Abstract
Background
Long-term outcome data are needed to define the role of bariatric surgery in type 2 diabetes (T2D). To address this, we collated diabetes outcomes more than a decade after laparoscopic adjustable gastric band (LAGB) surgery.
Method
Clinical and biochemical measures from 113 obese T2D patients who underwent LAGB surgery in 2003 and 2004 were analyzed. Diabetes remission was defined as HbA1c < 6.2% (44 mmol/mol) and fasting glucose < 7.0 mmol/L.
Results
Seventy-nine patients had weight data at 10 years and attained a median [Q1, Q3] weight loss of 16 [10, 21] percent. Sixty patients attended a follow-up assessment. Their baseline HbA1c of 7.8 [7.1, 9.3] percentage units (62 [54, 78] mmol/mol) had decreased to 6.6 [6.1, 8.4] (49 [43, 68] mmol/mol) despite no significant change in glucose-lowering therapy. Eleven patients (18%) were in diabetes remission and another 18 had HbA1c ≤ 6.5%. Significant improvements in physical measures of quality of life, blood pressure, and lipid profile were also observed but there was no change in the proportion of patients with albuminuria and a significant decline in estimated glomerular filtration rate. Twelve patients in the follow-up cohort (20%) required anti-reflux medication after surgery and 26 (43%) underwent gastric band revision surgery.
Conclusion
Weight loss for over 10 years after LAGB surgery delivers clinically meaningful improvements in HbA1c, blood pressure, lipids, and quality of life at the cost of a high rate of revision surgery and increased use of anti-reflux medication. These findings support the use of bariatric surgery as a long-term treatment for weight loss and wellbeing in patients with T2D.
Study Registration
Registered with the Australian Clinical trials registry as ACTRN12615000089538.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment guidelines for type 2 diabetes (T2D) highlight the importance of weight loss [1], a recommendation supported by several randomized trials of at least 5 years’ duration that showed intensive lifestyle modification [2, 3] and bariatric surgery [4,5,6] improved glycemia and quality of life in overweight and obese T2D populations. The magnitude of benefit from weight loss broadly correlates with its degree and duration [7, 8], leading proponents of bariatric surgery to call for its widespread use as a diabetes treatment. However, this view is not endorsed by treatment guidelines [9] because long-term diabetes outcome data for bariatric surgery are sparse and often confounded by selection and observer biases [10].
Of the three main bariatric operations, sleeve gastrectomy and gastric bypass are favored over gastric band surgery on account of their superior weight loss and relative ease of aftercare in most centers [11]. However, gastric band surgery is safer than the other common bariatric operations [12] and delivers good long-term weight loss in dedicated centers [13]. These features, combined with its adjustability and reversibility, make gastric band surgery an appealing option for a significant proportion of obese patients. In addition, because gastric band surgery does not alter anatomy or the gut hormone profile, it provides insight into the potential effectiveness of non-surgical weight loss therapies, particularly those centered on dietary modification and calorie restriction.
During 2003 and 2004, we performed primary gastric band surgery on 919 patients, of whom 113 had T2D. The purpose of this study was to describe the rates of diabetes remission and long-term outcomes of these 113 patients and to describe the relationship between weight loss and measures of diabetes control and complications.
Method
Study Design and Setting
This was a retrospective single-arm cohort study with prospective patient follow-up more than 10 years after gastric band surgery at The Centre for Bariatric Surgery, Melbourne. The study was approved by Monash University Human Research Ethics Committee and registered with the Australian Clinical trials registry as ACTRN12615000089538.
Participants
We included 113 patients who underwent primary laparoscopic adjustable gastric band (LAGB) surgery between January 2003 and December 2004 and who had T2D at the time of surgery, defined as a prior diagnosis of T2D, fasting glucose > 7 mmol/L or HbA1c > 6.5% (48 mmol/mol). Indefinite surgical aftercare to review and adjust the band was provided to all participants through the Centre for Bariatric Surgery, Melbourne. All 60 patients in the “included” cohort provided written informed consent to attend follow-up assessments.
Data Collection
Baseline clinical data and progressive weight data, entered prospectively by treating clinicians, were extracted from LapBase (LapBase Pty Ltd., Melbourne), an electronic database. Clinical data more than 10 years after LAGB were obtained during face-to-face patient interviews between March 2015 and May 2016. Sitting blood pressure was measured with a SureSign VS2 automated sphygmomanometer (Philips, Andover, MA) after the patient had been resting for at least 5 min. Quality of life scores from the SF-36 questionnaire were standardized to the Australian population mean ± SD of 50 ± 10 as previously described [14]. Biochemistry was performed at accredited community laboratories, the majority of which were operated by Melbourne Pathology and Dorevitch Pathology (both located in Melbourne, Australia). Beta-cell function was calculated from fasting glucose and C-peptide measures using the HOMA2 calculator [15].
Diabetes Remission
We defined diabetes remission according to our prior study [16] as fasting glucose < 7.0 mmol/L and HbA1c < 6.2% (44 mmol/mol). Because we routinely recommend metformin to our T2D patients after LAGB, patients taking metformin monotherapy (N = 4) were included in the remission population. Diabetes remission was also classified according to ADA recommendations [17], which required participants not to be taking any glucose-lowering drugs. Complete remission was defined as HbA1c < 6% and fasting glucose (FG) < 5.6 mmol/L, and partial remission as HbA1c < 6.5% and FG < 7.0 mmol/L.
Statistical Analyses
Data were complete unless otherwise stated. Average weight and HbA1c over time were determined using the trapezoidal rule. Excess weight was the weight in kg above a body mass index (BMI) of 25 kg/m2.
The approxfun function in the R software package (www.r-project.org) was used to calculate 6-monthly weight and HbA1c for each participant, thereby enabling generation of time-course curves. Linear regression analyses were performed using the lm function in R after interpolating two missing C-peptide values with the group median. Statistical analyses were performed using Prism v7.0b (Graphpad San Diego, CA). Paired-group comparisons of categorical and continuous data used a Fisher’s exact test and Mann Whitney U test, respectively. A two-tailed p value below 0.05 was considered significant.
Results
The participant flow chart is presented in Fig. 1. Of 919 patients undergoing LAGB surgery at our center between January 2003 and December 2004, 113 had T2D. Of these patients, 79 had weight data beyond 10 years and 60 (29 male, 31 female) agreed to present for clinical assessment and blood testing in 2015 and 2016. Baseline characteristics of this “included” cohort and the 53 “excluded” patients are provided in Table 1. Body weight, body mass index (BMI), blood pressure, and HbA1c did not differ significantly between the two patient groups. However, when compared to the excluded cohort, included patients had a greater duration of follow-up in the aftercare clinic and were significantly more likely to have undergone LAGB revision surgery to re-site or replace the band. Full details of revision surgery for both groups are provided in Supplemental Table 2.
Ten-year weight data were available for 79 of the 113 patients (all 60 in the included cohort and 19 in the excluded cohort), equating to 70% of the overall population. The median [Q1, Q3] weight loss of these 79 patients 10 years after LAGB was 16 [10, 21] percent. Figure 2a presents percent weight loss over 10 years following LAGB surgery according to patient cohort. The included cohort achieved maximal weight loss of 20% at 2 years and sustained more than 15% weight loss out to 10 years, with median weight loss of 17 [12, 22] percent at follow-up, equating to a median excess weight loss of 43 [24, 60] percent. When averaged over the available follow-up data of median duration 12 [11, 12] years, the included cohort achieved median weight loss of 16 [12, 22] percent (Fig. 2b), equating to 42 [31, 56] percent of their excess weight. The median duration of clinic follow-up for excluded patients was shorter at 7 [5, 11] years (p < 0.0001) and their weight loss was lower over the first 6 years (Fig. 2a), but the difference was not statistically significant. Their average weight loss over the duration of clinic attendance was 15 [9, 20] percent (Fig. 2b).
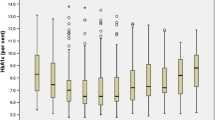
Weight loss over 10 years following LAGB surgery. Weight loss for the 60 included (filled circles) and 53 excluded (open circles) patients are shown. Included patients had all been followed for more than 10 years after surgery whereas 34 of the 53 excluded patients had stopped attending clinic before 10 years. a Median percent weight loss over half-yearly intervals. b Per-patient percent weight loss averaged over the duration of clinic follow-up by calculating the area under the weight loss curve and dividing by follow-up time. The median and interquartile range of each group is indicated. c Median HbA1c over half-yearly intervals for the 60 included patients
The clinical characteristics of the 60 included patients at baseline and after more than 10 years’ follow-up are provided in Table 2. Median BMI decreased from 43 to 34 kg/m2 and was associated with significant reductions in HbA1c, total cholesterol, LDL cholesterol, triglycerides, and blood pressure. Analysis of median HbA1c over time (Fig. 2c) revealed that improvements in HbA1c mirrored weight loss, with the HbA1c nadir of 6.2% (44 mmol/mol) 2 years after surgery corresponding to the time of maximal weight loss. The correlation coefficient (Spearman R) between the half-yearly median values of HbA1c and percent weight loss was highly significant (R = − 0.962, 95% CI − 0.985 to − 0.907, P < 0.0001).
At follow-up, diabetes remission (fasting glucose < 7.0 mmol/L and HbA1c < 6.2%) was observed in 11 participants (18%), 4 of whom continued to take metformin. Remitters were younger than non-remitters at the time of surgery (43 [36, 51] vs. 50 [44, 54] years; P = 0.0193) and their duration of diabetes was significantly lower (1 [0, 3] vs. 2 [1, 9] years; P = 0.0171). When the more stringent ADA criteria for diabetes remission [17] were applied, complete diabetes remission was observed in five patients and partial remission in three. Another five patients taking metformin as their sole glucose-lowering drug met the metabolic criteria for complete (N = 1) or partial (N = 4) remission. Overall, 29 patients (48%) attained very good glucose control with HbA1c ≤ 6.5% (48 mmol/mol) at the final follow-up visit.
The number of medications used to control glucose and blood pressure did not differ significantly at baseline and follow-up. Analysis of glucose-lowering medications revealed similar frequency of insulin use at both time points, with a shift away from glitazones and sulfonylureas at baseline to dipeptidyl peptidase 4 (DPP4) inhibitors, sodium-glucose co-transporter-2 (SGLT2) inhibitors, and exenatide at follow-up. Use of lipid-lowering and anti-reflux medications was more frequent at follow-up, being used by 65 and 38% of patients, respectively. This contributed to a non-significant increase in the number of prescription medications from 4 [2, 6] at baseline to 5 [3, 7] after more than a decade. The number of patients experiencing macro- or microvascular events increased over time, but there were no significant changes in the number of patients affected by specific events. Microalbuminura did not regress following LAGB surgery and the median estimated glomerular filtration rate (eGFR) declined significantly from 62 [54, 78] to 49 [43, 68] mL/min/1.73m2, with one patient commencing peritoneal dialysis 11 years after LAGB surgery.
Quality of life was assessed at baseline and follow-up using the SF-36 survey, with scores standardized to the Australian population mean ± SD of 50 ± 10. The outcomes for the group of 60 included patients are presented in Fig. 3. Despite the passage of more than a decade, significant improvements in the composite physical health score and in the scores for the domains of physical function, role physical, general health, and vitality were observed.
We performed linear regression analysis to identify factors associated with HbA1c at the final visit, undertaken at a median of 12 years after LAGB surgery. The input variables were percent weight loss and number of glucose-lowering medications at follow-up, sex, and baseline measures of number of glucose-lowering medications, age, diabetes duration, HbA1c, and beta-cell function derived from fasting glucose and C-peptide (HOMAB [15]). Percent weight loss and baseline HbA1c were the only factors independently associated with the final HbA1c, accounting for 19 and 6% of its variance, respectively (Supplemental Table 1).
Quality of life outcomes at baseline and more than 10 years after LAGB. Composite scores for physical and mental health and scores of each of the eight health domains assessed by the SF-36 survey at baseline (white) and follow-up (gray) are shown. The median is indicated by the horizontal line in the box depicting the interquartile range. Survey scores are standardized to the Australian population mean ± SD of 50 ± 10. A single asterisk and three asterisks indicate P < 0.05 and P < 0.001, respectively. Data for 17 patients were missing at baseline and for two of these patients at follow-up
Discussion
This is the first report of long-term diabetes outcomes following LAGB. Our cohort sustained substantial weight loss over more than a decade, with associated improvements in HbA1c and quality of life. At follow-up, diabetes remission was observed in 11 (18%) out of the 60 participants, with 29 patients (48%) achieving HbA1c ≤ 6.5%. These glucose outcomes are similar to those seen 5 years after sleeve gastrectomy and gastric bypass surgery in randomized trials, in which around 40% of the participants attained HbA1c ≤ 6.5% (5, 6).
The 10-year weight outcomes of our cohort were similar to those seen in the general population of obese patients at our [13] and other [18, 19] LAGB centers and was comparable to the 10-year weight loss observed in obese Swedes with T2D undergoing fixed or adjustable gastric band surgery [20]. This degree of weight loss far exceeded the 6% seen after a decade of intensive lifestyle intervention for overweight and obese people with T2D [2]. The substantial 43% rate of revision surgery over the 12-year follow-up period was nonetheless comparable to that seen in our prior series of obese people [13], but lower than that observed in the other LAGB cohort followed for more than 10 years [18].
Randomized trials of bariatric surgery for T2D [4,5,6] have shown that substantial, sustained weight loss improves HbA1c out to 5 years. We extend these findings by showing glycemic benefit out to 12 years, when HbA1c and fasting glucose improved more than 1% and 1 mmol/L, respectively, despite similar intensity of glucose-lowering therapy. In contrast, lifestyle intervention for weight loss to achieve 6% weight loss at 10 years did not deliver sustained improvement in HbA1c [2]. Greater weight loss may therefore be required to achieve glycemic benefit in the longer term, as suggested by time-course studies of obese people with metabolic syndrome in which more than 11% weight loss was required for the majority to revert from hyper- to normoglycemia [21]. Furthermore, our finding that weight loss after more than a decade is the predominant factor associated with the HbA1c at follow-up is consistent with recent analyses from the Swedish Obese Subjects (SOS) study reporting that the degree of weight loss after bariatric surgery correlated strongly with fasting glucose and diabetes remission after ten or more years [7, 20].
The intensity of glucose-lowering therapy and overall medication use did not change significantly between the baseline and final clinical assessment, a finding consistent with the outcomes of SOS participants with T2D who underwent bariatric surgery [22]. This finding contrasts with clinical trial outcomes showing decreased use of glucose-lowering medications after 5 years [4,5,6], but is consistent with high rates of diabetes relapse following surgical-induced remission [23]. Possible explanations for this discrepancy include differences in study populations and their diabetes management and the shorter duration of follow-up in the clinical trial cohorts. Glucose-lowering medication is therefore probably an important component of long-term diabetes management in “real life” cohorts undergoing bariatric surgery. One implication is that the glycemic and weight loss benefit of bariatric surgery might be improved even further with modern regimens that incorporate newer drugs such as including glucagon-like peptide (GLP) agonists and sodium-glucose co-transporter (SGLT) antagonists.
The reduction in LDL cholesterol of 0.4 mmol/L after a median of 12 years’ follow-up is probably explained by the corresponding increase in statin use because LAGB has no significant effect on LDL cholesterol concentration [4, 16, 24]. Higher use of anti-reflux medication is consistent with reports of increased gastro-oesophageal reflux symptoms following LAGB [25], particularly in patients with proximal dilatation [26], which affected many of the patients who underwent revision surgery. Ongoing innovations in the LAGB technique and post-surgical follow-up may reduce the impact of this significant complication.
Vascular complications of diabetes continued to occur in our patients, albuminuria persisted and eGFR declined significantly at follow-up. These observations suggest factors other than body weight and blood glucose are important determinants of diabetes complications in T2D patients undergoing LAGB surgery. They also highlight the importance of multifactorial medical therapy, which has a proven role in preventing diabetes complications [27].
After a median of 12 years after LAGB surgery, we observed significant improvements in several aspects of quality of life. Randomized trials have shown similar improvements at 5 years [4,5,6], with the Italian RCT [6], which achieved the greatest weight loss (30%), also showing improvement in mental health. More modest weight loss through lifestyle modification did not prevent age-related declines in quality of life in the Look AHEAD study [28], suggesting that sustained weight loss of more than 10% body weight might be needed to preserve and improve quality of life in obese people with T2D. This finding is extremely relevant for health payers because the economic benefit of bariatric surgery for T2D is primarily driven by the quality-adjusted life-year (QALY) gain from weight loss with protection from diabetes complications delivering relatively modest QALY gains and cost savings [20, 29, 30].
Several limitations warrant discussion. This was an uncontrolled study and any conclusions regarding potential benefits or harms of LAGB rely on comparison to earlier studies. Although we strived to follow the entire cohort of 113 patients, weight data were only available for 70% and nearly half did not provide follow-up diabetes outcome data. It is likely that the excluded cohort, enriched for people who had died, had band explant or chronic illness, would have had less substantial weight loss and lower quality of life after 10 years when compared to the included cohort. The generalizability of our findings may also be questioned by the ongoing decline in popularity of LAGB surgery [11] and the evolution of GLP- and SGLT-based therapies, which were not available when our patients underwent LAGB surgery. The degree to which these newer therapies improve diabetes outcomes and weight loss after bariatric surgery is an uncertain but important area of further research. Despite these limitations, it is important to document long-term LAGB outcomes as they are likely to be reproduced by other interventions that primarily restrict caloric intake without altering gut absorption and endocrine function. Furthermore, there is likely to be ongoing use of LABG, particularly in niche areas such as low BMI or the extremes of age.
In summary, after more than a decade, LAGB surgery for obese people with T2D delivered substantial, sustained weight loss that correlated with improved glucose control without significant changes in medication burden. Revision surgery and gastric reflux symptoms are common LAGB complications that should be discussed with candidate patients.
References
American Diabetes A. 7. Obesity management for the treatment of type 2 diabetes. Diabetes Care. 2017;40(Suppl 1):S57–63.
Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54.
Rejeski WJ, Ip EH, Bertoni AG, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366(13):1209–17.
Wentworth JM, Burton P, Laurie C, et al. Five-year outcomes of a randomized trial of gastric band surgery in overweight but not obese people with type 2 diabetes. Diabetes Care. 2017;40(4):e44–e5.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73.
Sjoholm K, Sjostrom E, Carlsson LM, et al. Weight change-adjusted effects of gastric bypass surgery on glucose metabolism: 2- and 10-year results from the Swedish Obese Subjects (SOS) study. Diabetes Care. 2016;39(4):625–31.
Gummesson A, Nyman E, Knutsson M, et al. Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(9):1295–1305.
Puzziferri N, Roshek 3rd TB, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–42.
Arterburn DE, Fisher DP. The current state of the evidence for bariatric surgery. JAMA. 2014;312(9):898–9.
Khorgami Z, Shoar S, Andalib A, et al. Trends in utilization of bariatric surgery, 2010–2014: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2017;13(5):774–8.
Flum DR, Belle SH, Longitudinal Assessment of Bariatric Surgery, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–54.
O'Brien PE, MacDonald L, Anderson M, et al. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013;257(1):87–94.
Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2(7):545–52.
Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2.
Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316–23.
Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133–5.
Himpens J, Cadiere GB, Bazi M, et al. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg. 2011;146(7):802–7.
Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93.
Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304.
Ooi GJ, Doyle L, Tie T, et al. Weight loss after laparoscopic adjustable gastric band and resolution of the metabolic syndrome and its components. Int J Obes. 2017;41(6):902–8.
Keating C, Neovius M, Sjoholm K, et al. Health-care costs over 15 years after bariatric surgery for patients with different baseline glucose status: results from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2015;3(11):855–65.
DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010;6(3):249–53.
Benetti A, Del Puppo M, Crosignani A, et al. Cholesterol metabolism after bariatric surgery in grade 3 obesity: differences between malabsorptive and restrictive procedures. Diabetes Care. 2013;36(6):1443–7.
Naik RD, Choksi YA, Vaezi MF. Consequences of bariatric surgery on oesophageal function in health and disease. Nat Rev Gastroenterol Hepatol. 2016;13(2):111–9.
Chen RY, Burton PR, Ooi GJ, et al. The physiology and pathophysiology of gastroesophageal reflux in patients with laparoscopic adjustable gastric band. Obes Surg. 2017. https://doi.org/10.1007/s11695-017-2662-1.
Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–91.
Rubin RR, Wadden TA, Bahnson JL, et al. Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: the Look AHEAD Trial. Diabetes Care. 2014;37(6):1544–53.
Wentworth JM, Dalziel KM, O'Brien PE, et al. Cost-effectiveness of gastric band surgery for overweight but not obese adults with type 2 diabetes in the U.S. J Diabetes Complicat. 2017;31(7):1139–44.
Hoerger TJ, Zhang P, Segel JE, et al. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care. 2010;33(9):1933–9.
Acknowledgements
We are grateful to our patients for their assistance. This work was supported by a competitive Diabetes Research Grant from Novo Nordisk, through Victorian State Government Operational Infrastructure Support and NHMRC Research Institute Infrastructure Support Scheme.
Author information
Authors and Affiliations
Contributions
JMW conceived the study. PEO, PRB, WAB, and SS provided surgical care and, with JMW, CL and CC collated the data. JMW and CC analyzed the data. JMW drafted the manuscript and all authors reviewed and edited it. JMW is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of Interest
Prof O’Brien and Prof Brown report being affiliated with the Centre for Obesity Research and Education. The Centre has received funding for research purposes from Allergan and Apollo Endosurgery, the manufacturers of the Lap-Band™. The grant was not tied to any specific research project, and neither Allergan nor Apollo Endosurgery has no control of the protocol, analysis and reporting of any studies. CORE also receives a grant from Applied Medical towards educational programs. Prof Brown reports financial support for a bariatric surgery registry from the Commonwealth of Australia, Apollo Endosurgery, Covidien, Johnson and Johnson, Gore and Applied Medical. Since initial submission of this paper, she has also received a speaker’s honorarium from Merck Sharpe and Dohme and a speaker’s honorarium and fees from participation in a scientific advisory board from Novo Nordisk. The Bariatric Registry and the honorariums are outside of the submitted work. Prof O’Brien has written a patient information book entitled “The Lap-Band Solution: A partnership for weight loss” which is given to patients without charge, but some are sold to surgeons and others, for which he receives a royalty. Dr. Burton reports personal fees from Covidien, outside the submitted work. No other author has a conflict of interest to declare.
Statement of Informed Consent
Informed consent was obtained from all individual participants who comprised the ‘Included Group’ in the study.
Statement of Human Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Wentworth, J.M., Cheng, C., Laurie, C. et al. Diabetes Outcomes More than a Decade Following Sustained Weight Loss After Laparoscopic Adjustable Gastric Band Surgery. OBES SURG 28, 982–989 (2018). https://doi.org/10.1007/s11695-017-2944-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2944-7