Abstract
Estrogenic activities and main causative fractions in three representative sections of Yangtze River (Nanjing section) were determined. The results showed that significant vitellogenin (VTG) and 17β-estradiol (E2) induction and gonad atrophy were observed. Estradiol equivalents of actual water samples from Jiangxinzhou section, Sanchahe section and Daqiao section were 0.3651, 0.1301 and 0.5060 ng L−1, respectively. Polar contaminants were responsible for the estrogenic activities in Jiangxinzhou section and Daqiao section while mid-polar and nonpolar contaminants resulted in majority of the estrogenic activity in Sanchahe section. To Jiangxinzhou section, Sanchahe section and Daqiao section, good positive correlations between VTG and E2 (the correlation coefficients were 0.737, 0.690 and 0.817, respectively) and good inverse correlations between VTG and gonado-somatic index (GSI; the correlation coefficients were −0.838, −0.540 and −0.794, respectively) were obtained, whereas the correlations between E2 and GSI were relatively poor (the correlation coefficients were only −0.557, −0.620 and −0.509, respectively).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endocrine disrupting chemicals (EDCs) have been of great concern in the past decades. A wide range of studies in laboratory settings have documented that even at very low concentrations (ng L−1) EDCs can have adverse effects on reproduction and subsequent population development in fish populations (Pawlowski et al. 2004; Brion et al. 2004). Environmental estrogens are the most important in EDCs and numerous natural and “xeno”estrogens have been identified in wastewater. Since the aquatic environment is the ultimate sink for most environmental pollutants originating from industrial, agricultural or municipal effluent, most cases of endocrine disruption and associated reproductive impairment have been reported in various species of fish in many countries (Hashimoto et al. 2000; Roy et al. 2003; Solé et al. 2003; Dick Vethaak et al. 2003; Hu et al. 2003).
Yangtze River is the largest river in China. Nanjing is an important city in Yangtze River Delta which is the most developed area in China. In Nanjing, a great deal of effluents from wastewater treatment plants (WWTPs), untreated sewage and upstream wastewater enter Yangtze River and many pollution zones have formed along the river. Many organic contaminants, with obvious genetic toxicity, have been detected in the river water (Li et al. 2006). In recent years, the number of fish has begun to decrease and fish have tended to smaller and younger. Fish illness has begun to prevail and the number of deformities has increased (Peng 2006). The aim of this study was to investigate the estrogenic activities and dominant causative fractions in the water samples taken from three representative sections of Yangtze River (Nanjing section) using solid phase extraction (SPE) fractionation and an in vivo bioassay focused on serum vitellogenin (VTG) level, serum 17β-estradiol (E2) concentration and gonado-somatic index (GSI). It would be a scientific reference to know and control environmental estrogen pollution in Yangtze River (Nanjing section) and protect and recover important fish resource.
Materials and Methods
C18-H solid phase extraction cartridge (5 g, 20 mL volume) was purchased from Beijing Zhenxiang Industrial Trade Co., Ltd. (Beijing, China). Purified goldfish vitellogenin and primary antibody (rabbit anti-goldfish vitellogenin) were obtained from College of Marine Life Sciences, Ocean University of China (Qingdao, China). (Phenylmethyl) sulfonyl fluoride (PMSF), heparin sodium and Tween-20 were purchased from Nanjing Sunshine Biotechnology Co., Ltd. (Nanjing, China) and their purities were >99%. Phosphate-buffered saline, non-fat milk, goat anti-rabbit IgG labeled with alkaline phosphatase (AP) were purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan, China). p-Nitrophenylphosphate (pNPP) was purchased from Shanghai Boyun Biotech Co., Ltd. (Shanghai, China). Coomassie brilliant blue G-250 (Ultra Pure Grade) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Bovine serum albumin was purchased from Shanghai Huixing Biochemistry Reagent Co., Ltd. (Shanghai, China) and the purity was >98%. All other chemicals were of analytical grade and were obtained from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China). E2 assay kit was purchased from Adlitteram Diagnostic Laboratories Inc. (USA).
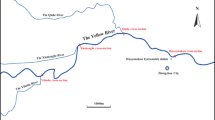
Based on the distribution of Yangtze River (Nanjing section) water system and effluent discharge from WWTPs in Nanjing, three representative sections were set near the input points of main branches and outlets of WWTPs. They were Jiangxinzhou section, Sanchahe section and Daqiao section. Distribution of the three representative sections was presented in Fig. 1. Water samples were collected in November 2008. All sampling equipments were disinfected with a weak bleach solution, after which they were rinsed with tap water and distilled water. A 12 L of water sample (approximate to saturation adsorption volume of the 5 g C18-H SPE cartridge) was collected from each section by water sampler. In order to prevent bacteria growth, methanol (5‰) was added into each water sample immediately.
After vacuum filtered through a 0.45 μm glass fiber filter to remove suspended substance, each water sample was extracted by a 5 g reverse-phase C18-H SPE cartridge which had been previously conditioned with 10 mL of methanol followed by 10 mL of deionized water. After freeze-dried to remove the residual water, the cartridge was eluted by a series of 5 mL volumes of methanol/water mixtures (0%, 25%, 50%, 75%, 80%, 85%, 90%, 95%, and 100% methanol), which were collected in separate vials (Desbrow et al. 1998). The same SPE cartridge was then sequentially eluted with solvents of low polarity to nonpolarity (diethyl ether, 50/50 diethyl ether/hexane, and finally hexane) to elute compounds of the widest polarity range that had not been removed previously by the 100% methanol. Under a gentle nitrogen stream, these fractionated samples were blown down to incipient dryness and redissolved in 1 mL of dimethyl sulfoxide (DMSO) for in vivo assay. 0.1 mL of each extracted fraction was extracted and mixed to make up a mixture. Pollutants in the 1.2 mL of mixture were equivalent to those in 1.2 L of water sample from Yangtze River. The fractions and mixtures were stored at −20°C in the dark until analysis.
Adult male goldfish (Carassius auratus) weighing 34.6 ± 2.8 g were obtained from Nanjing Fuzimiao Flower and Bird Market. The fish were acclimatized for 2 weeks in dechlorinated municipal water prior to experimentation, during which time they were fed with commercial fish food (Nanjing, China) once a day. Sewage and uneaten food were removed every other day by suction. A 50% water change was performed every other day. Water temperatures ranged from 14 to 16°C. Fish were not fed for 24 h prior to the experiments.
Each randomly assigned fish was treated with 100 μL of different polar fraction or mixture by intraperitoneal injection. Each fraction or mixture was delivered in triplicate. A dechlorinated municipal water control and a solvent control (receive DMSO only) were included in the experimental design. The concentration–response curve of E2 for VTG induction was determined by delivering 100 μL of a dilution series of E2 ranged from 1 × 10−2 ng L−1 to 1 × 104 ng L−1 to fish. Fish were then maintained in 30-L aquariums (40 × 25 × 30 cm) containing 20 L of dechlorinated municipal water under constant aeration for 7 day and were not fed throughout the experiment. Temperature was the same as for acclimation described above.
Blood (about 1 mL) was collected via heparinized 2 mL syringe from the dorsal aorta and immediately added to 0.1 mM PMSF (5‰) as protease inhibitor. The samples were immediately centrifugateed for 10 min (4000×g) at 4°C. The serum was removed and stored at −80°C until analysis.
Serum VTG levels were measured following a competitive enzymelinked immunosorbent assay (ELISA), as described by Rempel et al. (2006). To decrease level of cross-reactivity, it was necessary to dilute the serum samples a minimum of 1:50. Goat anti-rabbit IgG-AP was used as the secondary antibody. VTG in the standards and diluted samples were measured in triplicate. Serum VTG levels were normalized to total protein per serum sample to control for potential survivorship differences between treatments and among replicates (Todorov et al. 2002). The total protein concentrations of the serum samples were determined using a Coomassie Protein Assay Kit (Bradford 1976), with bovine serum albumin as the standard. Measurements were conducted on a microplate reader at 595 nm.
Serum E2 concentrations were determined by competitive enzyme immunoassays conducted using commercial kits. Assay procedure was performed according to the instruction. The minimum detectable concentration of E2 in this assay was estimated to be 1.0 pg mL−1.
The fish was weighed and gonad was removed and weighed after blood was sampled. GSI (%) was calculated by 100 × gonad wet weight (g)/wet weight of fish (g).
The working range of the ELISA was from 20% to 85% of the 0 ng mL−1 standard absorbance value. Only the sample dilutions that fell within the working range of the standard curve should be used. All statistical analyses were performed with SPSS 13.0. Differences between solvent control and treatments were analyzed by one-way ANOVA followed by Dunnett′s test with statistical significance at p < 0.05. Correlations of the three biomarkers were analyzed by two-tailed bivarite correlation.
Results and Discussion
No mortalities or deviants were observed in fish receiving any dilution of E2, which indicated that the dilution series of E2 were not acutely toxic at the tested concentrations and that the fish were not unduly stressed. The levels of serum VTG were below the detection limit in the control and solvent control. E2 induced serum VTG in a concentration-dependent manner (Fig. 2), which had been reported in previous studies (Thompson et al. 2000; Tilton et al. 2002; Brion et al. 2004; Sun et al. 2009). 1 × 10−2 ng L−1 E2 failed to induce detectable VTG expression. VTG levels ranging from 0.0207 to 0.2253 ng μg−1 protein were detected in 1 × 10−1–1×104 ng L−1 E2 treatment groups. The lowest observed effective concentration (1 × 10−1 ng L−1) of E2 was lower than that for adult male zebrafish (Danio rerio) after 3 weeks of exposure to E2 (> 5 ng L−1; Brion et al. 2004) and that for male sheepshead minnow (Cyprinodon variegatus) after 16 d of exposure to E2 (200 ng L−1; Folmar et al. 2000). Experimental fish, exposure manner and condition (such as exposure time and exposure temperature etc.) may be responsible for the discrepancy. Commonly used indicators of estrogen exposure in laboratory and field studies could not be directly compared across species due to differences in sensitivity (Thompson et al. 2000). Significant increases of VTG levels were observed at E2 concentrations equal to or higher than 1 × 103 ng L−1. The Logistic regression model was selected for the curve estimation (Sun et al. 2009). Estradiol equivalents (EEQs) of extracted mixtures and actual water samples in the three representative sections were derived from the equation.
From Fig. 3, serum VTG induced by extracted mixtures from Jiangxinzhou section, Sanchahe section and Daqiao section were 0.1003, 0.0818 and 0.1067 ng μg−1 protein, respectively. From the regression equation of concentration–response curve of E2, EEQs of actual water samples from Jiangxinzhou section, Sanchahe section and Daqiao section were 0.3651, 0.1301 and 0.5060 ng L−1, respectively. Compared with the EEQ results of surface waters in domestic and foreign countries, the estrogenic activities in the three representative sections of Yangtze Rriver (Nanjing section) were low to moderate level. Pojana et al. (2007) reported that the average EEQs of water from four sampling stations in the Venice lagoon, a highly urbanized coastal water ecosystem receiving both industrial and municipal wastewater effluents were 16, 20, 17 and 21 ng L−1. Oh et al. (2000) reported that the EEQ of the downstream of the Kumho river was 7.43 ng L−1 and upstream of Kum river was 2.05 ng L−1. The EEQs of Taihu water were estimated in the range of 2.2–12.1 ng L−1 (Shen et al. 2001).
As shown in Fig. 3, no detectable VTG induction was observed in any of the control or solvent control group. Injection with all of the extracted fractions and mixtures resulted in significant induction of different levels of VTG in male goldfish with the exception of 0%, 80%, 85% and 90% methanol fractions from Sanchahe section. The potencies for VTG induction were observed higher in fractions from Jiangxinzhou section and Daqiao section than those from Sanchahe section. To Jiangxinzhou section, Sanchahe section and Daqiao section, the inductions of serum VTG were most significant in fish receiving 25–85% methanol fractions, 95% methanol–hexane fractions and 90% methanol–diethyl ether fractions, respectively. The maximal VTG induction was observed in response to treatment with the 85% methanol fraction for Jiangxinzhou section (0.1339 ng μg−1 protein), the hexane fraction for Sanchahe section (0.1053 ng μg−1 protein) and the 95% methanol fraction for Daqiao section (0.1528 ng μg−1 protein).
The induction effects of fractions and mixtures on serum E2 were presented in Fig. 4. Serum E2 concentrations in the water controls did not differ significantly from that in the solvent controls (p > 0.05). Therefore, serum E2 concentrations of injected fish were compared with that of solvent control. Elevated serum E2 concentrations were observed in all groups receiving fractions or mixtures with the exception of hexane fraction from Jiangxinzhou section, 80% and 90% methanol fractions from Sanchahe section and hexane fraction from Daqiao section. Additionally, serum E2 were significantly up-regulated in male goldfish receiving 50%, 80%, 85% methanol fractions and mixture from Jiangxinzhou section, 50% methanol and diethyl ether fractions from Sanchahe section and 90% methanol fraction and mixture from Daqiao section when compared with the solvent controls (p < 0.05). The maximal E2 induction was observed in response to treatment with the 80% methanol fraction for Jiangxinzhou section (423.02 pg mL−1), the diethyl ether fraction for Sanchahe section (342.24 pg mL−1) and the 95% methanol fraction for Daqiao section (338.72 pg mL−1). The highest induction concentration was 103.65% above that of solvent control. As the levels of VTG induction increased, the overall E2 concentrations were elevated. Change of serum E2 concentrations matched corresponding change of serum VTG levels basically for each section (Figs. 3, 4).
Serum E2 concentrations in fish receiving extracted fractions or mixtures. Each value represents the mean ± S.D. c control, sc solvent control, 0–100% 0–100% methanol/water mixtures, d.e. diethyl ether, d.e./h. 50/50 diethyl ether/hexane, h. hexane * Value significantly different over the solvent control (sc) at p < 0.05
GSI determined in adult male goldfish receiving fractions or mixtures were shown as Table 1.
From Table 1, no significant difference in GSI was observed between controls and solvent controls. Therefore, GSI of injected fish were compared with that of solvent control. Gonad atrophies were observed in all groups receiving fractions or mixtures with the exception of hexane fraction from Jiangxinzhou section, 85% and 100% methanol fractions from Sanchahe section and 85% methanol fraction from Daqiao section. The atrophies were significant in fish receiving 25%, 85% methanol fractions and mixture from Jiangxinzhou section, 25% methanol and hexane fractions from Sanchahe section and 95%, 100% methanol, 50/50 diethyl ether/hexane fractions and mixture from Daqiao section when compared with the solvent controls (p < 0.05). The minimal GSI was observed in response to treatment with 85% methanol fraction for Jiangxinzhou section (1.12%), 25% methanol fraction for Sanchahe section (0.78%) and 95% methanol fraction for Daqiao section (0.54%). The most decrease rate was 71.28%. In the three sections, almost all fish with low GSI (p < 0.05) exhibited high serum VTG levels (Fig. 3). But not all fractions or mixtures induced serum VTG led significant decreases in GSI.
From Figs. 3, 4 and Table 1, significant VTG and E2 induction and gonad atrophy were observed in this study. The effects on fish had been documented in many studies on surface water (Hashimoto et al. 2000; Tilton et al. 2002; Solé et al. 2003). VTG expression under physiological conditions is female-specific and normally silenced in male and juvenile fish. But males or juveniles can be induced to synthesize VTG after exposure to estrogens or estrogen-mimics that can activate estrogen receptor (ER; Tilton et al. 2002). Under normal conditions, there are relatively low levels of E2 in male fish. “Xeno”estrogens present at the water can up-regulate aromatase (an invertase who is responsible for the conversion of male hormone to female hormone) activity in both sexes, so serum E2 concentration can be elevated (Solé et al. 2003). Reduction in GSI may be due to inhibition of testicular growth or atrophy of the testis (Milnes et al. 2006). The in vivo results showed estrogen exposures in the three representative sections of Yangtze River (Nanjing section). Effluents from the WWTPs may be main contributors to the estrogenic activities in Jiangxinzhou section and Daqiao section and the estrogenic pollution in Sanchahe section is possibly attributed to the inflow of pollutants in Qinhuai River. A number of investigations suggested that the final effluents of WWTPs were mainly responsible for the increasing estrogenic activity in the aquatic environment (Metcalfe et al. 2001; Coors et al. 2004). In the effluents, estradiol (E2), bisphenol A (BPA), estrone (E1) and nonylphenol (NP), etc. were frequently detected as potential causative substances. These chemicals had been demonstrated to cause significant ecotoxicological effects, such as sex ratio changes, reduction of fecundity and fertilization rate on fish and other wild lives. In addition to the effluents from WWTPs, main surface runoff may be an important source of estrogenic pollution in surface waters. In this study, the water sample from Sanchahe section was estrogenic. Sanchahe section was near to the input point of Qinhuai River to Yangtze River. Qinhuai River is a main riverway throughout Nanjing city. With economic development, many factories and residential areas have been built along the river. Untreated industrial wastewater and sewage flow into the river and pollute the water seriously. Industrial and municipal wastewaters without treatment were important source of estrogenic activity in surface water had been reported. Ma et al. (2007) investigated estrogenic potencies of the effluents from WWTPs, factories and hospitals and some receiving rivers in Beijing. The results suggested that EEQ levels in river water in Beijing were likely attributable to untreated municipal and industrial wastewaters discharged directly into the river.
Polar fractions from Jiangxinzhou section and Daqiao section and mid-polar and nonpolar fractions from Sanchahe section were main contributors to the estrogenic activities. Polarity of main environmental estrogens in the three sections was Jiangxinzhou > Daqiao > Sanchahe. This may be correlative with different source of pollutants. Jiangxinzhou wastewater treatment plant is main domestic wastewater in origin and lack significant industrial inputs. Chengbei wastewater treatment plant receives both industrial and domestic wastewater, but domestic wasterwater is dominant in origin. Industrial wastewater constitutes main source of Qinhuai River. It seems estrogenic activity in domestic wastewater is mainly due to polar pollutants while that in industrial wastewater is mainly due to mid-polar or nonpolar pollutants. Maybe it is because steroids and alkylphenols frequently presented in domestic wastewater are polar while industrial products such as organochlorine pesticides, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) are low polar. Desbrow et al. (1998) developed a fractionation system combined with an in vitro assay to detect estrogenic activities and isolate and identify the major estrogenic chemicals present in seven sewage-treatment work effluents receiving primarily domestic input. The results showed that the majority of the estrogenic activity was eluted by 50–85% methanol and three sterols [E2, E1 and 17α-ethynylestradiol (EE2)] were isolated from the estrogenic fractions. Results from a corresponding study on wastewater samples from a WWTP receiving both industrial wastewater and domestic wastewater also displayed the polar fractions exhibited greater contributions to total estrogenic activities than the mid-polar and nonpolar fractions (Sun et al. 2008).
Almost all fish with significantly elevated E2 concentrations or decreased GSI (p < 0.05) expressed high VTG levels (Figs. 3, 4; Table 1). To Jiangxinzhou section, Sanchahe section and Daqiao section, the correlation coefficients of VTG and E2 were 0.737, 0.690 and 0.817, respectively, the correlation coefficients of VTG and GSI were −0.838, −0.540 and −0.794, respectively and the correlation coefficients of E2 and GSI were −0.557, −0.620 and −0.509, respectively. Good positive correlations between VTG and E2 and good inverse correlations between VTG and GSI were obtained for the three sections, whereas the correlations between E2 and GSI were relatively poor. VTG is one of the proteins produced in response to E2 in fish and contaminants that can raise serum E2 have the potential to raise the levels of VTG (Tilton et al. 2002). Significantly elevated serum E2 concentrations and expressions of serum VTG were also observed in male channel catfish (Ictalurus punctatus) after 21 d of exposure to wastewater from two treatment facilities (Tilton et al. 2002). A significant decrease in GSI in response to the increased VTG induction had been reported in many studies. Hashimoto et al. (2000) reported that high concentrations of serum VTG were observed more often in male flounder (Pleuronectes yokohamae) whose GSI were lower than 2%. Significantly decreased GSI had also been observed in fathead minnow (Pimephales promelas) expressed highest VTG levels (Pawlowski et al. 2004).
In summary, significant VTG and E2 induction and gonad atrophy are observed in adult male goldfish (Carassius auratus) after injection with fractions extracted from the three representative sections of Yangtze River (Nanjing section), which indicates fish in Yangtze River (Nanjing section) can be exposed to environmental estrogens and at risk of feminization. Effluents from WWTPs and main surface runoff should be responsible for the estrogenic activities in the three representative sections. Among the three endpoints examined, serum VTG proves to be the most sensitive biomarker in indicating estrogen exposure. Good correlations between VTG and E2, VTG and GSI show serum E2 concentration and GSI should be detected as additional endpoints when male fish is deployed as a biomonitor for environmental estrogens.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brion F, Tyler CR, Palazzi X, Laillet B, Porcher JM, Garric J, Flammarion P (2004) Impacts of 17β-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile- and adult-life stages in zebrafish (Danio rerio). Aquat Toxicol 68:193–217
Coors A, Jones PD, Glesy JP, Ratte HT (2004) Assessing the elimination of estrogenic activity in advanced wastewater treatment with a reporter genebased bioassay. Water Sci Technol 50:181–188
Desbrow C, Routledge EJ, Brighty GC, Sumpter JP, Waldock M (1998) Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ Sci Technol 32:1549–1558
Dick Vethaak A, Lahr J, Kuiper RV, Grinwis GCM, Rankouhi TR, Giesy JP, Gerritsen A (2003) Estrogenic effects in fish in The Netherlands: some preliminary results. Toxicology 181–182:147–150
Folmar LC, Hemmer M, Hemmer R, Bowman C, Kroll K, Denslow ND (2000) Comparative estrogenicity of estradiol, ethynyl estradiol and diethylstilbestrol in an in vivo, male sheepshead minnow (Cyprinodon 6ariegatus), vitellogenin bioassay. Aquat Toxicol 49:77–88
Hashimoto S, Bessho H, Hara A, Nakamura M, Iguchi T, Fujita K (2000) Elevated serum vitellogenin levels and gonadal abnormalities in wild male founder (Pleuronectes yokohamae) from Tokyo Bay, Japan. Mar Environ Res 49:37–53
Hu JY, An LH, Sun XH, Zhang HF, Deng BS (2003) Investigation of vitellogenin in wild crucian (Carassius auratus) Tianjin. China Environ Sci 23:281–284
Li ZL, Li YQ, Chen HG, Xu Y, Kong ZM, Liu ZT (2006) Evaluation of genotoxic effects induced by organic pollutants in the Yangtze River in Nanjing section. Res Environ Sci 19:105–108
Ma M, Rao KF, Wang ZJ (2007) Occurrence of estrogenic effects in sewage and industrial wastewaters in Beijing, China. Environ Pollut 147:331–336
Metcalfe CD, Metcalfe TL, Kiparissis Y, Koenig BG, Khan C, Hughes RJ, Croley TR, March RE, Potter T (2001) Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes). Environ Toxicol Chem 20:297–308
Milnes MR, Bermudez DS, Bryan TA, Edwards TM, Gunderson MP, Larkin ILV, Moore BC, Guillette LJ Jr (2006) Contaminant-induced feminization and demasculinization of nonmammalian vertebrate males in aquatic environments. Environ Res 100:3–17
Oh SM, Choung SY, Sheen YY, Chung KH (2000) Quantitative assessment of estrogenic activity in the water environment of Korea by the E-SCREEN assay. Sci Total Environ 263:161–169
Pawlowski S, van Aerle R, Tyler CR, Braunbecka T (2004) Effects of 17α-ethinylestradiol in a fathead minnow (Pimephales promelas) gonadal recrudescence assay. Ecotoxicol Environ Saf 57:330–345
Peng G (2006) Present state and protection countermeasure for fishery resources in the Yangtze River in Jiangsu section. Fish Econ Res 23:18–21
Pojana G, Gomiero A, Jonkers N, Marcomini A (2007) Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environ Int 33:929–936
Rempel MA, Reyes J, Steinert S, Hwang W, Armstrong J, Sakamoto K, Kelley K, Schlenk D (2006) Evaluation of relationships between reproductive metrics, gender and vitellogenin expression in demersal flatfish collected near the municipal wastewater outfall of Orange County, California, USA. Aquat Toxicol 77:241–249
Roy LA, Armstrong JL, Sakamoto K, Steinert S, Perkins E, Lomax DP, Johnson LL, Schlenk D (2003) The relationships of biochemical endpoints to histopathology and population metrics in feral flatfish species collected near the municipal wastewater outfall of Orange County, California, USA. Environ Toxicol Chem 22:1309–1317
Shen JH, Gutendorf B, Vahl HH, Shen L, Westendorf J (2001) Toxicological profile of pollutants in surface water from an area in Taihu Lake, Yangtze Delta. Toxicology 166:71–78
Solé M, Raldua D, Piferrer F, Barceló D, Porte C (2003) Feminization of wild carp, Cyprinus carpio, in a polluted environment: plasma steroid hormones, gonadal morphology and xenobiotic metabolizing system. Comp Biochem Physiol C Toxicol Pharmacol 136:145–156
Sun QF, Deng SB, Huang J, Shen G, Yu G (2008) Contributors to estrogenic activity in wastewater from a large wastewater treatment plant in Beijing, China. Environ Toxicol Pharmacol 25:20–26
Sun LW, Zha JM, Wang ZJ (2009) Interactions between estrogenic chemicals in binary mixtures investigated using vitellogenin induction and factorial analysis. Chemosphere 75:410–415
Thompson S, Tilton F, Schlenk D, Benson WH (2000) Comparative vitellogenic responses in three teleost species: extrapolation to in situ field studies. Mar Environ Res 51:185–189
Tilton F, Benson WH, Schlenk D (2002) Evaluation of estrogenic activity from a municipal wastewater treatment plant with predominantly domestic input. Aquat Toxicol 61:211–224
Todorov JR, Elskus AA, Schlenk D, Lee Ferguson P, Brownawell BJ, McElroy AE (2002) Estrogenic responses of larval sunshine bass (Morone saxatilis × M. Chrysops) exposed to New York city sewage effluent. Mar Environ Res 54:691–695
Acknowledgments
This research was financially supported by the Key Program of National Natural Science Foundation of China (50830304), China’s National Basic Research Program (2008CB418203) and the Key Project of Chinese Ministry of Education (109076).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, W.T., Lu, G.H., Wang, C. et al. Study on Environmental Estrogen Pollution in Yangtze River (Nanjing Section) by an In Vivo Bioassay. Bull Environ Contam Toxicol 84, 406–412 (2010). https://doi.org/10.1007/s00128-010-9944-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-010-9944-9