Abstract
Aims/hypothesis
Obesity and dysglycaemia are major risk factors for type 2 diabetes. We determined if obese people undergoing laparoscopic adjustable gastric banding (LAGB) had a reduced risk of progressing from impaired fasting glucose (IFG) to diabetes.
Methods
This was a retrospective cohort study of obese people with IFG who underwent LAGB. Weight and diabetes outcomes after a minimum follow-up period of 4 years (mean ± SD 6.1 ± 1.7 years) were compared with those of Australian adults with IFG from a population-based study (AusDiab).
Results
We identified 281 LAGB patients with baseline IFG. Their mean ± SD age and BMI were 46 ± 9 years and 46 ± 9 kg/m2, respectively. The diabetes incidence for patients in the lowest, middle and highest weight loss tertile were 19.1, 3.4 and 1.8 cases/1,000 person-years, respectively. The AusDiab cohort had a lower BMI (28 ± 5 kg/m2) and a diabetes incidence of 12.5 cases/1,000 person-years. This increased to 20.5 cases/1,000 person-years when analysis was restricted to the 322 obese AusDiab participants, which was higher than the overall rate of 8.2 cases/1,000 person-years seen in the LAGB group (p = 0.02). Multivariable analysis of the combined LAGB and AusDiab data suggested that LAGB was associated with ∼75% lower risk of diabetes (OR 0.24 [95% CI 0.10, 0.57], p = 0.004).
Conclusions/interpretation
In obese people with IFG, weight loss after LAGB is associated with a substantially reduced risk of progressing to diabetes over ≥4 years. Bariatric surgery may be an effective diabetes prevention strategy in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective strategies to prevent type 2 diabetes are fundamental to addressing the huge health and economic burden of this disease [1]. People with impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) are at high risk of developing type 2 diabetes [2], and obesity increases the risk of progressing from IGT to diabetes [3, 4].
In moderately overweight populations with IGT, intensive lifestyle intervention [5–8] or drug therapy with metformin [5, 6], acarbose [9] or pioglitazone [10] reduces the risk of progression to type 2 diabetes by 30–70%. There is less evidence to guide diabetes prevention strategies for people with severe obesity (BMI >35 kg/m2). Torgerson et al [11] showed that orlistat reduced the risk of diabetes by 37% over 4 years in a predominantly normoglycaemic population of severely obese people. In addition, a trial designed to determine the effects of phentermine/topiramate combination on body weight in a population with a mean BMI of 36 kg/m2 reported that 2 years of drug therapy achieved ∼10% weight loss and reduced the risk of diabetes by ∼65% [12].
Bariatric surgery is an effective and durable weight loss treatment [13] that has been proven in three randomised controlled trials to induce remission of type 2 diabetes more commonly than a conventional medical treatment programme [14–16]. This raises the possibility of using bariatric surgery to prevent diabetes in high-risk severely obese populations. This proposition was strengthened by a recent report by the Swedish Obese Subjects (SOS) investigators [17]. Using a case–control study of non-diabetic people with a BMI >34 kg/m2 (mean ± SD 42 ± 5 kg/m2), the investigators showed that people undergoing gastroplasty, gastric band or gastric bypass surgery achieved an average weight loss of 20 kg after 15 years and ∼80% reduction in diabetes incidence, compared with a lifestyle intervention delivered to an equally obese control group.
To clarify the potential benefit of substantial weight loss in preventing type 2 diabetes, we determined the relationship between weight loss after laparoscopic adjustable gastric banding (LAGB) and the rate of progression from IFG to type 2 diabetes. We also compared the overall LAGB patient outcomes with those of a contemporaneous community-dwelling cohort of predominantly non-obese people with IFG who participated in the AusDiab Study [18].
Methods
Study design and participants
We performed a longitudinal cohort study of all patients treated for obesity by authors P. O’Brien and S. Skinner at the Centre for Bariatric Surgery in Melbourne between October 1995 and August 2007. Postoperative management mandated regular medical review to assess weight loss and to adjust the band. The median (interquartile range) number of clinic attendances in follow-up years 1, 2, 3, 4 and 5 was 8 (6–10), 6 (2–9), 3 (1–7), 2 (0–6) and 2 (0–4), respectively.
Data sources and measurement
All patients had fasting plasma glucose (FPG), lipid profile and HbA1c measured up to 3 months before LAGB. Follow-up FPG levels were ordered as required by the family physician or bariatric physician. These assays were performed by clinical laboratories in Melbourne, and results were included if generated at least 4 years after surgery. Nearly all follow-up FPG levels were obtained from an electronic clinical record (LapBase; LapBase Pty Ltd, Melbourne, VIC, Australia), with five FPG values obtained after patients were telephoned and asked to have the test (see Fig. 1). Of the 281 follow-up FPG values, 56 were obtained between 4 and 5 years of follow-up, and the rest after 5 years. If multiple FPG results were available, we used the first one obtained after 5 years of follow-up. Follow-up weight data were collated from LapBase. For correlations between weight loss and glycaemic status, weight records within 3 months of the follow-up FPG were used.
The AusDiab dataset comprised clinical and biochemical data obtained in 1999/2000 and again in 2004/2005 from over 11,000 community-dwelling Australians aged over 25 years [18]. The AusDiab subgroup used in this study comprised people with complete data who had IFG but not diabetes at baseline. To match for age, AusDiab participants over 55 years old were excluded.
The study was approved by the Monash University Human Research and Ethics Committee and was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12613000548730).
Definitions
IFG was defined as FPG of 5.6–6.9 mmol/l (100–125 mg/dl) in the context of no history of diabetes or of glucose-lowering drug use [19]. Diabetes was defined as a patient report of being diagnosed by another practitioner during the 5 year follow-up period or FPG of ≥7.0 mmol/l based on the single value described above.
Statistical analysis
Percentage weight loss measures from each patient were linearly interpolated at specific time points (0, 3, 6, 12, 18, 24, 30, 36, 42, 48, 54 and 60 months) using the approxfun function in R (www.r-project.org). For time points outside the measured range for a given patient, the final observed value was carried forward. Average weight loss over 5 years was calculated for each patient and used to compare the weight loss curves of different groups. Multivariable logistic regression with generalised linear models was performed separately for each cohort, and on the combined dataset using R. The significance of each variable was assessed by analysis of deviance using a χ 2 test. Other data were analysed using GraphPad Prism (v5.0a) software. Continuous variables were assessed using the t test. Comparison of rates of diabetes used Fisher’s exact test and χ 2 test for trend, and assumed that the duration of follow-up was the same in each group. A p value of <0.05 was considered significant.
Results
Figure 1 provides a flow chart for the participant selection. Of a total of 3,174 patients who underwent LAGB between October 1995 and August 2007, 333 (248 women and 85 men) had IFG (5.6–6.9 mmol/l) and no history of diabetes or of glucose-lowering drug use. Of these 333 people, 281 (84%; 210 women and 71 men) had follow-up weight and FPG data. Their baseline characteristics are given in Table 1.
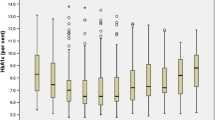
After 5 years, the LAGB cohort lost an average of 25 kg, representing 19% weight loss and a mean BMI reduction from 46 to 37 kg/m2. Figure 2a shows the weight loss curves of the 14 and 169 patients who respectively did and did not develop diabetes after a minimum follow-up period of 4 years (mean ± SD 6.1 ± 1.7 years). These weight trajectories were significantly different (p = 0.003), whereas there were no significant differences between the weight trajectories of women compared with men, or of the 281 people with follow-up FPG compared with the 37 people with weight outcome data but no follow-up FPG. During the first 5 years of patient follow-up, revision surgery to resite the band was performed in 25% of women and 12% of men (p < 0.02), and LAGB explant was performed in 14 patients (5%), of whom one proceeded to biliopancreatic diversion after 39 months of follow-up.
Weight loss and its effect on FPG. (a) Weight trajectories of LAGB patients who did (n = 14; squares) and who did not (n = 267; circles) develop diabetes after a minimum of 4 years follow-up. Data are mean percentage weight loss and 95% CI. Numbers of patients with weight data at each time point are indicated below the x axis. (b) Correlation between weight loss and ∆FPG. Data from patients in the LAGB group who did not develop diabetes (n = 267; one outlier with −62% weight loss omitted), with line of best fit (m = −0.023 ± 0.003) and 95% CIs depicted in solid and dashed lines, respectively
We divided the LAGB group into weight loss tertiles to determine the relationship between weight loss and risk of diabetes. In the 281 LAGB patients, weight loss correlated with baseline body weight, but not with any other baseline patient characteristic (Table 1). Fourteen patients developed diabetes during the study period, corresponding to an incidence rate of 8.2 cases/1,000 person-years. There were 12 cases of diabetes in women and two in men, corresponding to incidence rates of 9.2 and 4.9 cases/1,000 person-years, respectively, a difference that was not statistically significant. Table 2 shows the relationship between weight loss tertile and progression to diabetes. There was one case of diabetes in a patient in the highest tertile of weight loss: a 67-year-old woman who developed diabetes after gallstone pancreatitis complicated by recurring pancreatic pseudocysts and portal vein thrombosis. Diabetes incidence progressively reduced with weight loss, with 19.1, 3.4 and 1.8 cases/1,000 person-years in tertiles 1, 2 and 3, respectively (p < 0.001 for trend). Regression analysis of weight change vs ∆FPG reflected these findings, with 10% weight loss corresponding to a 0.23 mmol/l reduction in FPG after ≥4 years (p < 0.001; Fig. 2b).
We then compared these results with those of 1,043 community-dwelling Australians (362 women and 681 men) recruited into the AusDiab cohort study in 1999/2000 [20] who had IFG and were followed for 5 years (Table 1). In these people, the mean ± SD age of 46 ± 7 years was comparable with that of the LAGB group, while BMI (28 ± 5 kg/m2) and the proportion of women (35%) were significantly lower (p < 0.001 for each comparison). After 5 years, 65 AusDiab participants developed diabetes, yielding a diabetes incidence of 12.5 cases/1,000 person-years, a rate that was not significantly different from the rate seen in LAGB patients. However, AusDiab diabetes incidence increased significantly (p < 0.001) to 20.5 cases/1,000 person-years when analysis was restricted to obese participants (n = 322; mean BMI 34 ± 4 kg/m2). This rate was greater than the overall LAGB rate of 8.2 cases/1,000 person-years (p < 0.02).
To adjust rates of progression to diabetes for possible relevant variables, we performed multivariable analyses. The input variables were baseline age, baseline BMI, baseline FPG, sex, percentage weight change, duration of follow-up and, for LAGB patients, revision surgery and/or band explant during the follow-up period. The outcome variable, progression to diabetes, was independently associated with poor weight loss and baseline FPG in the LAGB cohort, and with baseline FPG, baseline BMI and female sex in the AusDiab group (Table 3). To determine whether LAGB was independently associated with progression to diabetes, we combined data from both groups, replacing percentage weight change with the presence or absence of LAGB surgery. In this group of 1,324 individuals, LAGB was associated with a reduced risk of diabetes of more than 75% (OR 0.239 [95% CI 0.095, 0.571], p = 0.004), with female sex and baseline FPG also significantly associated with progression to diabetes (Table 3).
Discussion
We report long-term weight and diabetes outcomes for obese people with IFG who underwent LAGB surgery. Surgery induced a substantial and sustained mean weight loss of 19% of body weight, with one-fifth of patients requiring revision surgery to resite the band. These outcomes are similar to those observed in the general population of obese people who have this operation [13]. The rate of progression from IFG to diabetes reduced significantly across the tertiles of weight loss. Taken together with the findings of the multivariable analysis, we conclude that weight loss in obesity complicated by IFG prevents progression to diabetes. This accords with the recent SOS findings [17], trials of weight loss drugs [11, 12] and other reports of remission of diabetes after medical [21] or surgically induced weight loss [14–16].
There are several reasons why the differences in diabetes incidence observed in the LAGB and AusDiab cohorts were underestimated. First, although surgical patients were followed-up on average 1 more year than AusDiab participants, we had to assume these variables were equal in order to perform statistical comparisons. Second, the risk of diabetes in severely obese people (BMI >35 kg/m2) is roughly double that of less obese people [22–24], making it likely that the diabetes incidence of the obese AusDiab cohort (mean BMI 34.3 kg/m2) underestimated the baseline diabetes risk in our LAGB population. This accords with a much greater (over threefold) diabetes incidence of 91 cases/1,000 person-years observed in morbidly obese people (n = 290, BMI 41 ± 4 kg/m2) in the SOS control group who had IFG [17]. On the other hand, using the same definition of diabetes, the 301 members of the SOS surgical group with baseline IFG achieved similar weight loss and a slightly increased rate of diabetes (15/1,000 person-years after 15 years) compared with our LAGB patients (8/1,000 person-years after 6 years). Finally, the relatively small numbers of men undergoing LAGB surgery and of obese AusDiab participants reduced our ability to detect differences between these groups and their relevant comparators.
LAGB is a common outpatient procedure [25], and yet has comparable durability and a superior record of safety and patient acceptability compared with the two major alternatives of sleeve gastrectomy and gastric bypass surgery [13, 26]. These features are important considerations for the design of prevention strategies for type 2 diabetes in obesity.
In conclusion, we show that the rate of progression from IFG to diabetes is substantially reduced in obese people who undergo LAGB surgery. Given the uncertain efficacy of lifestyle intervention in severely obese people at risk of diabetes, these findings strengthen the case for a randomised trial to determine whether LAGB surgery is a safe and cost-effective approach to preventing type 2 diabetes in this population.
Abbreviations
- FPG:
-
Fasting plasma glucose
- IFG:
-
Impaired fasting glucose
- IGT:
-
Impaired glucose tolerance
- LAGB:
-
Laparoscopic adjustable gastric banding
- SOS:
-
Swedish Obese Subjects
References
Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414:782–787
American Diabetes Association (2013) Standards of medical care in diabetes–2013. Diabetes Care 36(Suppl 1):S11–S66
Edelstein SL, Knowler WC, Bain RP et al (1997) Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 46:701–710
Burke JP, Williams K, Narayan KM, Leibson C, Haffner SM, Stern MP (2003) A population perspective on diabetes prevention: whom should we target for preventing weight gain? Diabetes Care 26:1999–2004
Ramachandran A, Snehalatha C, Mary S et al (2006) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 49:289–297
Knowler WC, Barrett-Connor E, Fowler SE et al (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346(6):393–403
Tuomilehto J, Lindstrom J, Eriksson JG et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350
Li G, Zhang P, Wang J et al (2008) The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371(9626):1783–1789
Chiasson JL, Josse RG, Gomis R et al (2002) Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359:2072–2077
DeFronzo RA, Tripathy D, Schwenke DC et al (2011) Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 364:1104–1115
Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L (2004) XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27:155–161
Garvey WT, Ryan DH, Look M et al (2012) Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 95:297–308
O’Brien P, McDonald L, Anderson M, Brennan L, Brown WA (2013) Long term outcomes after bariatric surgery: fifteen year follow up after gastric banding and a systematic review of the literature. Ann Surg 257:87–94
Dixon JB, O’Brien PE, Playfair J et al (2008) Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. J Am Med Assoc 299:316–323
Mingrone G, Panunzi S, De Gaetano A et al (2012) Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366(17):1577–1585
Schauer PR, Kashyap SR, Wolski K et al (2012) Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366(17):1567–1576
Carlsson LM, Peltonen M, Ahlin S et al (2012) Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 367(8):695–704
Magliano DJ, Barr EL, Zimmet PZ et al (2008) Glucose indices, health behaviors, and incidence of diabetes in Australia: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 31(2):267–272
American Diabetes Association (2010) Standards of medical care in diabetes - 2010. Diabetes Care 33(Suppl 1):S11–S61
Dunstan DW, Zimmet PZ, Welborn TA et al (2002) The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 25:829–834
Gregg EW, Chen H, Wagenknecht LE et al (2012) Association of an intensive lifestyle intervention with remission of type 2 diabetes. J Am Med Assoc 308:2489–2496
Colditz GA, Willett WC, Stampfer MJ et al (1990) Weight as a risk factor for clinical diabetes in women. Am J Epidemiol 132:501–513
Hu FB, Manson JE, Stampfer MJ et al (2001) Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 345:790–797
Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC (1994) Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17(9):961–969
Cobourn C, Mumford D, Chapman MA, Wells L (2010) Laparoscopic gastric banding is safe in outpatient surgical centers. Obes Surg 20:415–422
Longitudinal Assessment of Bariatric Surgery C, Flum DR, Belle SH et al (2009) Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 361:445–454
Acknowledgements
We are grateful to our patients and the participants of the AusDiab Study. We thank M. Mehmet and A. McNeil for assistance with collating biochemical data. Also, for their invaluable contribution to the set-up and field activities of AusDiab, we are enormously grateful to A. Allman, B. Atkins, S. Bennett, A. Bonney, S. Chadban, M. de Courten, M. Dalton, D. Dunstan, T. Dwyer, H. Jahangir, D. Jolley, D. McCarty, A. Meehan, N. Meinig, S. Murray, K. O’Dea, K. Polkinghorne, P. Phillips, C. Reid, A. Stewart, R. Tapp, H. Taylor, T. Welborn, T. Whalen, F. Wilson and P. Zimmet.
Funding
The work was funded primarily by the Monash University Centre for Obesity Research and Education. It was supported by Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS. JES is supported by a National Health and Medical Research Council Fellowship (586623). The AusDiab Study, co-coordinated by the Baker IDI Heart and the Diabetes Institute, gratefully acknowledges the generous support given by: National Health and Medical Research Council (NHMRC grant 233200); Australian Government Department of Health and Ageing; Abbott Australasia; Alphapharm; AstraZeneca; Bristol-Myers Squibb; City Health Centre-Diabetes Service – Canberra; Department of Health and Community Services – Northern Territory; Department of Health and Human Services – Tasmania; Department of Health – New South Wales; Department of Health – Western Australia; Department of Health – South Australia; Department of Human Services – Victoria; Diabetes Australia; Diabetes Australia Northern Territory; Eli Lilly Australia; Estate of the Late Edward Wilson; GlaxoSmithKline; Jack Brockhoff Foundation; Janssen-Cilag; Kidney Health Australia; Marian & FH Flack Trust; Menzies Research Institute; Merck Sharp & Dohme; Novartis Pharmaceuticals; Novo Nordisk Pharmaceuticals; Pfizer; Pratt Foundation; Queensland Health; Roche Diagnostics Australia; Royal Prince Alfred Hospital, Sydney; Sanofi Aventis; Sanofi-synthelabo; and the Victorian Government’s OIS Program. The Centre for Obesity Research and Education (CORE) at Monash University receives grants from Allergan and Applied Medical for research and educational support. The grants are not tied to any specified research projects and the grantors have no control over the protocol, analysis and reporting of any studies.
Contribution statement
All authors contributed to conception and design, acquisition of data, analysis and interpretation of data. JMW and PEO drafted the manuscript and revised it with all other authors, who also approved the final version. JMW takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Duality of interest
All authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
John M. Wentworth and Tamishta Hensman contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wentworth, J.M., Hensman, T., Playfair, J. et al. Laparoscopic adjustable gastric banding and progression from impaired fasting glucose to diabetes. Diabetologia 57, 463–468 (2014). https://doi.org/10.1007/s00125-013-3129-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3129-0