount of GSSG in L. gibba L. frond, particularly after the fourth day, may be an expression of the increase in GSH-dependent responses.
In the present study, vitamin levels were decreased due to the high energy demand arising from the stress conditions caused by xenobiotics. Chemicals increased the levels of MDA by increasing the formation of free radicals or by reducing the defense capacity against cellular peroxidation reactions. It can be also suggested that xenobiotics change quantities of reduced GSH, GSSG, and GSH/GSSG. Alteration in GSH, GSSG, GSH/GSSG levels shows an adaptation to pollutants and has been proven to play a critical role in maintaining cellular homeostasis.
It can be concluded that MDA, GSH, GSSG, GSH/GSSG and vitamins (A, E and C) in L. minor L. and L. gibba L. can be considered biomarkers to control water quality. Measurement of the levels of antioxidant biomarkers such as MDA, GSH, GSSG, GSH/GSSG, and vitamin (A, E and C), shows that they may be promising indicators of an oxidant impact on aquatic plants.
References
APHA, AWWA, WPCF (2005) American Public Health Association, American Water Works Association, Water Pollution Control Federation, Washington
Appenroth KL, Krech K, Keresztes A, Fischer W, Koloczek H (2010) Effects of nickel on the chloroplasts of the duckweeds Spirodela polyrhiza and Lemna minor and their possible use in biomonitoring and phytoremediation. Chemosphere 78:216–223
Barim O, Karatepe M (2010) The effects of pollution on the vitamins A, E, C, beta-carotene contents and oxidative stress of the freshwater crayfish, Astacus leptodactylus. Ecotoxicol Environ Saf 73:138–142
Bergmann BA, Cheng J, Classen J, Stomp AM (2000) In vitro selection of duckweed geographical isolates for potential use in swine lagoon effluent renovation. Bioresour Technol 73:13–20
Cerhata D, Bauerova A, Ginter E (1994) Determination of ascorbic acid in blood plant using high-performance liquid chromatography and its correlation with spectrophotometric. Caska Slov Farm 43:166–168
Chen Y, Wang C, Wang Z (2005) Residues and source identification of persistent organic pollutants in farmland soils irrigated by effluents from biological treatment plants. Environ Int 31:778–783
Cnubben NHP, Rietjens IMCM, Wortelboer H, van Zanden J, Van Bladeren PJ (2001) The interplay of glutathione-related processes in antioxidant defense. Environ Toxicol Pharmacol 10:141–152
Dickinson DA, Forman HJ (2002) Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci 973:488–504
Doganlar ZB (2013) Metal accumulation and physiological responses induced by copper and cadmium in Lemna gibba, L. minor and Spirodela polyrhiza. Chem Speciat Bioavailab 25:79–88
Doyotte A, Cossu C, Jacquin MC, Babut M, Vasseur P (1997) Antioxidant enzymes, glutathione and lipid peroxidation of experimental or field exposure in the gills and the digestive gland of the fresh water bivalve Unio tumidus. Aquat Toxicol 39:93–110
Gagne F, Andre C, Cejka P, Hausler R, Fournier M, Blaise C (2008) Immuno-toxic effects on freshwater mussels of a primary-treated wastewater before and after ozonation: a pilot plant study. Ecotoxicol Environ Saf 69:366–373
Halliwell B (1994) Free radical antioxidants in human disease. Curiosity cause or consequence. Lancet 344:721–724
Hamoutene D, Mathieu A, Hofmann P, Salaun JP, Lafaurie M (1995) Preparation and characterization of subcellular fractions suitable for studies of xenobiotic metabolism from leaf sheaths of a marine seagrass: Posidonia oceanica (Linnaeus) Delile. Mar Environ Res 39:249–253
Hercberg S, Galan P, Prezıosı P, Alfarez MJ, Vazquez C (1998) The potential role of antioxidant vitamins in preventing cardiovascular diseases and cancers. Nutrition 14:513–520
Hou W, Chen X, Song G, Wang Q, Chang CC (2007) Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (L. minor). Plant Physiol Biochem 45:62–69
Karatas F, Öbek Kamıslı EF (2009) Antioxidant capacity of Lemna gibba L. exposed to wastewater treatment. Ecol Eng 35:1225–1230
Lackner R (1998) Oxidative stress in fish by environmental pollutants. Fish Ecotoxicol 86:203–224
Miller KW, Lorr NA, Yang CS (1984) Simultaneous determination of plasma retinol a-tocoferol, iycopene, a-carotene, and b-carotene by high performance liquid chromatography. Anal Biochem 138:340–345
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Morel Y, Barouki R (1999) Repression of gene expression by oxidative stress. Biochem J 342:481–496
Nunesa BAR, Gaioc F, Carvalhod L, Guilherminoa (2008) Behaviour and biomarkers of oxidative stress in Gambusia holbrooki after acute exposure to widely used pharmaceuticals and a detergent. Ecotoxicol Environ Saf 71:341–354
Ortega-Villasante C, Rellan-Alvarez R, Del Campo FF, Carpena Ruiz RO, Hernandez LE (2005) Cellular damage induced by cadmium and mercury in Medicago sativa. J Exp Bot 56:2239–2251
Penn MR, Pauer JJ, Mihelcic JR (2004) Environmental and ecological chemistry—vol. II—biochemical oxygen demand. Encyclopedia of Life Support Systems (EOLSS), Oxford
Petala M, Kokokiris L, Samaras P, Papadopoulos A, Zouboulis A (2009) Toxicological and ecotoxic impact of secondary and tertiary treated sewage effluents. Water Res 43:5063–5074
Prasad MN, Malec P, Waloszek A, Bojko M, Strzałka K (2001) Physiological responses of Lemna trisulca to cadmium and copper bioaccumulation. Plant Sci 161:881–889
Qiu-jin X, Yue-gang N, Xiang-can JIN, Chang-zhou Y, Jin L, Gao-ming J (2007) Effects of chitosan on growth of an aquatic plant (Hydrilla verticillata) in polluted waters with different chemical oxygen demands. J Environ Sci 19:217–221
Radic S, Stipanicev D, Cvjetko P, Sırac S, Pevalek-Kozlina B, Pavlica M (2011) Duckweed Lemna minor as a tool for testing toxicity and genotoxicity of surface waters. Ecotoxicol Environ Saf 74:182–187
Rama Devi S, Prasad MNV (1998) Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Sci 138:157–165
Razinger J, Dermastia M, Drinovec L, Drobne D, Zrimec A, Koce JD (2007) Antioxidative responses of duckweed (Lemna minor L.) to short-term copper exposure. Environ Sci Pollut Res Int 14:194–201
Rijstenbil JW, Derksen JWM, Gerringa LJA, Poortvliet TCW, Sandee A, Van der Berg M (1994) Oxidative stress induced by copper: defense and damage in the marine planktonic diatom Ditylum brightwellii, grown in continuous cultures with high and low zinc levels. Mar Biol 119:583–590
Stryer L (1995) Biochemistry, 4th edn. W.H. Freeman, New York, pp 452–455
Tavazzi B, Lazzarino G, Di-Pierro D, Giardina B (1992) Malondialdehyde production and Ascorbate decrease are associated to the reperfusion of the isolated postischemic rat heart. Free Radic Biol Med 13:75–78
Wang B, Zhou LP, Li W (2002) Preliminary studies on the purifying function and its physiological reaction of Potamogeton crispus under different water qualities. J Wuhan Bot Res 20(2):150–152
Yamamoto Y, Hachia A, Matsumoto H (1997) Oxidative damage to membranes by a combination of aluminium and iron in suspension-cultured tobacco cells. Plant Cell Physiol 38:1333–1339
Zhang JF, Lıub H, Sun YY, Wang XR, Wu JC, Xue YQ (2005) Responses of the antioxidant defenses of the Goldfish Carassius auratus, exposed to 2,4-dichlorophenol. Environ Toxicol Pharmacol 19:185–190
Zhao Z, Cai Y, Fu M, Bai Z (2008) Response of the soils of different land use types to drought: eco-physiological characteristics of plants grown on the soils by pot experiment. Ecol Eng 34:215–222
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tatar, S.Y., Obek, E. & Cikcikoglu Yildirim, N. Antioxidant Response in Duckweed After Exposure to Secondary Effluent from Municipal Wastewater Treatment Plant, Elazığ, Turkey. Bull Environ Contam Toxicol 99, 399–404 (2017). https://doi.org/10.1007/s00128-017-2133-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2133-3