Abstract
Estrogenic activities of river water from four representative cross-sections of the Yellow River (Zhengzhou section) and their effects on reproduction and development of fish were assessed. MVLN assay showed estradiol equivalents of river water from Yiluohe, Xinmanghe, Qinhe and Huayuankou cross-sections were 1.09 ± 0.11, 0.72 ± 0.01, 1.19 ± 0.19 and 0.80 ± 0.04 ng/L, respectively. Significant vitellogenin (VTG) inductions were observed in adult male Japanese madaka (Oryzias latipes) after 30 days of exposure to river water from Yiluohe and Qinhe cross-sections (p < 0.05). Hepatic-somatic index was significantly elevated in fish exposed to water from Qinhe cross-section (p < 0.05). A significant delay in time to hatching was observed in embryos treated by water from Xinmanghe cross-section (p < 0.05). Significant lower survivals were observed in fish treated by water from Yiluohe and Xinmanghe cross-sections after a full life cycle exposure (p < 0.05). Exposure of water from Yiluohe and Qinhe cross-sections induced significantly elevated VTG levels in the first sexually mature male fish (p < 0.05). Both the in vitro and in vivo bioassay demonstrate endocrine disrupting chemicals exist in the Yellow River (Zhengzhou section) and fish in Yiluohe and Qinhe cross-sections can be at a risk of reproductive and developmental impairment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
There is heightened concern worldwide about endocrine disrupting chemicals that can result in reproductive abnormalities and developmental toxicity in fish even at ng/L level (Lei et al. 2013, 2014). Endocrine disrupting chemicals have been detected in many natural water bodies that receive sewage effluent and industrial wastewater discharges (Furuichi et al. 2004; Lei et al. 2009; Lu et al. 2010). Steroid estrogens such as estrone (E1), 17β-estradiol (E2) and estriol (E3) which exhibit the highest estrogenic potencies usually are regarded as the main causation for endocrine disrupting activities in the aquatic environment (Furuichi et al. 2004; Lu et al. 2010).
The Yellow River is the second longest river in China and is often called “the Mother River of China”. But in recent years she has suffered from low water yields and water pollution. Estrogenic compounds such as E1, E2, 4-t-octylphenol (4-t-OP), 4-nonylphenols, bisphenol A and triclosan (TCS) have been detected in the river water sampled from 15 sites from upper reach to lower reach of the Yellow River (Wang et al. 2012). The species and number of fish have begun to decrease and fish tend to be smaller (Ru et al. 2010). Zhengzhou is the capital of Henan province and is located along middle-lower reach of the Yellow River. Yiluohe river, Xinmanghe river and Qinhe river are three big tributaries of the Yellow River (Zhengzhou section) and they receive large domestic and industrial wastewater discharges (Fu 2011; Gong et al. 2006; Shen et al. 2012). According to Environmental Quality Standards for Surface Water in China (GB3838-2002), water quality of Yiluohe river, Xinmanghe river and Qinhe river were reported class IV, inferior class V and inferior class V, respectively (Yellow River Basin Water Resources Protection Bureau 2015). It is crucial to understand endocrine disrupting activity in the Yellow River (Zhengzhou section) and its potential adverse effects on reproduction and development of aquatic organisms.
The objective of this study was to investigate the endocrine disrupting activities of four representative cross-sections in the Yellow River (Zhengzhou section) and endocrine disrupting effects on fish using combined in vitro MVLN cell assay and in vivo bioassays. Estradiol equivalents (EEQs) of the four representative cross-sections were expected to be determined. Vitellogenin (VTG) inductions, gonadosomatic index (GSI) and hepatosomatic index (HSI) were assessed in adult male Japanese medaka (Oryzias latipes) after exposure to collected river water from the four representative cross-sections in the lab. In another in vivo bioassay, time to hatching, hatchability, fry survival and VTG inductions were assessed in fish following a full life cycle exposure.
Materials and Methods
Methanol, acetonitrile, ethyl acetate and hexane were all HPLC grade and purchased from J.T. Baker (USA). Oasis HLB (6 mL, 500 mg) and Silica (6 mL, 500 mg) solid phase extraction (SPE) cartridges were purchased from Waters (USA). E2 (98 % pure) and dimethyl sulfoxide (DMSO, 99.5 % pure) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM)/High Glucose and DMEM/High Modified (-phenol red) were purchased from Hyclone (USA). Fetal bovine serum (FBS), dextran/charcoal-stripped free-hormone FBS, penicillin streptomycin, 0.05 % trypsin–EDTA and 0.5 % trypsin–EDTA (no phenol red) were purchased from Gibco (USA). The CellTiter-Blue® cell viability assay reagent and luciferase Assay system were obtained from Promega Corporation (USA). Japanese Medaka VTG enzyme-linked immunosorbent assay (ELISA) kit was purchased from Shanghai Fanke industrial Co., Ltd. (Shanghai, China).
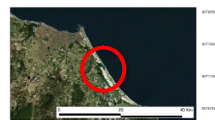
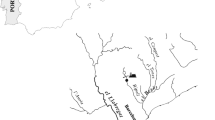
The location of the four representative cross-sections in the Yellow River (Zhengzhou section) is shown in Fig. 1. Yiluohe cross-section, Xinmanghe cross-section and Qinhe cross-section were assigned about 200 m downstream of the inlets of the three tributaries into the Yellow River respectively. Huayuankou cross-section was assigned near Huayuankou hydrometric station because it is an important water source for people’s daily life and industrial consumption in Zhengzhou city. Single sampling point was assigned about 2 m far from the shore and 0.5 m depth from the water surface in each representative cross-section. All of the sampling equipment was disinfected with a weak bleach solution, after which it was rinsed with tap water and then distilled water. 20 L of river water was collected from each representative cross-section every time. Water samples were collected every other day during May–August 2015. After vacuum filtering through a 0.45 μm glass fiber filter to remove suspended particles within 12 h, the collected river water was stored at 4°C until usage.
River water from each representative cross-section was extracted basically according to the method reported by Chang et al. (2009) that mainly targeted steroid hormones. In order to concentrate more steroid hormones, river water collected from each representative cross-section was extracted through an Oasis HLB cartridge until saturation. The Oasis HLB cartridge was previously conditioned with 6 mL ethyl acetate, 6 mL acetonitrile and 12 mL distilled water at a flow rate of 5–10 mL/min. The cartridge was washed with 10 mL of distilled water, and then was dried under a flow of nitrogen. 15 mL of ethyl acetate and 6 mL of ethyl acetate/acetonitrile (1:1, v/v) were used to elute the analytes. The eluates were dried and redissolved in 0.2 mL of ethyl acetate and 1.8 mL of hexane. The mixed solution was applied to a silica cartridge, which had been preconditioned with 4 mL water-saturated ethyl acetate and 4 mL hexane/ethyl acetate (90:10, v/v). After rinsed with 3 mL of hexane/ethyl acetate (90:10, v/v), the cartridge was eluted with 3 mL of hexane/ethyl acetate (38:62, v/v) and the eluate was dried and reconstituted with 0.25 mL of DMSO for MVLN assay. According to Chang et al. (2009), recoveries of the steroid hormones ranged from 75 % to 100 %.
MVLN cells are derived from human breast carcinoma MCF-7 cell line and are stably transfected with luciferase reporter gene under the control of estrogen responsive elements of the Xenopus VTG A2 gene for the detection of estrogen receptor (ER)-mediated activity (Pons et al. 1990). The MVLN cell line was kindly provided by J.P. Giesy (Michigan State University, USA). MVLN cells were maintained in DMEM containing 10 % FBS, 100 U/mL of penicillin and 100 µg/mL of streptomycin and cultured in a humidified atmosphere of 5 % CO2 at 37°C.
Before MVLN assay, cytotoxicities of the river water extracts were assessed by measuring mitochondrial activity of viable cells on the basis of chemical reduction of resazurin to resorufin. Cells were starved in steroid-free (SF) medium (phenol red-free DMEM supplemented with 5 % free-hormone FBS, 100 U/mL of penicillin and 100 µg/mL of streptomycin) for 24 h to minimize the basal hormonal activity before seeding. Exposure solutions were prepared by serial dilution of each water extract (1/60, 1/300 and 1/600) in SF medium. After 48 h of exposure, concentration of resorufin was measured with a fluorometer at an excitation wavelength of 530 nm and emission wavelength of 590 nm. All the exposure solutions were tested in triplicate.
Exposure solutions used in the MVLN assay should have no cytotoxicity to the MVLN cells. Cells were starved, seeded and exposed to the exposure solutions for 48 h as described above. A concentration range of 1 pM–0.5 nM E2 was used for positive control and standard calibration. All the samples were tested in triplicate. Luciferase activity was measured with the luciferase assay system according to the manufacturer’s protocol. The maximal induction of E2 (corrected by solvent control) was set as 100 %, and the solvent control-corrected responses of other concentrations of E2 and exposure solutions were converted to percentage of the maximum level. Dose-response curve for E2 was fitted to a Slogistic1 function (Origin 8.5, Microsoft, USA). For each representative cross-section, EEQ of the water extract could be estimated on basis of the Slogistic1 model. The EEQ of water extract was divided by the concentration factor obtained by SPE, resulting in the EEQ of the river water.
Ten-month-old adult Japanese madaka (O. latipes) were kindly provided by Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences. The fish were kept in dechlorinated tap water at a constant temperature of 25 ± 2°C, with a photoperiod of 16:8 h (light:dark). The fish were fed with newly hatched brine shrimp twice a day.
Randomly assigned five 10-month-old adult male Japanese madaka were kept in a 10-L glass tank containing 8 L of river water from each representative cross-section under constant aeration for 30 days. A semi-static test was conducted by replacing 2/3 of river water in each tank once a day. After 30 days of exposure, all fish were frozen to death by keeping them on ice for 2 min and the body weight was measured. The livers and gonads were removed and weighted. GSI (%) was calculated as 100 × gonad wet weight (g)/wet weight of fish (g). HSI (%) was calculated as 100 × liver wet weight (g)/wet weight of fish (g). Each liver was homogenized in cold PBS (0.01 mol/L, pH 7.2–7.4) with volume (mL):mass (g) = 9:1 and centrifuged for 15 min (1800×g) at 4°C. The supernatants were removed and stored at −80°C for VTG analysis. Negative control and positive control using fish in dechlorinated tap water and in E2 at an environmentally relevant concentration of 1 ng/L were performed.
Fertilized eggs were collected from glass tanks of the brood stock maintained in dechlorinated tap water, in the morning daily, and separated carefully. Randomly assigned 50 healthy eggs were placed uniformly in a 100 mm petri dish containing 50 mL of river water from each representative cross-section and incubated on a 16:8 h light:dark photoperiod cycle at 25 ± 2°C. The experiment was performed with three replicates of each treatment running simultaneously. 2/3 of the river water in each petri dish was renewed daily. Dead eggs were removed timely. Time to hatching and hatchability were recorded. The hatched fish were immediately transferred to a 500 mL of beaker containing the same river water. 2/3 of the river water in each beaker was renewed daily too. Dead fish were recorded and removed immediately. With growth of the fish, they were transferred to a 2- and 10-L of glass tank containing the same river water in turn until sexual maturity. Survival (%) was calculated as 100 × live fish/hatched fish. For each treatment group, randomly three first sexually mature male fish were collected from the 10-L of glass tank and frozen to death on ice for 2 min. Because livers of the fish were small, the whole bodies of fish were weighted and homogenized in cold PBS as described above. The supernatants were removed and stored at −80°C for VTG analysis. Negative control and positive control were also performed during the full life cycle exposure.
All data were expressed as mean ± standard deviation (SD). Data from different treatments were checked for normality and equal variance. Data were compared by one-way analysis of variance (ANOVA) and statistically different treatments were identified by Dunnett’s t test. All differences were considered significant at p < 0.05. All statistical analyses were performed using the SPSS statistical package (ver. 13.0, SPSS Company, Chicago, IL, USA).
Results and Discussion
E2 induced clear concentration-dependent increases of relative luciferase activities in the MVLN assay, as shown in Fig. 2. The Slogistic1 regression equation was fitted as followed:
where, y is the relative luciferase activity, x is lg C (concentration) of E2 (mol/L).
Effects of river water extracts on cell viability are shown in Fig. 3. Cell viabilities of water extract treatment groups were presented as the percentages relative to that of DMSO treated control. From Fig. 3, no significant cytotoxicity was observed. For each representative cross-section, cell viability increased progressively with increasing exposure concentration of the exposure solution. Cell viability was significantly elevated after exposure to the highest concentration of exposure solution (1/60 extract) from Yiluohe cross-section (p < 0.05). MVLN cells are derived from human breast carcinoma MCF-7 cell line. MCF-7 cells are estrogen-dependent cells and estrogens such as E2, OP and TCS have proved to be able to stimulate proliferation of MCF-7 cells (Lee et al. 2014). MVLN cell viability increases in this study may indicate there are some estrogenic chemicals in the four representative cross-sections.
Cell viability of MVLN after exposure to exposure solutions from Yiluohe (Y), Xinmanghe (X), Qinhe (Q) and Huayuankou (H) cross-sections (n = 3). 1–3 represent exposure solutions prepared by serial dilution (1/600, 1/300 and 1/60) of water extract from each cross-section. Asterisk indicates cell viability that is significantly different from control (p < 0.05)
As shown in Fig. 4, for each representative cross-section, the exposure solutions induced concentration-dependent responses of relative luciferase activities. Exposure solution of 1/300 extract induced higher luciferase activity than that of 1/600 extract. But exposure solution of 1/60 extract induced lower luciferase activity even than solvent control. From Fig. 3, no significant cytotoxicity was observed after treatment with exposure solution of 1/60 extract from each cross-section. Lower luciferase activity in these treatment groups might be due to direct adverse influence of the high concentration of water extract on the activity of luciferase. From Fig. 4, for each representative cross-section, it was exposure solution of 1/600 extract that could be sure having no adverse influence on the activity of luciferase. So the relative luciferase activity induced by exposure solution of 1/600 extract was selected to calculate EEQ of the river water. EEQs of river water from Yiluohe, Xinmanghe, Qinhe and Huayuankou cross-sections were calculated as 1.09 ± 0.11, 0.72 ± 0.01, 1.19 ± 0.19 and 0.80 ± 0.04 ng/L, respectively. The rank of final calculated EEQs of the four representative cross-sections was not consistent with that of the relative luciferase activity responses shown in Fig. 4. Different concentration factors obtained by SPE for the four representative cross-sections should be responsible for the inconsistence. Actually, due to different pollutant levels and water quality characters, volume of river water extracted through the Oasis HLB cartridges was a little different for the four representative cross-sections, which resulted in the different concentration factors obtained.
According to Keller et al. (2015), the potential risk for fish endocrine disruption could be assessed based on the EEQ concentrations and river could be classified according to one of the following categories: “no risk” [(EEQ) < 1 ng/L], “at risk” [1≤(EEQ) < 10 ng/L] and “high risk” [(EEQ) ≥ 10 ng/L]. The threshold between no risk and at risk was based on the predicted no effect concentrations (PNEC) for population level effect endpoints. According to the categories, fish exposed to river water from Yiluohe cross-section or Qinhe cross-section can be at a risk of endocrine disruption and that exposed to river water from Xinmanghe cross-section or Huayuankou cross-section can be regarded as no risk for endocrine disruption. Based on the location of the four representative cross-sections shown in Fig. 1, risk levels of the four representative cross-sections did not change along the flow of the Yellow River. Maybe nearby pollution source rather than upriver pollutant input ought to be responsible for the estrogenic activities in the four representative cross-sections.
One mortality was observed in the adult male fish exposed to river water from Yiluohe cross-section and Xinmanghe cross-section, respectively. An intersex individual (having simultaneous presence of ovarian and testicular tissue in the gonad) and a spine curvature fish was found in the test group treated by river water from Qinhe cross-section and Yiluohe cross-section, respectively. Effect of river water from the four representative cross-sections on VTG, GSI and HSI of adult male Japanese madaka were shown in Fig. 5a–c. Yolk protein precursor VTG is specifically synthesized in the liver of mature females. But males or juveniles can be induced to synthesize VTG by exposure to estrogens or estrogen-mimics that can activate ER (Tilton et al. 2002). VTG has proved to be the most sensitive biomarker in indicating estrogen exposure (Pawlowski et al. 2004; Song et al. 2010). From Fig. 5a, VTG levels were significantly elevated in fish treated with 1 ng/L E2 and river water from Yiluohe and Qinhe cross-sections after 30 days of exposure (p < 0.05). GSI and HSI are general measurements of the overall condition of fish or the growth status of the specific tissue (Huang et al. 2015). From Fig. 5b, no significant GSI change was observed in any treatment group when compared with the control (p > 0.05). Typically, GSI of male fish is reduced after exposure to estrogenic or antiandrogenic contaminants because of suppression of normal seasonal growth of the testes, or atrophy (Milnes et al. 2006). Combined with the EEQs obtained in the MVLN assay, maybe levels of estrogens in the four representative cross-sections and the positive control were not high enough to result in gonad atrophy. The results were consistent with previous studies. Lv et al. (2006) reported that it had no significant changes in the GSI of male Chinese loaches (Misgurnus anguillicaudatus) exposed to E2 at low concentrations of 0.5, 1.5 and 10 ng/L continuously for 8 weeks. Shappell et al. (2010) also reported GSI of male fathead minnows (Pimephales promelas) did not decrease when compared with the controls after 21 days of exposure to 9, 20 and 44 ng/L of E2. From Fig. 5c, HSI was significantly elevated in fish exposed to river water from Qinhe cross-section (p < 0.05), which induced the highest levels of VTG in male fish liver. Estrogen induced HSI increases in male fish have been reported in other studies and it was stated that increases of liver weight might be due to VTG accumulation, which may have adverse effects on fish growth (Lei et al. 2013; Sun et al. 2009).
VTG (a), GSI (b) and HSI (c) of adult male Japanese madaka after 30 days of exposure to river water from the four representative cross-sections (n = 5 for Control (C), E2, Qinhe (Q) and Huayuankou (H) cross-sections; n = 4 for Yiluohe (Y) and Xinmanghe (X) cross-sections). Asterisks indicate values that are significantly different from control values (p < 0.05)
Time to hatching, hatchability and survival of Japanese madaka after a full life cycle exposure to river water from the four cross-sections are presented in Table 1. As shown in Table 1, exposure of 1 ng/L of E2 significantly promoted time to hatching of eggs (p < 0.05), while exposure to river water from Xinmanghe cross-section led to a significant delay in time to hatching (p < 0.05). Octanol–water partition coefficient (logKow) of E1, E2 and E3 are 3.43, 3.94 and 2.81, respectively (Lei et al. 2009). Due to lipophilicity, steroid estrogen or other high lipophilic chemicals in the river water can be expected to partition into lipid compartments (e.g. membranes) and get into the developing embryo from water to interfere with normally embryonic development process (Lei et al. 2014). From Table 1, no significant difference in hatchability was observed in any treatment group when compared with the control (p > 0.05). The result was consistent with a previous study in which no difference of hatchability was recorded in pejerrey fish (Odontesthes bonariensis) after exposure to E2 and ethinylestradiol (Gárriz et al. 2015). After about 90 days post hatch, all the treatment groups reached the first sexual maturity, which were judged by the occurrence of eggs in the tanks. Survivals of the first sexually mature fish treated with 1 ng/L of E2, river water from Yiluohe and Xinmanghe cross-sections were significantly lower than that of the control (p < 0.05). It seemed survival of fish in the control group (56.3 %) was a little lower than that achieved in other studies (>75 %) (Overturf and Huggett 2015; Song et al. 2011). Maybe it was because exposure duration used in this study (full life cycle, 90 days) was much longer than that used in other studies (partial life cycle, ≤28 days).
From Fig. 6, after a full life cycle exposure, VTG levels were significantly elevated in the first sexually mature male fish treated with river water from Yiluohe and Qinhe cross-sections, as well as those treated with 1 ng/L of E2 (p < 0.05). The result was consistent with that gained from 30 days of exposure of the adult male fish. It further confirmed the endocrine disruption risk of fish in Yiluohe and Qinhe cross-sections.
Domestic and industrial wastewater discharges are regarded the main causation of water pollution in Yiluohe river and Qinhe river (Gong et al. 2006; Shen et al. 2012). Three industrial wastewater outlets locate in Xinmanghe river and wastewater from paper mill and gourmet powder factory are discharged into the river through pipeline continuously (Fu 2011). Huayuankou is the important water source for Zhengzhou city. Strong endocrine disrupters such as E1, E2 and E3 are excreted mainly in urine and a small proportion in feces of humans and mammals, hence occur most frequently in domestic wastewater (Hanselman et al. 2003). Maybe it can explain why there was endocrine disruption risk in Yiluohe and Qinhe cross-sections and no obvious endocrine disruption risk in Xinmanghe and Huayuankou cross-sections.
In summary, in vitro and in vivo bioassay both demonstrated endocrine disrupting chemicals do exist in the Yellow River (Zhengzhou section). Fish in Yiluohe and Qinhe cross-sections can be at a risk of reproductive and developmental impairment. Though no obvious endocrine disruption risk was observed in Xinmanghe cross-section, there may be some other chemicals affecting fish hatch and survival in it. River water from Huayuankou cross-section could not induce any significant changes of biological parameters in fish.
References
Chang H, Wan Y, Hu JY (2009) Determination and source apportionment of five classes of steroid hormones in urban rivers. Environ Sci Technol 43:7691–7698
Fu MT (2011) Analysis on monitoring of water quality of functional areas in Manghe river and investigation of sewage outlets to the river. Yellow River 33:64–66
Furuichi T, Kannan K, Giesy JP, Masunaga S (2004) Contribution of known endocrine disrupting substances to the estrogenic activity in Tama River water samples from Japan using instrumental analysis and in vitro reporter gene assay. Water Res 38:4491–4501
Gárriz Á, Menéndez-Helman RJ, Miranda LA (2015) Effects of estradiol and ethinylestradiol on sperm quality, fertilization, and embryo-larval survival of pejerrey fish (Odontesthes bonariensis). Aquat Toxicol 167:191–199
Gong XL, Hou HR, Deng XY (2006) Evaluation of surface water pollution in Yiluohe basin. Yellow River 28:76–77
Hanselman TA, Graetz DA, Wilkie AC (2003) Manure-borne estrogens as potential environmental contaminants: a review. Environ Sci Technol 37:5471–5478
Huang B, Sun WW, Li XM, Liu JL, Li Q, Wang RM, Pan XJ (2015) Effects and bioaccumulation of 17β-estradiol and 17α-ethynylestradiol following long-term exposure in crucian carp. Ecotoxicol Environ Saf 112:169–176
Keller VDJ, Lloyd P, Terry JA, Williams RJ (2015) Impact of climate change and population growth on a risk assessment for endocrine disruption in fish due to steroid estrogens in England and Wales. Environ Pollut 197:262–268
Lee HR, Hwang KA, Nam KH, Kim HC, Choi KC (2014) Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem Res Toxicol 27:834–842
Lei BL, Huang SB, Zhou YQ, Wang DH, Wang ZJ (2009) Levels of six estrogens in water and sediment from three rivers in Tianjin area, China. Chemosphere 76:36–42
Lei BL, Kang J, Yu YX, Zha JM, Li W, Wang ZJ (2013) β-estradiol 17-valerate affects embryonic development and sexual differentiation in Japanese medaka (Oryzias latipes). Aquat Toxicol 134–135:128–134
Lei BL, Kang J, Yu YX, Zha JM, Li W, Wang ZJ, Wang YP, Wen Y (2014) Long-term exposure investigating the estrogenic potency of estriol in Japanese medaka (Oryzias latipes). Comp Biochem Physiol C 160:86–92
Lu GH, Song WT, Wang C, Yan ZH (2010) Assessment of in vivo estrogenic response and the identification of environmental estrogens in the Yangtze River (Nanjing section). Chemosphere 80:982–990
Lv XF, Shao J, Song MY, Zhou QF, Jiang GB (2006) Vitellogenic effects of 17 beta-estradiol in male Chinese loach (Misgurnus anguillicaudatus). Comp Biochem Physiol C 143:127–133
Milnes MR, Bermudez DS, Bryan TA, Edwards TM, Gunderson MP, Larkin ILV, Moore BC, Guillette LJ Jr (2006) Contaminant-induced feminization and demasculinization of nonmammalian vertebrate males in aquatic environments. Environ Res 100:3–17
Overturf MD, Huggett DB (2015) Responses to various exposure durations of levonorgestrel during early-life stages of fathead minnows (Pimephales promelas). Aquat Toxicol 161:33–40
Pawlowski S, van Aerle R, Tyler CR, Braunbeck T (2004) Effects of 17α-ethinylestradiol in a fathead minnow (Pimephales promelas) gonadal recrudescence assay. Ecotoxicol Environ Saf 57:330–345
Pons M, Gagne D, Nicolas CJ, Mehtali M (1990) A new cellular model of response to estrogens: a bioluminescent test to characterize (anti)estrogen molecules. Biotechniques 9:450–459
Ru HJ, Wang HJ, Zhao WH, Shen YQ, Wang Y, Zhang XK (2010) Fishes in the mainstream of the Yellow River: assemblage characteristics and historical changes. Biodivers Sci 18:169–174
Shappell NW, Hyndman KM, Bartell SE, Schoenfuss HL (2010) Comparative biological effects and potency of 17α- and 17β-estradiol in fathead minnows. Aquat Toxicol 100:1–8
Shen GX, Song XY, Zhang D (2012) Hydrochemistry characteristics of Qin River (Henan section) in winter. J Water Resour Water Eng 23:43–46
Song WT, Lu GH, Wang C, Zhang HZ, Xu S, Qin J (2010) Study on environmental estrogen pollution in Yangtze River (Nanjing section) by an in vivo bioassay. Bull Environ Contam Toxicol 84:406–412
Song WT, Lu GH, Qi PD, Wang C (2011) Estrogenic effects of water from the Yangtze River (Nanjing section) on goldfish (Carassius auratus) after an early life stage exposure. J Environ Sci 23:1179–1185
Sun LW, Zha JM, Wang ZJ (2009) Effects of binary mixtures of estrogen and antiestrogens on Japanese medaka (Oryzias latipes). Aquat Toxicol 93:83–89
Tilton F, Benson WH, Schlenk D (2002) Evaluation of estrogenic activity from a municipal wastewater treatment plant with predominantly domestic input. Aquat Toxicol 61:211–224
Wang L, Ying GG, Chen F, Zhang LJ, Zhao JL, Lai HJ, Chen ZF, Tao R (2012) Monitoring of selected estrogenic compounds and estrogenic activity in surface water and sediment of the Yellow River in China using combined chemical and biological tools. Environ Pollut 165:241–249
Yellow River Basin Water Resour Prot Bur (2015) Bulletin of water resources quality of the key water function zone of Yellow River basin. http://www.yrbwrpb.gov.cn/GBShow.aspx?nid=13&InfoId=12
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NSFC51409096, NSFC51404101) and Scientific Research Fund of Henan Provincial Education Department (13A610314).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, W.T., Wang, Z.J. Occurrence and Biological Effects of Endocrine Disrupting Chemicals in the Yellow River (Zhengzhou Section). Bull Environ Contam Toxicol 97, 763–769 (2016). https://doi.org/10.1007/s00128-016-1930-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1930-4