Abstract
Combined with the dating analysis of 210Pb and 137Cs, the historical concentration and risk of As, Cd, Cr, Cu, Hg, Ni, Pb and Zn in the core sediment of Weishan Lake were determined. Pollution concentrations, enrichment factor, geo-accumulation index, and ecological risk index of these metals and C/N ratio indicate that the natural inputs prevailed till early 1900s. From late 1970s onwards, the risk posed by these metals was significantly increased. Cu and Hg might cause adverse biological effects. The increase of soil erosion and wastewater discharge in the watershed promoted input and accumulation of these metals in the lake. The coal and petroleum industry in the catchment led the more accumulation of Hg, Cd and Cu in the lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Lacustrine pollution caused by heavy metals has been a major global concern due to rapid urbanization and industrial development (Hansen 2012). Heavy metals enter lakes through weathering of rocks, soil erosion and anthropogenic activities, and are finally deposited in the sediment (Bacardit et al. 2012). Sediments serve both as reservoirs and as potential sources for heavy metals (releasing heavy metals to the water body). Sediment-associated heavy metals have the potential to directly affect the sediment-dwelling organisms. Such heavy metals can adversely affect wildlife and human health due to their accumulation in the food chain (Alves et al. 2014). Therefore, a study of the heavy metals in lake sediments can provide vital information regarding their historical status and the risk posed by these metals to the lacustrine ecosystem (Ra et al. 2011; Ma et al. 2013). With this information, the efficiency of pollution management strategies can be evaluated and more suitable regulatory actions can be implemented to reduce potential health and environmental risks caused by the lacustrine pollution.
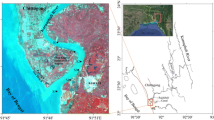
Nansi lake (34°27′–35°20′N, 116°34–117°21′E) is the largest freshwater lake in North China, having an area of 1266 km2 and an average depth of 1.46 m. It includes four lakes, namely Nanyang Lake, Dushan Lake, Zhaoyang Lake and Weishan Lake (see Fig. 1). After the building of a dam in 1960, Nansi Lake was divided into upper and lower lakes (Zhang et al. 2003). Nanyang, Dushan and Zhaoyang lakes are located in the upper lake, while the Weishan Lake is located in the lower lake. As the largest buffering reservoir in the East Route of China’s South-North Water Transfer Project, the lake plays an important role for the security of agriculture and drinking water supply. There has been a rapid economic growth of Jining city, Zaozhuang city and Xuzhou city near Nansi Lake watershed since 1970s. Due to this growth, excessive pollutants contained in municipal wastewater, agricultural run-off and industrial wastewater discharged into the Nansi Lake (Zhang et al. 2007). This excessive discharge of pollutants has led to the accumulation of As, Hg, Cd and Pb in surficial sediments of river mouths and the dam of the Nansi Lake (Lv et al. 2014). The surficial sediment in upper lake had a higher ecological risk than that in the lower lake due to the more anthropogenic activities (Yang et al. 2015). However, these researches mostly focused on the distribution and risk of heavy metals in the surficial sediments of Nansi Lake in recently (Liu and Zhang 2013). Literature on the study of historical accumulation and risk of heavy metals in the lake is scarce. The historical environmental change and pollution of the lake can be well-recorded in the core sediment from the relatively stable sedimentary environment (Oldfield and Appleby 1984; Ma et al. 2013). Thus, based on the above consideration, a core sediment owning more stable sedimentary environment and less anthropogenic disturbance from Weishan Lake located in the downstream lake area of Nansi Lake was sampled. The historical input and the subsequent risk posed by As, Cd, Cr, Cu, Hg, Ni, Pb and Zn in the lake were assessed and analyzed based on the change of concentration, enrichment factor (EF), geo-accumulation index (I geo), and ecological risk index (RI) of heavy metals in the core sediment.
Materials and Methods
The study carried out in the autumn of 2012 at the Weishan Lake. The lake is located in the downstrearm of the dam. Zaozhuang city in Shangdong province and Xuzhou city in Jiangsu province located around the lake (see Fig. 1). In view of the effect of hydrological characteristics on sedimentary environment, the sampling area were far from river mouths, water facilities, aquaculture, tourist and living activities to avoid any other kind of disturbance (Fig. 1). A 60-cm-long sediment core was collected using a Beeker type core sampler (Product code: 04.23.SA) (100 cm length × 57 mm i.d.; Eijkelkamp Co., Giesbeek, The Netherlands) from the center of the Weishan Lake (34°36′6″N, 117°12′38″E), where the water depth was approximately 3.5 m. The core sediment was immediately sliced at intervals of 2-cm in the field. All samples were freeze-dried, ground into a fine homogenized powder and then stored at −20 °C until further processing.
Total organic carbon (TOC) and total nitrogen (TN) were analyzed by combusting samples at 950 °C using a Perkin-Elmer PE 2400 Series II elemental analyzer. For elemental analyses, approximately 0.5 g sediment sample was digested using HClO4, HNO3 and HF at 180 °C for 24 h (Ra et al. 2011). Then each digested sample was diluted with 2% HNO3 to certain volume to analyze by instruments based on GB/T 17136-17141-1997 in China (Cao et al. 2014). The Inductively coupled plasma optical emission spectrometer (ICP-OES) (Thermo Electron Co., USA) was used to analyze Fe and total phosphate (TP). Inductively coupled plasma atomic emission spectrometry (ICP-AES) (IRIS Intrepid II, Thermo Electron Co., USA) was used to analyze Cr, Ni, Cu and Zn. Inductively coupled mass spectrometry (ICP-MS) (X Series II, Thermo Electron Co., USA) was used to analyze As, Cd and Pb. The cold vapor atomic fluorescence spectrometry (CV-AFS) was used to determine the concentration of total Hg in samples and the blanks by using F732-V Hg detector. The detection limits (µg g−1) were found to be 10 for TP and 1000 for Fe, 5 for Cr and Ni, 0.5 for Cu, 2 for Zn, 0.02 for As and Cd, 2 for Pb and 0.001 for Hg. The recoveries obtained in the above mentioned reference materials ranged from 95.5 to 105.1%.
The radioisotope activity concentrations (such as 210Pb, 226Ra and 137Cs) in the sediment core were determined at 2-cm intervals using a well-type high-purity Germanium (HPGe) gamma detector (GCW3523, Canberra Inc., USA) to date the core sediment (Liu et al. 2005). The 210Pb activity in the sediment samples was determined by the γ emissions at 46.5 keV, while the activity of 226Ra was determined by the γ-rays emitted at 352 keV. The activity of 137Cs was determined by γ emissions at 662 keV. 210Pbex was obtained by subtracting the 226Ra activity from the total 210Pb activity (Piazza et al. 2009).
Results and Discussion
Figure 2 shows the vertical distributions of excess 210Pb and 137Cs for the core sediment samples. The activity of 137Cs showed a peak at a depth of 22 cm, recording the maximum atmospheric fallout from the testing of thermonuclear weapons in 1963 (Piazza et al. 2009). The 210Pbex activity in the core sediment declined from 45.67 Bq/kg at the surface to 1.00 Bq/kg at 28 cm. The excess 210Pb activity profile showed a definite exponential decay with respect to depth. The 210Pb geochronology was calculated from a constant rate of supply (CRS) model, which is represented by Eq. (1).
where A 0 and A z are the 210Pbex accumulation fluxes at the surficial layer of the sediment core and at depth z respectively. λ is the 210Pbex radioactive decay constant (0.03114 y−1). Thus, relatively accurate dates were obtained from the 210Pb dating, which were also verified by 137Cs dating (see Fig. 2). The 60-cm core covers approximately 140 years sedimentary history between 1870 and 2012, while the sediment deposition rate ranged from 0.22 to 0.74 cm y−1, with a mean value of 0.42 cm y−1.
Figure 3 shows the vertical distributions of Fe, TOC, TN, TP and C/N ratio for the core sediment. The Fe contents varied from 4.73% to 6.89%, showing a slightly increasing trend from the bottom to the top. The TN contents varied from 0.45 to 1.38 mg/g, showing a fluctuating increase from the bottom to the top. The TP contents varied from 0.17 to 0.57, showing no obvious change with respect to the depth. The higher values of TN and TP at the upper layer might be attributed to excessive use of fertilizers in agriculture and erosion during land use in the past 10 years (Shao et al. 2013). The TOC contents varied from 0.10% to 2.47%, showing a depth profile very similar to that of C/N (1.23–23.28). Higher values of TOC (>1) and C/N (>10) were observed at the surface of the core indicating the input of terrestrial organic matter (Kendall et al. 2001), which might attribute to increased soil erosion due to the change of land use in recently (Shao et al. 2013). Since 2000s, some point source pollution controlled measurements implemented to improve water quality in Nansi Lake, which have reduced the chemical oxygen demand (COD) of water in the lake to some degree (Wu et al. 2010). However, the refractory organics still continuously accumulated in the sediment (Yu et al. 2012).
Figure 4 shows the vertical distribution of heavy metals in the core sediment. All heavy metal in the core sediment showed similar increasing trends from bottom to the surface. The higher concentrations of Cd (>0.17 µg/g), Cr (>95.82 µg/g), Cu (>79.01 µg/g), Hg (>0.10 µg/g) and Zn (>93.66 µg/g) were found at 0–16 cm depth (1978–2012), whereas the maximum concentrations of As, Ni, and Pb were observed to be 14.94 µg/g at 0–2 cm depth (2012), 64.18 µg/g at 12–14 cm depth (1980) and 35.59 µg/g at 16–18 cm depth (1973) respectively. The presence of Cu, Zn Cr, Cd and Hg is mainly due to the coal combustion and industrial effluents (Hou et al. 2013). The higher concentrations of Cd, Cr, Cu and Hg in the upper layer of the core sediment in the lake might be attributed to the high use of large quantity of coal since the “reform and opening” began in around 1978. There are actually two famous coal ore mining companies operating in the region. These are Zaozhuang coal mining and Xuzhou coal mining, both of which operate near Weishan Lake. Each of these coal mines produces more than 20 million tons of raw coal per year. The anthropogenic activities and the industrial input during various time periods might have caused the variation in the abundance characteristics of the heavy metals.
The relationship among the geochemical variables in the sediment core and the sources of these elements was evaluated by principal component analysis (PCA) (Reid and Spencer 2009; Ma et al. 2013). According to the PCA, two components were obtained, accounting for 76.09% of the total variance (see Fig. 5). Component 1 accounted for 65.09% of total variance to form an element group of Cd, Cr, Cu, Hg, Zn, Fe, Pb, Ni, As and TOC with high loadings (>0.6) which indicating a common origin. In the zone of component 1, the group was separated two part I and part II based on the positive and negative value of loading in the direction of component 2. Cd, Cu and Hg are mainly from the discharge of coal and petroleum industrial effluents (Ruiz-Fernández et al. 2007). These metals plotted in the same area on principal component 1 scores with Fe, Zn, Cr, Pb, Ni, As and TOC which indicated that these elements are mainly from the input of industrial activities. TOC had a good relationship with other elements (>0.5) confirmed organic carbon plays a major role in accumulating metals in sediments (Sheykhi and Moore 2013). Component 2, with a variance of 11.00%, was highly correlated (>0.6) with TP and TN, which identified as the allochthonous inputs from agricultural non-point sources such as fertilizer usage and terrestrial soil erosion (Zhang et al. 2003; Ma et al. 2013). As compounds were widely used as the pesticides, and phosphate fertilizers also is an important source of As (Lv et al. 2014). The runoff and soil erosion from agricultural land can be also an important source of As in sediments of Nansi Lake, which have a little difference in scores of Component 1 (0.6) and Component 2 (0.0) from other heavy metals. TOC and Fe are generally concentrated in the fine fractions, and Pb is mainly from the release of coal combustion and traffic tool, which might suggest that the elements located in I zone (Component 1 > 0.6 and Component 2 > 0.0) are from the atmospheric deposition.
The enrichment factor (EF) and geo-accumulation index (I geo) were used to investigate the change in pollution levels of heavy metals with respect to depth and sedimentary process in the core sediment. The EF value was calculated using the formula given in Eq. (2).
where (Me/Fe)sample is the metal to Fe ratio in the sample of interest, while (Me/Fe)background is the natural background value of metal to Fe ratio (Sinex and Wright 1988). The I geo was calculated by using Eq. (3).
where C n is the measured concentration of element N in the samples and B n is a parameter, which is either directly measured in texturally equivalent uncontaminated sediments or adapted from the literature. In the current study, the natural geochemical background of the soil in the Shandong province in China was taken as the background value for the lake. The background values of As, Cd, Cr, Cu, Hg, Ni, Pb, Zn and Fe were found to be 9.30, 0.08, 66.00, 24.00, 0.02, 25.80, 63.50 μg/g and 2.72% respectively (SEPA of China 1990). The calculated results of the EF and I geo are shown in Table 1. The order of enrichment of different elements in the lake was found to be: Hg > Cu > Ni > Cd > Zn > Cr > As > Pb. The EF values were below 2 and were found to be in the range of 0.62–0.83 (for As), 0.59–1.10 (for Cd), 0.56–0.68 (for Cr), 1.06–1.47 (for Cu), 0.81–1.10 (for Ni), 0.43–0.59 (for Pb) and 0.44–0.70 (for Zn) respectively. These values indicated that the degree of pollution caused by these metals can be categorized from no pollution to slight (degree of) pollution. However, the EF values of Hg exceeded 2 at the depth of 0–18 cm (corresponding to the time of the 1970s–2012), indicating a moderate degree of pollution (Pekey 2006). The order of I geo of different elements in the lake was found to be: Hg > Cu > Ni > Cd > As > Zn > Cr > Pb. The I geo values were below 0 or 1 and were found to be in the range of −0.21–0.10 (for As), −0.47–0.71 (for Cd), −0.51–0.05 (for Cr), 0.02–0.73 (for Ni), −0.69 to −0.12 (for Pb) and −0.84–0.12 (for Zn). These values indicated that the degree of pollution caused by these metals can be categorized from no pollution to moderate pollution. However, the I geo values of Cu were between 1 and 2 at the depth of 0–18 cm (corresponding to the time of the 1970s–2012), indicating a moderate degree of pollution. The I geo values of Hg were close to 2 at the depth of 0–14 cm (corresponding to the time of the 1978–2012), indicating a moderate to high degree of pollution (Hao et al. 2013). Some heavy metals, such as Cd, Cu and Hg, were easily accumulated and exhibited a relatively higher ecological risk in the upper layers (0–14 cm) of the sediment core compared to the lower layers. In spatial distribution, As, Pb, Cd and Hg in surficial sediments from river mouths and surrounding the dam in the lake had a higher risk (Lv et al. 2014). The characteristics of coal ore production, its usage near the lake and the rapid economic development since 1978 (Li and Zhang 2012) might have led to the accumulation of these metals.
The ecological risk index (RI) was applied with the aim of achieving a broader assessment of ecological risks caused by heavy metals in the lake, which is based on the toxicity of heavy metals and the response of the environment (Hakanson 1980; Lv et al. 2014). The RI of a given contaminant is defined as:
where T i is the toxic-response factor for a given substance, C ni is threshold effect level in sediments, C si is present concentration values. The toxic-response factors for As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn were 10, 30, 2, 5, 40, 5, 5, and 1 respectively (Qiao et al. 2005; Sheykhi and Moore 2013). RI of heavy metals is in the range of 194–353 showing ecological risk caused by heavy metals existing in the lake (Table 1). In the upper layers (0–14 cm) of sediment core, the value of RI is higher than 300 indicating high ecological risk, and that in lower layer sediment is between 150 and 300 indicating moderate ecological risk (Hakanson 1980). The results indicate urbanization and industrialization in the watershed since the 1970s has significantly increased the contaminant risk level of the lake. The concentration profiles of the heavy metals’ residues, EF, I geo and RI values in the core sediments with respect to both the depth and the date indicate that the risks to environment and humans have increased with an increase in anthropogenic activities, particularly since the onset of “reform and opening” in 1978. Due to a significant increase in the concentrations of these metals and the subsequent risk these posed, continuous monitoring and control of the sources (of these metals) should be carried out in the lake. In this regard, special focus should be given to Hg.
References
Alves RIS, Sampaio CF, Nadal M, Schuhmacher M, Domingo JL, Segura-Munoz SI (2014) Metal concentrations in surface water and sediments from Pardo River, Brazil: Human health risks. Envrion Res 133:149–155
Bacardit M, Krachler M, Camarero L (2012) Whole-catchment inventories of trace metals in soils and sediments in mountain lake catchments in the central pyrenees: apportioning the anthropogenic and natural contributions. Geochim Cosmochim Acta 82:52–67
Cao XZ, Shao Y, Deng WJ, Wang H, Wang SL (2014) Spatial distribution and potential ecologic risk assessment of heavy metals in the sediments of the Nansi Lake in China. Environ Monit Assess 186:8845–8856
Hakanson L (1980) An ecological risk index for aquatic pollution control: a sedimentological approach. Water Res 14(1):975–1000
Hansen AM (2012) Lake sediment cores as indicators of historical metal(loid) accumulation—a case study in Mexico. Appl Geochem 27:1745–1752
Hao LB, Sun LJ, Zhao YY, Lu JL (2013) Sedimentary records of evolution of heavy metals in Songhua Lake, Northeast China. Clean Soil Air Water 41(10):1010–1017
Hou DK, He J, Lu CW, Ren LM, Fan QY, Wang JH, Xie ZL (2013) Distribution characteristics and potential ecological risk assessment of heavy metals (Cu, Pb, Zn, Cd) in water and sediments from Lake Dalinouer, China. Ecotox Environ Safe 93:135–144
Kendall C, Silva SR, Kelly VJ (2001) Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States. Hydrol Process 15:1301–1346
Li S, Zhang ZL (2012) Spatial distribution and pollution assessment of heavy metals in surface sediments of Nansihu Lake. Water Resour Protect 28: 6–11. (In Chinese)
Liu L, Zhang ZL (2013) Spatial distribution, sources and pollution assessment of heavy metals in the surface sediments of Nansihu Lake. J Hydroecol 34:7–15. (In Chinese)
Liu GQ, Zhang G, Li XD, Li J, Peng XZ, Qi SH (2005) Sedimentary record of polycyclic aromatic hydrocarbons in a sediment core from the Pearl River Estuary, South China. Mar Pollut Bull 51:912–921
Lv JS, Zhang ZL, Li S, Liu Y, Sun YY, Dai B (2014) Assessing spatial distribution, sources, and potential ecological risk of heavy metals in surface sediments of the Nansi Lake, Eastern China. J Radioanal Nucl Chem 299:1671–1681
Ma L, Wu JL, Abuduwili JLL (2013) Geochemical evidence of the anthropogenic alteration of element composition in lacustrine sediments from Wuliangsu Lake, north China. Quatern Int 306:107–113
Oldfield F, Appleby PG (1984) Empirical testing of 210Pb-dating models for lake sediments. In: Haworth EY, Lund J.W.G. (eds), Lake sediments and environmental history. Leicester University, Leicester pp. 93–124
Pekey H (2006) Heavy metals pollution assessment in sediments of the Izmit Bay, Turkey. Environ Monit Assess 123:219–231
Piazza R, Ruiz-Fernández AC, Frignani M, Vecchiato, Bellucci LG, Gambaro A, Pérez-Bernal LH, Páez-Osunaa F (2009) Historical PCB fluxes in the Mexico City Metropolitan Zone as evidenced by a sedimentary record from the Espejo de los Lirios lake. Chemosphere 75:1252–1258
Qiao S, Jiang J, Xiang W, Tang J (2005) Distribution of heavy metals in sediments in lakes in Wuhan with assessment on their potential ecological risk. Resour Environ Yangtze Basin 14: 353–357. (In Chinese)
Ra K, Bang JH, Lee JM, Kim KT, Kim ES (2011) The extent and historical trend of metal pollution recorded in core sediments from the artificial Lake Shihwa, Korea. Mar Pollut Bull 62:1814–1821
Reid M, Spencer K (2009) Use of principal components analysis (PCA) on estuarine sediment datasets: the effect of data pre-treatment. Environ Pollut 157:2275–2281
Ruiz-Fernández AC, Hillaire-Marcel C, Páez-Osuna F (2007) 210Pb chronology and trace metal geochemistry at Los Tuxtlas, Mexico, as evidenced by a sedimentary record from the Lago Verde crater lake. Quatern Res 67:181–192
Shao B, Jia YF, Ji XM (2013) The research on the coordinate development between economy and water environment in Nansi Lake watershed. Shanddong University Press, Jina, pp. 6–60. (In Chinese)
Sheykhi V, Moore F (2013) Evaluation of potentially toxic metals pollution in the sediments of the Kor river, southwest Iran. Environ Monit Assess 185:3219–3232
Sinex SA, Wright DA (1988) Distribution of trace metals in the sediments and biota of Chesapeake Bay. Mar Pollut Bull 19:425–431
The State Environmental Protection Agency (SEPA) of China. (1990) China’s soil element background values. China Environmental Science Press, Beijing.
Wu ZH, Mu JB, Xie G, Lu CG, Zhu J (2010) Analysis of water environment quality variation trends in Nansi Lake and its joined rivers. Res Environ Sci 23:1167–1173. (In Chinses)
Yang LY, Wang LF, Wang YQ, Zhang W (2015) Geochemical speciation and pollution assessment of heavy metals in surface sediments from Nansi Lake, China. Environ Monit Assess 187:261
Yu T, Zhang Y, Meng W, Hu XN (2012) Characterization of heavy metals in water and sediments in Taihu Lake, China. Environ Monit Assess 184:4367–4382
Zhang Z, Shen J, Liu E, Sun Q, Jiang L (2003) Formation and water environmental evaluation of the Nansihu Lake. J Geog Sci 13:241–249. (In Chinses)
Zhang ZL, Xin LJ, Liang CL (2007) The analysis of hydrological characteristics and processes of ecosystem in Lake Nansi during the past 50 years. Geographical Res 26: 957–966. (In Chinses)
Acknowledgements
The study was funded by projects of Beijing Natural Science Foundation of China (No. 8152028). The research was also supported by the Open Research Fund Program of Key Laboratory of Urban Stormwater System and Water Environment (Beijing University of Civil Engineering and Architecture), Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, L., Guo, W., Wang, S. et al. Historical Characteristics of Contamination and Risk of Heavy Metals in Weishan Lake, China. Bull Environ Contam Toxicol 98, 776–783 (2017). https://doi.org/10.1007/s00128-017-2073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2073-y