Abstract
Purpose
It is plausible that offspring born to mothers using tobacco during pregnancy may have increased risk of mood disorders (depression and bipolar disorders); however, mixed results have been reported. We conducted a systematic review and meta-analysis to investigate the magnitude and consistency of associations reported between prenatal tobacco use and mood disorders in offspring.
Methods
We systematically searched EMBASE, SCOPUS, PubMed and Psych-INFO for studies on mood disorders and prenatal tobacco use. Methodological quality of studies was assessed with the revised Newcastle–Ottawa Scale. We estimated pooled relative risk (RR) with inverse variance weighted random-effects meta-analysis. We performed leave-one-out analyses, and stratified analyses by a subgroup (depression and bipolar disorder). Potential publication bias was assessed by inspection of the funnel plot and Egger’s test for regression asymmetry. This study protocol was prospectively registered in PROSPERO (CRD42017060037).
Results
Eight cohort and two case–control studies were included in the final meta-analysis. We found an increased pooled relative risk of mood disorders in offspring exposed to maternal prenatal tobacco use RRs 1.43 (95% CI 1.27–1.60) compared to no prenatal tobacco use. Similarly, the pooled relative risks of bipolar and depressive disorders in offspring were 1.44, (95% CI 1.15–1.80) and 1.44, (95% CI 1.21–1.71), respectively. Moreover, the pooled estimated risk of mood disorders was not significantly attenuated in the studies that reported sibling comparison results [RR = 1.21 (95% CI 1.04–1.41)].
Conclusion
Taken together, there was strong evidence for a small (RR < 2) association between prenatal tobacco use and mood disorders in offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mood disorders, also known as affective disorders, are a group of mental health disorders consisting of bipolar and major depressive disorders [1, 2] which can impair the psycho-social functioning of individuals and significantly affect their quality of life [3]. A 6% lifetime prevalence of depression has been reported globally [4] and the global mental health survey conducted across 11 countries in America, Europe and Asia using the World Health Organization Composite International Diagnostic Interview (WHO-CIDI) version 3.0 reported a 2.4% lifetime prevalence of bipolar disorders [5].
Observational studies and randomized controlled trials have been unable to confirm the causes of mood spectrum disorders [6]. However, it has been hypothesized that the imbalances of certain neurotransmitters which are important regulators of the bodily functions [7], genetic factors [5] and environmental factors [1] can significantly contribute to mood disorders. In addition, it has been reported that maternal lifestyle behaviors during pregnancy may result in mental and behavioral problems in offspring via early programming of the developing brain [8].
Tobacco use during pregnancy is one of such behaviours, which may increase risk of mood or other mental disorders through direct pathways [9, 10]. For example, tobacco modulates nicotinic acetylcholine receptors in the brain and results in alterations in the neurodevelopmental trajectory of widespread pathways [9]. Further, a systematic review conducted to test the association between smoking and depressive disorders revealed adverse associations in more than a third of the included studies [11]. However, the level to which observed offspring mental health problems constitute a direct effect of exposure of tobacco remains unclear [12,13,14].
Tobacco is a commonly used legal drug during pregnancy [15] with epidemiological studies indicating this exposure may increase the risk of bipolar [16,17,18] and depressive disorders [19,20,21,22] in offspring. However, additional studies have produced inconsistent findings. For example, no association was found with internalizing behaviours, namely depression and withdrawal, among children in one study [23], while another study found a higher risk of depression only among prenatally exposed boys but no increased risk in females [24]. There is also suggestion of associations in the opposite direction. For instance, a retrospective cohort study conducted in the USA found that prenatal tobacco exposure was linked with lower risk of mood disorders [25]. Variability in assessment methods of mental health outcomes may explain these inconsistencies. Therefore, we conducted a systematic review and meta-analysis to assess the magnitude and consistency of associations reported between prenatal tobacco use and mood disorders in offspring.
Methods
Research design
This systematic review and meta-analysis followed the standards of quality for reporting a Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [26, 27] and the Preferred Reporting Items for Systematic review and Meta-Analysis guidelines (PRISMA) [28]. The literature search strategy, study selection, data extraction, and synthesis were compiled with a pre-defined protocol which was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number of CRD42017060037 (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=146976).
Literature search strategy
We systematically searched the following electronic databases with no language and date limits: EMBASE, SCOPUS, PubMed and Psych-INFO. An extensive search of these databases was conducted in August 2019. The search terms and keywords were: “(cigarette use OR cigarette smoking OR cigarette exposure OR tobacco use OR tobacco exposure OR nicotine use OR nicotine exposure OR substance use OR substance exposure) AND (prenatal OR antenatal OR pregnancy OR maternal) AND (offspring OR adolescents OR youths OR young OR child OR childhood OR young adults) AND (mental disorders OR internalizing behaviours OR depression OR bipolar disorder OR mood disorders OR depressive disorders OR severe mental illness OR hypomania OR mania OR mental illness OR mental disorder OR psychiatric disorders OR psychiatric morbidity)”.
Eligibility criteria
The following inclusion criteria were set to include the studies in this systematic review and meta-analysis: (1) case–control or cohort studies, (2) the exposure of interest was prenatal tobacco use, (3) the outcome of interest was mood disorders, namely bipolar and major depressive disorders, (4) measured outcomes using odds ratio (OR) or relative risk (RR) estimates with 95% confidence intervals (CIs) or data to calculate these were reported. We were interested in offspring outcomes, namely depression and bipolar disorders, rather than a group of behavioral problems such as internalizing behaviours (anxious/depressed/withdrawal). Case reports, editorials, comments, abstracts of meeting or conferences, letters and studies conducted on animals were excluded from the review.
Data extraction
Two reviewers (BD and GA) independently conducted an electronic database searching and screening of titles as well as abstracts. The data extraction was performed based on the standardized data extraction form. Data were extracted systematically from each study: the first author name, year of publication, study characteristics including study design, measurement of bipolar or depressive disorder, trimester in which smoking initiated, country in which the study was conducted, confounders, point estimates of risk such as odds ratios (OR) or relative risk (RR) with 95% Confidence Intervals (CI) in accordance with the PRISMA guidelines [28]. Any sources of mental health outcomes, either self-report or maternal report or clinical report, were included in the review. Reviewer conflicts and issues raised during data extraction were resolved by discussion.
Study quality
The methodological quality of all selected studies was assessed using the revised Newcastle–Ottawa Scale (NOS) [29]. The quality assessment was done by two independent reviewers (BD and GA). NOS is a scale which is recommended for the quality assessment of observational studies such as cohort and case–control studies. It uses three standard grading categories such as high quality (scored 7–9), moderate quality (scored 4–6), and low quality (scored 0–3). These points were calculated using the following items namely: group selection (four items), comparability between the groups (one item), and outcome and exposure assessment (three items). Based on the scale, a maximum of one star could be given for each item in the group selection, outcome and exposure assessment categories as well as a maximum of two stars could be given for comparability. Conflicting scores among two reviewers were resolved by consensus and discussion.
Data synthesis and analysis
A meta-analysis was conducted using a Comprehensive Meta-Analysis (CMA) software version 3.0 [30]. All studies that reported an effect size were included in the meta-analysis. If multiple estimates were presented in the studies, RR were reported in this review. Only three studies conducted a separate analysis for the effects of moderate (< 10 cigarettes per day) and high tobacco smoking (≥ 10 cigarettes per day) during pregnancy on offspring mood disorders. We have included the estimates of high prenatal tobacco use of these studies in our pooled analysis to ensure sufficient exposure contrast. We have combined the included studies using inverse variance weighted random-effect meta-analysis model to estimate the association between exposure and account for heterogeneity across the studies [31]. We performed a subgroup and sensitivity analysis to identify the potential source of heterogeneity. We further conducted an additional analysis for those studies that reported sibling comparison results. We stratified analyses by outcomes (depression and bipolar disorders). To identify studies that were influential on the pooled estimate, we ran a leave-one-out sensitivity analysis, whereby one study was removed at a time and the pooled estimate was re-estimated on the remaining studies [32]. The magnitude of statistical heterogeneity between studies was evaluated using the Q-and I2-statistic [30]. The scores of 25%, 50% and 75% were considered to refer low, moderate and high heterogeneity between studies, respectively [33]. Potential publication bias was assessed by inspection of the funnel plot and Egger’s test for regression asymmetry [34].
Results
Study selection
A total of 3987 articles were identified by our initial literature search. Seventeen additional studies were obtained via a manual search from the reference lists of other studies. Of these, 585 were duplicates, depression and bipolar disorders were not measured as an outcome in 12 studies, in 10 studies depression was as assessed as an internalizing behaviours, and 3380 studies were found not to be related to the subject from title and abstract review. A total of 39 articles were retrieved for further screening and resulting in a total of 10 studies for a meta-analysis (Fig. 1).
Characteristics of included studies
The studies included in the systematic review and meta-analysis were published between September 2000 [35] and October 2017 [36, 37]. Among the included studies, four studies were conducted in the USA [18, 25, 35, 37], one in Sweden [16], two in Finland [20, 38], one in Denmark [39], one in Brazil [22] and one study was based in the UK and combined the data of the four birth cohorts including Avon Longitudinal Study of Parents and Children (ALSPAC, UK), Nord-Trondelag Health Study (HUNT, Norway), the Pelotas 1982 birth cohort (Brazil) and Swedish Sibling Health Cohort (Sweden) [36]. Eight were cohort studies [16, 18, 20, 22, 25, 35, 36, 39] and two were nested case–control studies [37, 38]. Three studies reported the additional sibling analysis results. Four studies assessed the risk of bipolar disorder in offspring exposed to prenatal tobacco use [16, 18, 37, 38] while seven studies assessed the risk of depression [20, 22, 25, 35,36,37, 39]. Four studies adjusted for maternal alcohol use during pregnancy. Five studies recruited the study participants from clinical setting, whereas five studies from population-based registers. The sample size of the included studies ranges from 77 to approximately 1,312,516 participants (Table 1).
Outcome measures
Out of 10 studies included in the systematic review, three studies used the International Classification of Diseases and Related Health Problems, 10th edition (ICD-10) manual [20, 38, 39], one study used both ICD-9 and 10 [16], two studies used the fourth revised version of the Diagnostic and Statistical Manual of Mental Disorders (DSM IV-TR) [18, 37], two studies used the Schedule for Affective Disorders and Schizophrenia (SADS) [25, 35], one study used the Mini-International Psychiatric Interview (MINI) [22] and one study based in UK and combined the data of four birth cohorts used the Clinical Interview Schedule-Revised (CIS-R) (ALSPAC), Hospital Anxiety and Depression Scale (HADS) (HUNT) and Mini-International Psychiatric Interview (MINI) (Pelotas) [36] to screen and diagnose mood disorders in offspring.
The studies included in the review have screened or diagnosed mood disorders namely depression and bipolar disorders in offspring at different follow-up periods. For example, bipolar disorder was diagnosed in offspring between ages 10 and 30 years [16, 18, 37, 38]. Depressive disorder was screened and diagnosed in offspring at age ranges from 8 to 41 years [20, 22, 25, 35,36,37, 39]. For instance, a study conducted in the USA assessed depression in offspring at age of 8–18 years [35]; whereas, the mean follow-up age of offspring in another similar study in the same country was 27.7 years [25] (Table 1).
Quality assessment of included studies
The revised Newcastle–Ottawa scale (NOS) was used to evaluate the quality of the included studies and the points were given based on the following criteria: Selection process (0–4 points), the comparability of the cohorts (0–2 points) and the identification of the exposures and the outcomes of research participants (0–3 points). The NOS score of ≥ 7 of 9 was considered of high quality in this review. Based on the averages of the scores given by two independent reviewers, all of the included studies scored ≥ 7 of 9 points (Supplementary file 1).
Prenatal tobacco exposure and risk of mood disorders
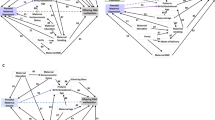
There was significant heterogeneity among the included studies (I2 = 81.22%; Q = 52.24; P value < 0.01), justifying our use of a random effect model. Prenatal tobacco use was associated with risk of mood disorders in offspring with a pooled adjusted RR of 1.43, (95% CI 1.27–1.60) (Fig. 2). Based on the stratification of the analysis by the type of outcomes in offspring, the pooled RR in offspring with bipolar disorder and depression was 1.44 (95% CI 1.15–1.80) and 1.44 (95% CI 1.21–1.71), respectively. We observed significant heterogeneity in bipolar disorder (I2 = 70.72%; Q = 10.25; P value = 0.02) as well as in depressive disorder (I2 = 86.03%; Q = 42.96; P value < 0.01).
Confounding variables in multivariable models
Apart from the studies conducted in Brazil [22] and in the USA [37], all other studies fully or partially adjusted for maternal psychiatric history, paternal psychiatric history, maternal lifetime psychopathology, parental psychiatric history and maternal mental illness before or during pregnancy. The majority of the studies adjusted for the following variables in common: maternal education, age, maternal race, parity, gestational age, offspring age and sex, family income, partner social class, partner support and planned pregnancy. Four studies adjusted for prenatal alcohol use whereas only one study adjusted for other prenatal substance use including alcohol. Further, one study [37] adjusted for Attention Deficit Hyperactivity Disorder (ADHD) in offspring, parental antisocial personality and maladaptive parenting style (Table 1).
Publication bias
In the overall meta-analysis of the risk of mood disorders among offspring exposed to prenatal tobacco use, both visual inspection of the funnel plot (symmetric) and Egger’s regression test provided no evidence of potential publication bias (B = 1.155, SE = 1.026, P = 0.289) (Fig. 3). Similarly, Egger’s test was not statistically significant for both subgroups: B = 1.196, SE = 2.864, P = 0.748 and B = 1.388, SE = 1.959, P = 0.518 for bipolar and depressive disorders, respectively.
Subgroup and sensitivity analysis
Associations did not substantially change by the specific outcome of interest (bipolar and depression), the study setting, adjustment for prenatal alcohol use, socio-economic positions and reported dose-response effects of prenatal tobacco use. We performed an outcome specific analysis using the type of outcomes in offspring. The risk of bipolar disorder RR = 1.44 (95% CI 1.15–1.80) was similar when compared to depressive disorder RR = 1.44 (95% CI 1.21–1.71). However, the risk of mood disorders was greater in the studies that recruited the study participants from a clinical setting RR = 1.55 (95% CI 1.33–1.81) when compared to those recruited from population-based registers RR = 1.21 (95% CI 1.10–1.33). Similarly, the risk of mood disorders was greater in the studies that did not adjust for the residual confounding by socio-economic positions such as maternal age, education, parental income and social class RR = 1.80 (95% CI 1.47–2.20) when compared to those adjusted for socio-economic positions RR = 1.36 (95% CI 1.20–1.53). The risk of mood disorders was not significantly differed when studies included the adjustment for the confounding effect of prenatal alcohol exposure. For example, the risk of mood disorders in offspring exposed to prenatal tobacco use was RR = 1.57 (95% CI 1.23–1.99) and RR = 1.36 (95% CI 1.14–1.63) in the subgroup analysis of studies that adjusted or not adjusted for prenatal alcohol use, respectively. Further, to identify the possible effects of mood disorders in offspring, we also applied the analysis to studies that reported dose-related effects of prenatal tobacco use. We observed a greater risk of mood disorders in offspring exposed to high prenatal tobacco use RR = 1.54 (95% CI 1.46–1.62) when compared to moderate prenatal tobacco use RR = 1.36 (95% CI 1.30–1.42) (Table 2). The risk of mood disorders was not significantly attenuated when we limit the analysis to the studies that reported sibling comparison results. The relative risk of mood disorders in offspring exposed to prenatal tobacco use was RR = 1.21 (95% CI 1.04–1.41) in the studies that reported sibling comparison results. Moreover, the pooled estimated RR varied between 1.33 (95% CI 1.22–1.43) and 1.47 (95% CI 1.27–1.70) after removal of a single study at a time, which indicated that the findings were not influenced substantially by any single study (Supplementary file 2).
Discussion
Main findings
This systematic review and meta-analysis explored the risk of mood disorders in offspring exposed to prenatal tobacco use reported by eight cohort studies and two nested case–control studies. We found some evidence for a small association (RR < 2) with mood disorders in offspring. We also noted that exposure to higher levels of prenatal tobacco use was associated with an increased risk of mood disorders in offspring when compared to moderate exposure. For all studies, outcomes in offspring were prospectively collected and measured using well-accepted standardized and validated screening and diagnostic tools.
Possible biological mechanisms
Although the mechanisms underlying the association between prenatal tobacco use and mood disorders in offspring are not yet confirmed, a number of plausible mechanisms have been proposed [9, 10, 40,41,42,43,44]. One suggested mechanism is that the deleterious effects of the many hazardous compounds present in tobacco smoke can cross the placenta, affect the developing brain and alter neurodevelopmental trajectories [10, 40,41,42,43]. This pathway is characterized by excessive stimulation of serotonergic and dopaminergic receptors and the corresponding over-stimulation during pregnancy may alter sensitivity [9, 44] leading to impaired neural growth and circuit formation [9]. Thus, nicotine may directly interact with neural circuits linked with mood regulation [45] and contribute to mood disorders in offspring.
Exposure to prenatal tobacco use may also be linked with epigenetic changes in the offspring [46,47,48], in which modifications impact DNA expression through the chromatin remodelling and DNA methylation [49], without altering DNA sequences [50]. The epigenetic changes associated with prenatal tobacco use may include epigenetic regulation of genes involved in the hypothalamic–pituitary–adrenocortical axis (HPA) [47]. This over-stimulation of the axis is often seen in persons with mood disorders [51] has been suggested as a possible explanation for the causal pathway of prenatal tobacco exposure [52]. This is also supported by animal models, which showed that prenatal exposure to nicotine can induce HPA-axis hypersensitivity in offspring rats through the intrauterine programming of up-regulation of hippocampal GAD67 [53] and this may result in depression-like behavior in adolescent female rats that exposed to prenatal nicotine use [54].
Potential for confounding
The risk of mood disorders in offspring exposed to prenatal tobacco use may be due to a range of confounding, namely psychiatric problems in mothers and families [55,56,57]. For example, in the Avon Longitudinal Study of Parents and Children (ALPAC), the association between prenatal tobacco use and child psychological problems at the age of 4 years disappeared after adjusting for maternal and paternal psychopathology along with other covariates, suggesting that the association was due to confounding influences not prenatal tobacco exposure [58]. Similarly, a study assessing the risk of bipolar disorder in offspring exposed to prenatal tobacco use found a risk association in an unadjusted model [OR 1.41 (95% CI 1.12–1.79)], whereas reported no evidence for an association after adjusting for maternal psychiatric history [38]. In contrast, in a population-based study that adjusted for maternal and parental history of severe mental illness [16], the risk of bipolar disorder was largely attenuated but the association remained significant. Further, a population-based longitudinal study of Finnish reported the increased risk association between prenatal tobacco use and depression in offspring even after adjusting for maternal psychiatric diagnoses before child birth [20]. Similarly, a study that assessed prenatal tobacco use and bipolar disorder in offspring showed an increased risk of bipolar disorder in offspring after adjusting for potential confounders, such as; lifetime psychopathology, diagnoses of schizophrenia or other psychotic disorders, affective disorder, and postpartum depression [18]. This is also supported by epidemiological evidence from sibling analysis, for example [39]. In our meta-analysis, the pooled estimated risk of mood disorders was not significantly attenuated in the studies that reported sibling comparison results [RR = 1.21 (95% CI 1.04–1.41)]. Moreover, these findings are supported by reports that mothers could pass liability genes to offspring that may translate to associations between prenatal tobacco use and offspring behaviors [59].
We noted that all studies included in the review except studies from the UK [36] and Brazil [22] did not use paternal smoking as a robustness analyses to demonstrate the maternal effect resulted from a biological mechanism. One UK-based study that combined data from ALSPAC, HUNT, and the Pelotas 1982 birth cohort reported no association for paternal prenatal tobacco use and offspring depression [36]. Further, this finding was corroborated by another study [22].
Epidemiologic evidence also suggested that children born to mothers smoking during pregnancy are more likely to be exposed to second-hand smoke in childhood and may develop adverse outcomes [60, 61]. The environmental, individual and familial factors which predispose children to post-birth tobacco smoke have been associated with increased risk of neurobehavioral disorders in offspring [11, 61, 62]. For example, a systematic review and meta-analysis conducted to test the association between smoking and resultant depressive disorders found adverse associations, through which tobacco smoking was linked with later depressive disorders in more than a third of the included studies [11]. This finding is complimented by evidence suggesting that prolonged exposure to tobacco use or smoke may increase the individual vulnerability to have depression in later life [63, 64]. Therefore, considering these factors in the analysis may enable to differentiate the effects of in utero exposure to tobacco smoke from second-hand or passive smoking during pregnancy that have influenced the expression of childhood behavioral problems [65, 66].
Furthermore, more comprehensive adjusting for residual confounding by socio-economic positions may statistically correct the estimate of the effects of prenatal tobacco use on offspring adverse mental health and behavioural outcomes [58]. Some of the studies included in the current meta-analysis accounted for a range of residual confounding by socio-economic position that may influence the link between prenatal tobacco use and risk of mood disorders in offspring [16, 20, 22, 36, 38, 39]. Evidence from epidemiologic studies have shown that women who use tobacco during pregnancy have lower educational attainment and socioeconomic status including family income compared to non-smoking pregnant women [24, 67,68,69,70,71]. These have also been found to be associated with internalizing behaviors such as depression in offspring [41, 60]. For instance, in a study that combined data of four birth cohorts, both prenatal tobacco use and depression in offspring were associated with lower maternal education and social class [36]. This is also corroborated by a population-based cohort study testing associations between maternal smoking during pregnancy and internalizing behaviours where a risk association is found in unadjusted analysis [OR 1.60 (95% CI 1.60–2.10)]; whereas, no association is seen after adjustment for parental educational attainment and family income [OR 1.22 (95% CI 0.90–1.63)] [72], suggesting parental socioeconomic positions accounted for the greater risk of internalizing behaviours in offspring [24]. In our meta-analysis, we also found a similar pattern in which the risk of mood disorder in offspring was moderately attenuated in the studies that adjusted for socio-economic position explaining some part of the reported association between prenatal tobacco use and mood disorders in offspring.
Differences among the studies included in the meta-analysis
Although we found an association between prenatal tobacco use and mood disorders in offspring, it should be noted that the variations between the included studies led to a moderate level of heterogeneity in this meta-analysis. The type of mood disorders in offspring, the adjustment for prenatal alcohol use, outcomes measured at different time points and with different assessment methods, residual confounding by socio-economic positions, the setting as well as the level of prenatal tobacco exposure may have contributed to variability in the risk of mood disorders in offspring exposed to prenatal tobacco use. Nevertheless, the pooled RR estimate remained similar after the removal of a single study at a time in our leave-one-out sensitivity analysis, which indicated that the findings were not influenced substantially by any single study. Further, the subgroup analysis and sensitivity analysis appeared to support the robustness of our findings.
Strength and limitations
This systematic review and meta-analysis has the following strengths: we have used a predefined search strategy and data extraction protocol, as well as the methodological quality of the included studies, was checked by two independent reviewers. By doing so, we have minimized possible reviewer bias. We conducted subgroup and sensitivity analysis as well as leave-one-out-sensitivity analysis to identify the small study effect and the risk of heterogeneity. Further, we also conducted an additional analysis for those studies that reported sibling comparison results. In addition, the outcomes in offspring were measured using the standard and validated screening and diagnostic tools such as the ICD 9/10, DSM IV, SADS, MINI, CIS-R and HADS that provided well-validated assessments of mood disorders in offspring. However, the following limitations should be taken into consideration while interpreting these results. First, we did not analyze gender-, age- and study design-specific effect estimates due to a lack of sufficient and consistent data from the included studies. Second, the confounding effect of lifetime maternal mental health problems or mental health problems during pregnancy was not consistently adjusted in the included studies. Third, only three studies reported the effects of moderate and high tobacco smoking during pregnancy on offspring mood disorders and this might reduce the precision of the estimate. Fourth, in some studies, the follow-up period may be too short to find validated and diagnosed mood disorders and this may be contributed to underreporting due to a later manifestation of the outcome. Fifth, the prenatal tobacco exposure periods varied and for some studies the time of exposure during pregnancy that was investigated was not reported. Further, majority of the included studies had no information about smoking cessation. The consequence of this is that there will be a fraction of women who might have been classified as non-smokers but stopped smoking when they became aware of their pregnancy usually around mid-first trimester, or they were classified as smokers yet did not smoke after becoming aware of their pregnancy.
Conclusion
Although the etiology of mood disorders has not been established, this systematic review and meta-analysis provided some evidence for a small (RR < 2) association between prenatal tobacco use and mood disorders in offspring. However, it should be noted that the sparsity of studies on the topic and the potential for bias limits more conclusive inference.
Availability of data and material
All data generated or analyzed during this review were included in this article and attached as supplementary files.
References
Sadock BJ, Sadock VA, Ruiz P (2015) Kaplan and Sadock’s synopsis of psychiatry 11th edition: behavioral sciences/clinical psychiatry, book chapter 8. Wolters Kluwer, Alphen aan den Rijn
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association, Washington, DC
Malhi GS, Mann JJ (2018) Depression. Lancet 392(10161):2299–2312
Kessler RC, Bromet EJ (2013) The epidemiology of depression across cultures. Annu Rev Public Health 34(1):119–138
Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA et al (2011) Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry 68(3):241–251
James SB, Alcott SV (2007) Mood disorders. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 10th edn. Lippincott Williams & Wilkins, Philadelphia, pp 530–533
Thase ME (2009) Neurobiological aspects of depression. In: Gotlib IH, Hammen CL (eds) Handbook of depression. The Guilford Press, New York, pp 187–217
Kim DR, Bale TL, Epperson CN (2015) Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr Psychiatry Rep 17(2):5
Slotkin TA, Tate CA, Cousins MM, Seidler FJ (2006) Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: serotonin receptors and cell signaling. Neuropsychopharmacology 31(11):2462–2475
Slotkin TA (1998) Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther 285(3):931–945
Fluharty M, Taylor AE, Grabski M, Munafò MR (2017) The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tobacco Res 19(1):3–13
Al Mamun A, O’Callaghan FV, Alati R, O’Callaghan M, Najman JM, Williams GM et al (2006) Does maternal smoking during pregnancy predict the smoking patterns of young adult offspring? A birth cohort study. Tob Control 15(6):452–457
D’Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman CM, Grann M et al (2010) Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in Sweden. Arch Gen Psychiatry 67(5):529–538
Knopik VS, McGeary J, Nugent N, Francazio S, Heath AC (2010) Smoking during pregnancy, maternal xenobiotic metabolism genes, and child externalizing behavior: a case-crossover design. Behav Genet 40(6):799–800
Lange S, Probst C, Rehm J, Popova S (2018) National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health 6(7):e769–e776
Quinn PD, Rickert ME, Weibull CE, Johansson ALV, Lichtenstein P, Almqvist C et al (2017) Association between maternal smoking during pregnancy and severe mental illness in offspring. JAMA Psychiatry 74(6):589–596
Sutin AR, Flynn HA, Terracciano A (2017) Maternal cigarette smoking during pregnancy and the trajectory of externalizing and internalizing symptoms across childhood: similarities and differences across parent, teacher, and self reports. J Psychiatr Res 91:145–148
Talati A, Bao Y, Kaufman J, Shen L, Schaefer CA, Brown AS (2013) Maternal smoking during pregnancy and bipolar disorder in offspring. Am J Psychiatry 170(10):1178–1185
Ashford J, van Lier PA, Timmermans M, Cuijpers P, Koot HM (2008) Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. J Am Acad Child Adolesc Psychiatry 47(7):779–787
Ekblad M, Gissler M, Lehtonen L, Korkeila J (2010) Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Arch Gen Psychiatry 67(8):841–849
Indredavik MS, Brubakk A-M, Romundstad P, Vik T (2007) Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr 96(3):377–382
Menezes AMB, Murray J, Laszlo M, Wehrmeister FC, Hallal PC, Goncalves H et al (2013) Happiness and depression in adolescence after maternal smoking during pregnancy: birth cohort study. PLoS One 8(11):e80370
Monshouwer K, Huizink AC, Harakeh Z, Raaijmakers QA, Reijneveld SA, Oldehinkel AJ et al (2011) Prenatal smoking exposure and the risk of behavioral problems and substance use in adolescence: the TRAILS study. Eur Addict Res 17(6):342–350
Dolan CV, Geels L, Vink JM, van Beijsterveldt CE, Neale MC, Bartels M et al (2016) Testing causal effects of maternal smoking during pregnancy on offspring’s externalizing and internalizing behavior. Behav Genet 46(3):378–388
Talati A, Wickramaratne PJ, Wesselhoeft R, Weissman MM (2017) Prenatal tobacco exposure, birthweight, and offspring psychopathology. Psychiatry Res 252:346–352
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. Jama 283(15):2008–2012
Reviews UOYCF (2009) Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. University of York, Centre for Reviews & Dissemination
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al (2017) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 27 Apr 2019
Borenstein M, Hedges L, Higgins J, Rothstein H (2005) Comprehensive meta-analysis version 2. Biostat, Englewood, p 104
Borenstein M, Hedges LV, Higgins JP, Rothstein HR (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1(2):97–111
Patsopoulos NA, Evangelou E, Ioannidis JP (2008) Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 37(5):1148–1157
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed) 327(7414):557–560
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res Ed) 315(7109):629–634
Hill SY, Lowers L, Locke-Wellman J, Shen S (2000) Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. J Stud Alcohol 61(5):661–668
Taylor AE, Carslake D, De Mola CL, Rydell M, Nilsen TIL, Bjørngaard JH et al (2017) Maternal smoking in pregnancy and offspring depression: a cross cohort and negative control study. Sci Rep 7(1):1–8
Biederman J, Martelon M, Woodworth KY, Spencer TJ, Faraone SV (2017) Is maternal smoking during pregnancy a risk factor for cigarette smoking in offspring? A longitudinal controlled study of adhd children grown up. J Atten Disord 21(12):975–985
Chudal R, Brown AS, Gissler M, Suominen A, Sourander A (2015) Is maternal smoking during pregnancy associated with bipolar disorder in offspring? J Affect Disord 171:132–136
Meier SM, Plessen KJ, Verhulst F, Mors O, Mortensen PB, Pedersen CB et al (2017) Familial confounding of the association between maternal smoking during pregnancy and internalizing disorders in offspring. Psychol Med 19:1–10. https://doi.org/10.1017/S0033291716003627
Lambers DS, Clark KE (1996) The maternal and fetal physiologic effects of nicotine. Semin Perinatol 20(2):115–126
Rogers JM (2009) Tobacco and pregnancy. Reprod Toxicol (Elmsf NY) 28(2):152–160
Levin ED, Slotkin TA (1998) Chapter 34—developmental neurotoxicity of nicotine. In: Slikker W, Chang LW (eds) Handbook of developmental neurotoxicology. Academic Press, San Diego, pp 587–615
Slikker W, Xu ZA, Levin ED, Slotkin TA (2005) Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction—developmental neurotoxicity of nicotine. Crit Rev Toxicol 35(8–9):703–711
Ernst M, Moolchan ET, Robinson ML (2001) Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry 40(6):630–641
Suter M, Ma J, Harris A, Patterson L, Brown KA, Shope C et al (2011) Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics 6(11):1284–1294
Richmond RC, Simpkin AJ, Woodward G, Gaunt TR, Lyttleton O, McArdle WL et al (2015) Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet 24(8):2201–2217
Stroud LR, Papandonatos GD, Rodriguez D, McCallum M, Salisbury AL, Phipps MG et al (2014) Maternal smoking during pregnancy and infant stress response: test of a prenatal programming hypothesis. Psychoneuroendocrinology 48:29–40
Knopik VS, Maccani MA, Francazio S, McGeary JE (2012) The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev Psychopathol 24(4):1377–1390
Tehranifar P, Wu H-C, McDonald JA, Jasmine F, Santella RM, Gurvich I et al (2018) Maternal cigarette smoking during pregnancy and offspring DNA methylation in midlife. Epigenetics 13(2):129–134
Klose RJ, Bird AP (2006) Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci 31(2):89–97
Varghese FP, Brown ES (2001) The hypothalamic-pituitary-adrenal axis in major depressive disorder: a brief primer for primary care physicians. Prim Care Companion J Clin Psychiatry 3(4):151–155
Mello AF, Mello MF, Carpenter LL, Price LH (2003) Update on stress and depression: the role of the hypothalamic-pituitary-adrenal (HPA) axis. Rev Bras Psiquiatr (Sao Paulo Braz 1999) 25(4):231–238
He X, Lu J, Dong W, Jiao Z, Zhang C, Yu Y et al (2017) Prenatal nicotine exposure induces HPA axis-hypersensitivity in offspring rats via the intrauterine programming of up-regulation of hippocampal GAD67. Arch Toxicol 91(12):3927–3943
Zhang C, Fan SJ, Sun AB, Liu ZZ, Liu L (2019) Prenatal nicotine exposure induces depression like behavior in adolescent female rats via modulating neurosteroid in the hippocampus. Mol Med Rep 19(5):4185–4194
Lieb R, Isensee B, Höfler M, Pfister H, Wittchen H-U (2002) Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry 59(4):365–374
Halligan SL, Murray L, Martins C, Cooper PJ (2007) Maternal depression and psychiatric outcomes in adolescent offspring: a 13-year longitudinal study. J Affect Disord 97(1–3):145–154
Hannigan LJ, Eilertsen EM, Gjerde LC, Reichborn-Kjennerud T, Eley TC, Rijsdijk FV et al (2018) Maternal prenatal depressive symptoms and risk for early-life psychopathology in offspring: genetic analyses in the Norwegian Mother and Child Birth Cohort Study. Lancet Psychiatry 5(10):808–815
Brion M-J, Victora C, Matijasevich A, Horta B, Anselmi L, Steer C et al (2010) Maternal smoking and child psychological problems: disentangling causal and noncausal effects. Pediatrics 126(1):e57–e65
Kuja-Halkola R, D’Onofrio BM, Larsson H, Lichtenstein P (2014) Maternal smoking during pregnancy and adverse outcomes in offspring: genetic and environmental sources of covariance. Behav Genet 44(5):456–467
Knopik VS (2009) Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol 34(1):1–36
Kabir Z, Connolly GN, Alpert HR (2011) Secondhand smoke exposure and neurobehavioral disorders among children in the United States. Pediatrics 128(2):263–270
Tiesler CMT, Heinrich J (2014) Prenatal nicotine exposure and child behavioural problems. Eur Child Adolesc Psychiatry 23(10):913–929
Markou A, Kosten TR, Koob GF (1998) Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18(3):135–174
Rose JE, Behm FM, Ramsey C, Ritchie JC Jr (2001) Platelet monoamine oxidase, smoking cessation, and tobacco withdrawal symptoms. Nicotine Tob Res 3(4):383–390
Schlotz W, Phillips DI (2009) Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun 23(7):905–916
Thapar A, Rutter M (2009) Do prenatal risk factors cause psychiatric disorder? Be wary of causal claims. Br J Psychiatry J Ment Sci 195(2):100–101
Al-Sahab B, Saqib M, Hauser G, Tamim H (2010) Prevalence of smoking during pregnancy and associated risk factors among Canadian women: a national survey. BMC Pregnancy Childbirth 10(1):24
Muhajarine N, D’Arcy C, Edouard L (1997) Prevalence and predictors of health risk behaviours during early pregnancy: Saskatoon Pregnancy and Health Study. Can J Public Health Revue Canadienne de Sante Publique. 88(6):375–379
Stewart DE, Streiner DL (1995) Cigarette smoking during pregnancy. Can J Psychiatry Revue Canadienne de Psychiatrie 40(10):603–607
Nagahawatte NT, Goldenberg RL (2008) Poverty, maternal health, and adverse pregnancy outcomes. Ann N Y Acad Sci 1136:80–85
Heaman MI, Chalmers K (2005) Prevalence and correlates of smoking during pregnancy: a comparison of aboriginal and non-aboriginal women in manitoba. Birth 32(4):299–305
Roza SJ, Verhulst FC, Jaddoe VWV, Steegers EAP, Mackenbach JP, Hofman A et al (2008) Maternal smoking during pregnancy and child behaviour problems: the Generation R Study. Int J Epidemiol 38(3):680–689
Acknowledgements
We are so grateful to the Curtin University for providing us a wide range of available online databases.
Funding
No external funding obtained for this systematic review and meta-analysis.
Author information
Authors and Affiliations
Contributions
BD conceived the hypothesis, developed the methodology, identified all potential studies, extracted the data, assessed quality, conducted a meta-analysis, and wrote the first draft of the manuscript. GA reviewed abstracts and assessed the methodological quality of the included studies. GP reviewed the protocol, reviewed data extraction, data analysis and contributed to subsequent drafts of the manuscript. KB reviewed data extraction, data analysis and contributed to subsequent drafts of the manuscript. RA reviewed the protocol, reviewed data extraction, and synthesis and contributed to subsequent drafts of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to disclose.
Code availability
Comprehensive Meta-Analysis (CMA) version 3.0 was used to analyze the data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duko, B., Ayano, G., Pereira, G. et al. Prenatal tobacco use and the risk of mood disorders in offspring: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol 55, 1549–1562 (2020). https://doi.org/10.1007/s00127-020-01949-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00127-020-01949-y