Abstract
Key message
The common bean locus Co - 4, traditionally referred to as an anthracnose-resistant gene, contains a cluster of predicted receptor-like kinases (COK-4 and CrRLK1-like), and at least two of these kinases are co-regulated with the plant’s basal immunity.
Abstract
Genetic resistance to anthracnose, caused by the fungus Colletotrichum lindemuthianum (Sacc. and Magnus) Briosi and Cavara, is conferred by major loci throughout the Phaseolus vulgaris genome, named Co. The complex Co-4 locus was previously reported to have several copies of the COK-4 gene that is predicted to code for a receptor-like kinase (RLK). In general, plant RLKs are involved in pathogen perception and signal transduction; however, the molecular function of COK-4 remains elusive. Using newly identified molecular markers (PvTA25 and PvSNPCOK-4), the SAS13 marker, COK-4 sequences and phylogeny, and the recently released bean genome sequence, we determined the most probable boundaries of the Co-4 locus: a 325-Kbp region on chromosome Pv08. Out of the 49 predicted transcripts in that region, 24 encode for putative RLKs (including 18 COK-4 copies) with high similarity to members of the Catharanthus roseus RLK1-like (CrRLK1L) protein family from different plant species, including the well-described FERONIA (FER) and ANXUR. We also determined that two RLK-coding genes in the Co-4 locus (COK-4-3 and FER-like) are transcriptionally regulated when bean plants are challenged with the flg22 peptide, a commonly used elicitor of plant immunity, or the bacterium Pseudomonas syringae pv. phaseolicola, the causal agent of halo blight. While COK-4-3 is activated during immune response, FER-like is downregulated suggesting that these genes could play a role in plant responses to biotic stress. These results highlight the importance of dissecting the regulation and molecular function of individual genes within each locus, traditionally referred to as resistance gene based on genetic segregation analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have the innate ability to recognize conserved microbial molecular patterns and establish immune responses that can be triggered by a broad range of pathogens or highly specific to a particular pathogen. These responses can be addressed in two major layers of plant immunity: pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) and effector-triggered immunity (ETI) (Jones and Dangl 2006; Spoel and Dong 2012). PTI is induced by perception of PAMPs through pattern-recognition receptors (PRRs) located at the plant cell surface, and ETI is mediated by resistance (R) genes leading to hypersensitive response (HR). All kinds of phytopathogens can potentially activate PTI and/or ETI (Thomma et al. 2011), which may result in systemic plant responses such as induced systemic resistance (ISR) or systemic-acquired resistance (SAR). These immune responses involve intricate metabolic pathways mediated by several plant hormones, such as jasmonic acid (JA) and salicylic acid (SA) (Thomma et al. 2011).
Among many pathosystems used to study the molecular processes involved in plant–pathogen interaction, Colletotrichum species have long served as models for hemibiotrophic fungal pathogens (O’Connell et al. 2012), being used in early studies to elucidate the role of phytoalexins in common bean (Phaseolus vulgaris L.) resistance to the fungus (Kuć 1982). Besides its scientific importance, common bean is also the most economically important species of the genus Phaseolus and the primary dietary protein source for several populations, mainly in developing countries (Broughton et al. 2003). Colletotrichum lindemuthianum (Sacc. and Magnus) Briosi & Cavara is the causal agent of anthracnose in common bean, one of the most serious diseases in this crop throughout the world—not only because of its seed-borne nature, but also because of the great variability of this pathogen (Melotto et al. 2000). This disease is responsible for great losses of common bean yield (up to 100 %) and, therefore, it is one of the longest studied diseases of this crop (Kelly and Vallejo 2004; Singh and Schwartz 2010; Ferreira et al. 2013).
Understanding resistance against anthracnose is one of the main goals in common bean breeding programs as genetic resistance is the most-efficient and environmentally friendly control of crop diseases (Dodds and Rathjen 2010). Until now, 14 single anthracnose resistance loci (Co-1 to Co-14) and 11 quantitative trait loci (QTL) were discovered in the common bean genome, out of which, 12 loci were identified in bean genotypes of Mesoamerican origin, and nine were identified in genotypes of Andean origin (Kelly and Vallejo 2004; Ferreira et al. 2013). The Co-4 locus, first described in the genotype TO (Fouilloux 1979; Awale and Kelly 2001), confers resistance against several races of C. lindemuthianum (Balardin and Kelly 1998). A second allele, Co-4 2, was identified in the resistant differential cultivar G2333 that possesses a combination of three independent resistance loci, Co-4 2, Co-5, and Co-7 (Young et al. 1998). The single dominant Co-4 2 allele provides greater resistance than the original Co-4, and it is recognized among the broadest-based resistance genes described in common bean (Balardin and Kelly 1998; Silverio et al. 2002). The molecular structure of the different alleles of Co-4 remains to be determined.
The genomic structure of the Co-4 locus has been defined using genetics and genomics tools. Sequencing of the bacterial artificial chromosome BAC 78L17 (Vanhouten and MacKenzie 1999), which was identified with the SAS13 molecular marker (Young et al. 1998), revealed that the Co-4 locus contains several putative orthologs of Pto-like kinase genes, named COK-4 (Melotto and Kelly 2001; Melotto et al. 2004). In silico analysis suggested that COK-4 are the members of the receptor-like kinase (RLK) family that encode proteins with a superfamily kinase domain, including AT-binding and transmembrane domains (Melotto and Kelly 2001).
Receptor-like kinase (RLKs) are important PRRs that play a role in self- and nonself-recognition, including the perception of hormones (Shiu and Bleecker 2001), PAMPs, and pathogen effectors. Several RLK proteins involved in plant immunity have been identified, including Xa21 (Song et al. 1995), Pto (Sessa and Martin 2000), FLAGELLIN SENSING 2 (FLS2) (Chinchilla et al. 2006), BRASSINOSTEROID INSENSITIVE 1-ASSOCIATED KINASE 1 (BAK1) (Chinchilla et al. 2007), among others. FLS2 is one of the well-studied RLKs (Zipfel et al. 2004), which is involved in PTI through the perception of the bacterial PAMP flagellin, acting together with BAK1, to activate downstream immune responses (Chinchilla et al. 2007). Thus, mounting evidence suggests that RLKs are part of basal plant immunity against fungal and bacterial pathogens.
Owing to its similarity to RLKs, we reasoned that COK-4 could be regulated by PAMPs and play a role in basal immunity against other phytopathogens in addition to C. lindemuthianum. We first defined the bean genomic region containing Co-4 using genetic and genomic analyses; the locus is now placed in a 325-Kbp region close to the telomere of the Pv08 chromosome. Out of the 24 RLK-coding genes at the Co-4 locus, 18 showed high nucleotide sequence similarity to the originally identified COK-4 from the bean genotype SEL 1308 (Melotto and Kelly 2001). Functional analysis of two kinases in this locus (referred to as COK-4-3 and FER-like) revealed that they are regulated upon leaf treatment with the PAMP flg22 and infection with Pseudomonas syringae pv. phaseolicola (Pph). These findings suggest that the Co-4 locus not only confers resistance against the anthracnose fungus, but it is also involved in the early stages of PTI in common bean.
Materials and methods
Mapping population and C. lindemuthianum pathogenesis assay
The common bean breeding line SEL 1308 was used as the source of the Co-4 2 allele in a cross with Black Magic, an anthracnose-susceptible black bean cultivar. Hybrid seeds were advanced to the F2 generation, and 98 randomly selected F2 individuals were used as a mapping population (Melotto and Kelly 2001). Plants were grown in controlled environment at 22 °C, 80 % relative humidity, and 16 h of daylight. Ten-day old seedlings were spray inoculated with race 73 of C. lindemuthianum, which is avirulent on bean plants carrying the Co-4 2 allele (Young et al. 1998). Inoculum preparation, inoculation methods, and disease symptoms evaluation were conducted as described by Young and Kelly (1996).
Molecular marker development
Simple sequence repeat (SSR) markers were developed based on the DNA sequence of the BAC 78L17 that was mapped to the Co-4 locus (Melotto et al. 2004). SSRs were searched in the BAC sequence using the SSRIT software (http://www.gramene.org/db/markers/ssrtool; Temnykh et al. 2001). Among the SSR markers (Table S1), PvTA25 showed polymorphism between the SEL 1308 and Black Magic and was used to genotype the F2-segregating population. The PCR reaction (25 μl) consisted of 1.5 mM MgCl2, 1 × enzyme buffer, 200 μM dNTP, 1U Taq polymerase (Promega, Madison, WI), 50 ng DNA, and 25 ng of each primer (Table S2). The PCR cycle was 2 min at 94 °C; plus 13 cycles of 30 s at 94 °C; 30 s at 70 °C (with 1 °C decrease per cycle); 2 min at 72 °C; and 20 cycles of 30 s at 94 °C; 30 s at 57 °C; and 2 min at 72 °C, followed by a final extension cycle of 7 min at 72 °C. PCR products were resolved in 6 % polyacrylamide gel fixed in 1 % acetic acid and 10 % ethanol solution for 10 min, followed by a wash with distilled water for 1 min. The gel was soaked in 1.5 % nitric acid for 3 min and rinsed with distilled water for 1 min. The gel was stained with 0.2 % silver nitrate for 20 min followed by a final wash with distilled water for 30 s each. Developing was conducted with a solution of sodium carbonate (30 g/L) and 37 % formaldehyde (0.54 ml/L). Blocking was performed with 5 % acetic acid.
Amplified fragment length polymorphism (AFLP) markers were identified by means of bulk segregant analysis (BSA; Michelmore et al. 1991). DNA from F2 individuals were bulked in resistant and susceptible pools based on the anthracnose response of each individual. Bulked DNA was digested with EcoRI and MseI restriction enzymes, followed by adaptor ligation, and pre-selective amplification using adaptor-specific primers containing one additional base (Table S2). Selective amplification was performed with primers containing two more random bases. PCR conditions were exactly the same as described by Hazen et al. (2002). Amplicons were resolved in 6 % polyacrylamide gel following the same protocol described for the SSR analysis. Amplicons showing polymorphism between the parents and the bulks were used to genotype the F2 individuals to estimate genetic distance between polymorphic markers and the Co-4 2 allele.
Single nucleotide polymorphism (SNP) markers were developed to assay polymorphisms observed in the COK-4 open reading frame (ORF) sequences of contrasting bean genotypes (Melotto and Kelly 2001). Primers were designed to detect both parental alleles of the COK-4 gene in the F2-mapping population. One forward primer was designed to anneal with both homologs, and two reverse primers were designed to specifically anneal to one of each homolog (Table S2). The PCR was optimized to amplify both homologs in the same reaction to detect heterozygous genotypes. The reaction consisted of 1 × enzyme buffer, 3.5 mM MgCl2, 200 µM dNTP, 1.5 U Taq DNA polymerase (Gibco), 15 ng of forward primer, 15 ng of the Black Magic homolog reverse primer, and 30 ng of the SEL 1308 reverse primer in a 30 µl reaction. The thermocycling profile consisted of one cycle of 94 °C for 4 min, 30 cycles of 94 °C for 10 s, 70 °C for 30 s, and 72 °C for 2 min, followed by an extension cycle of 72 °C for 7 min.
Genetic linkage analysis
In addition to the newly developed markers, individual F2 plants from the Black Magic × SEL 1308 population were also screened with the previously found SCAR markers linked to the Co-4 locus: SAS13 (Young et al. 1998), SBB14, and SH18 (Awale and Kelly 2001). The amplification conditions were the same as described in the respective publications for each marker. Statistical probability for single locus inheritance of molecular markers in the F2 population was estimated with the Chi-square test. A linkage map around the Co-4 locus was obtained using the MAPMAKER 3.0b software (Lander et al. 1987) with thresholds of 3.0 LOD score value and 37.5 centiMorgan (cM) of maximum genetic distance. Loci were ordered using the “order” command, and the final order was tested using the “ripple” command with a window of six markers. Finally, multipoint distance estimates were obtained using the “map” command, with the cM distances among markers, and the Co-4 locus being calculated by the Kosambi mapping function. The linkage map diagram was created using the MapChart 2.2 program (Voorrips 2002).
Physical localization of DNA markers and sequence analysis
All molecular markers with known sequence and tight linkage to Co-4 were used to define the physical location of this locus in the G19833 reference bean genome sequence v1.0 available at Phytozome (http://www.phytozome.net/; Schmutz et al. 2014). The markers used were: PvTA25 and PvSNPCOK-4 from this study; SAS13 (a 978-bp sequence obtained from the genotype SEL 1308; Melotto et al. 2004); SH18 and SBB14 kindly provided by James Kelly and Halima Awale; and SCARY20 (phaseolusgenes ID 548) and SCARC08 (phaseolusgenes ID 334) (Queiroz et al. 2004; http://phaseolusgenes.bioinformatics.ucdavis.edu/). The SEL 1308 COK-4 ORF (NCBI accession number GI:9796477; http://blast.ncbi.nlm.nih.gov/) and the whole sequence of the BAC 78L17 (NCBI accession number GI:38194906) were also aligned to the bean genome. Alignment between DNA marker and the reference genome sequences was performed using BLASTN with default parameters (E-value <1 × 10−5 and identity ≥70 %) to define marker location. In addition, pairwise alignments between each marker and the Co-4 region were performed using the BLASTN tool available at NCBI (bl2seq; Tatusova and Madden 1999) to refine the E-value for each marker. All the predicted transcripts in the Co-4 locus were obtained from the Phytozome website, and the putative functions of the genes were inferred based on Pfam annotations also available through the Phytozome database.
Phylogenetic analysis
First, the top 100 hits of putative paralogs of the COK-4 kinase in common bean were identified using the predicted COK-4 protein from the bean line SEL1308 (COK-4_SEL1308) as query against the common bean proteome database available at Phytozome (BLASTP, threshold E-value ≤ 1 × 10−20 and identity >30 %). These 100 sequences and the SEL 1308 COK-4 were aligned with CLUSTALW as part of the MEGA 5.05 software (Tamura et al. 2011). The conserved catalytic tyrosine kinase domain of these predicted bean kinases was identified by searching the COK-4 protein sequence against the NCBI protein-conserved domain database (CDD) (Marchler-Bauer et al. 2013). The phylogenetic tree was created by means of MEGA 5.05 using the maximum parsimony method, and bootstrap support values were obtained from 1000 replications.
The phylogeny analysis described above was also performed with the top 100 hits of COK-4_SEL1308 against the non-redundant (nr) protein database of all species currently available at NCBI (BLASTP, threshold E-value ≤ 1 × 10−20 and identity >30 %).
Pathogenesis assay
Seeds of the bean genotypes G2333 and the halo blight susceptible control Beluga were germinated on filter paper in a growth chamber at 28 °C and 12-h photoperiod for 3 days. Seedling were transplanted to 1:1:1 v:v:v mixture of growing medium (Redi-earth plug and seedling mix, Sun Gro), fine vermiculite, and perlite and grown in controlled environmental chambers at 28 °C, 60 ± 5 % relative humidity, and a 12-h photoperiod under light intensity of 100 μmol/m2/s. Pseudomonas syringae pv. phaseolicola (Burkn.) Downs (Pph) strain NPS3121 was grown in low-salt Luria–Bertani medium (Katagiri et al. 2002) at 30 °C supplemented with 100 µg/ml rifampicin. Young, fully expanded primary leaves were dip inoculated into 1 × 108 CFU/ml aqueous suspension containing 0.03 % Silwet L-77 (Lehle Seeds Co., Round Rock, TX). Inoculum preparation and bacterial population counts in the leaf apoplast were performed as previously described (Katagiri et al. 2002). Statistical significance of the mean difference between the bean genotypes was detected with two-tailed Student’s t test. Typical disease symptoms were observed and photographed at 7 days after inoculation.
Callose deposition assay
G2333 seeds were germinated and grown as described above. Fully expanded, first trifoliate leaves were syringe infiltrated with 1 µM of flg22 (Alpha Diagnostics, Inc., Santa Monica, CA) or water. Infiltrated leaves were collected 12 and 24 h post-infiltration and incubated in 90 % ethanol at 37 °C in an orbital shaker (30 rpm). After the chlorophyll had been removed, leaves were rinsed in 50 % ethanol followed by a final rinse in water. Cleared leaves were stained with 0.1 µM aniline blue for 30 min and maintained in 50 % glycerol. Images (12–15 per sample) were captured by a Nikon Eclipse 80i fluorescent microscope (Nikon Corporations, Shinagawa-ku, Tokyo) equipped with DAPI filter (358-nm excitation and 461-nm emission) and a digital camera. Callose deposits were counted using the DotCount v1.2 software (Reuter 2012; http://reuter.mit.edu/software/dotcount/), using an image intensity threshold of 100 and dot sizes ranging from 5 to 500. Experiments were performed two times independently.
Gene expression analysis
Gene-specific primers were designed based on the common bean gene sequences from the Phytozome database (Table S3). The efficiency of each primer set was verified using a fivefold serial dilution of G2333 cDNA. Linear regression between the amounts of cDNA template and the corresponding C T values were calculated for each primer set to obtain the correlation coefficient (R 2 > 0.95) according to Schmittgen and Livak (2008).
Fully expanded primary leaves of G2333 were dip inoculated with either 1 × 108 CFU/ml Pph suspension with 0.03 % Silwet or 0.03 % Silwet alone (mock inoculation). Leaves were collected at 6, 12, and 24 h post-inoculation (hpi). In addition, young first trifoliate leaves were immersed in 5 µM flg22 or water control for 30 min. Leaves were maintained in high humidity inside a growth chamber set at 28 °C and 12-h photoperiod, and were collected 6, 12, or 24 h after flg22 treatment. Total RNA was extracted from leaves using the RNAeasy Plant mini kit (Qiagen, Valencia, CA) following the manufacturer’s recommendations. The total RNA was quantified using a NanoDrop spectrophotometer (Thermo 367 Scientific, Rockford, IL).
Reverse transcription (RT) was performed using the Takara RNA PCR kit (Clontech, Montain View, CA), 150 ng/µl of total RNA, and 0.125 µM of oligo-dT primer, according to the manufacturer’s protocol. RT reaction was carried out at 50 °C for 30 min and at 95 °C for 5 min. Quantitative PCR (qPCR) reactions were carried out using 1 µl of cDNA (RT reaction above), 200 mM of each primer (Table S3), and iTaq Fast SYBR green supermix (BioRad, Hercules, CA) reagents in a final volume of 20 µl. qPCR cycles consisted of one cycle of 95 °C for 5 min, 40 cycles of 95 °C for 10 s, 60 °C for 30 s, followed by the dissociation-curve default parameters using the Applied Biosystems 7300 thermocycler (Applied Biosystems, Foster City, CA). The gene expression levels of the treated samples relative to control samples were determined by means of the 2−∆∆CT method (Livak and Schmittgen 2001). The P. vulgaris INSULIN DEGRADING ENZYME (PvIDE; Phvul.001G133200) gene (Borges et al. 2011) was used as the reference gene for the amount of RNA template across different reactions. The genes analyzed were FLS2-like (LRR) (Phvul.005G149200), FLS2-like (RLK) (Phvul.002G196200), COK-4-3 (Phvul.008G026900), FER-like (Phvul.008G030800), NB-LRR (Phvul.008G031200), and FUL-like (Phvul.008G027800). All experiments were performed in three biological replicates, and statistical analysis was conducted using the two-tailed Student’s t test.
Results
Molecular markers define the genetic boundaries of the Co-4 locus
Several markers closely linked to the anthracnose resistance Co-4 locus of common bean have been identified (Kelly and Vallejo 2004; Ferreira et al. 2013); however, the genomic boundaries of this locus were still elusive. Thus, we sought to refine its genetic structure by saturating this locus with new molecular markers and determine the segregation ratios of all possible polymorphic markers using the SEL 1308 × Black Magic F2 population (Melotto and Kelly 2001). A SSR marker was developed based on the sequence of the previously identified clone (BAC 78L17) that spans part of the complex Co-4 locus (Melotto et al. 2004). A 149-bp TA-repeat marker, named PvTA25, showed a co-dominant polymorphism between the parents of the mapping population (SEL 1308 and Black Magic) as well as polymorphisms among bean lines carrying contrasting alleles at the Co-4 locus (Fig. 1a). All three lines known to carry the Co-4-resistant allele (G2333, SEL 1308, and TO) showed the same PvTA25 marker allele, while the susceptible genotypes Black Magic and SEL 1360 shared a PvTA25 DNA fragment of higher molecular weight (Fig. 1a). A dominant single locus AFLP marker, named ETGCMGGT(135), was also identified to be linked to the Co-4 locus, with the presence or the absence of a 135-bp PCR amplicon in the resistant and susceptible genotypes, respectively (Fig. 1b). Finally, an allele-specific SNP marker, named PvSNPCOK-4, was developed by aligning the COK-4 sequences from SEL 1308 and Black Magic. Segregation analysis of this marker revealed its co-dominant nature, in which heterozygous COK-4 individuals carried both the 700-bp susceptible and 1000-bp resistance alleles (Fig. 1c).
Molecular marker banding pattern in resistant and susceptible genotypes of common bean. G2333, SEL 1308, and TO are homozygous anthracnose-resistant genotypes, Black Magic and SEL1360 are homozygous anthracnose-susceptible genotypes. a, b Silver-stained polyacrylamide gel showing polymorphisms (scored bands are indicated by the arrows) detected with the PvTA25 SSR primers (a) and the ETGCMGGT(135) AFLP (b) markers. c Ethidium bromide-stained agarose gel showing PCR-amplified DNA fragments obtained with the PvSNPCOK-4 marker (700-bp lower band and 1000-bp upper band); legends on top of the lanes indicate the genotype of F2 individuals from the cross between SEL 1308 and Black Magic. d Predicted genetic distance among the markers linked to the Co-4 locus. The numbers on the top are estimated distances between molecular markers in centiMorgans (cM) calculated with the MapMaker software considering LOD score >3 as threshold. The linkage map diagram was created using the MapChart 2.2 program (Voorrips 2002) and the scale was set as 10 mm/cM
The PvTA25, AFLP ETGCMGGT(135), and PvSNPCOK-4 markers, as well as the previously identified SAS13 molecular marker, segregated as a single locus in our mapping population as determined by Chi-square statistical analysis (Table 1) and were closely linked to Co-4 as determined by linkage mapping analysis (Fig. 1d). The previously identified SBB14 and SH18 markers (Awale and Kelly 2001) were also tested in our segregating population, and they showed a low Chi-square P value, indicating that they might not be a single locus (Table 1); nonetheless, both were found to be linked to Co-4 based on linkage mapping analysis (Fig. 1d). The genetic order of all markers around the Co-4 locus was estimated using the genotypic and phenotypic data of F2 individuals. SAS13, PvTA25, and PvSNPCOK-4 were the closest markers to Co-4, all within 0.7 cM from each other, whereas ETGCMGGT(135) and SBB14 markers mapped at 6.6 cM away from Co-4, and SH18 mapped at 10.4 cM away from Co-4 (Fig. 1d).
Physical location of markers linked to Co-4 in the bean genome
The farthest marker away from the Co-4 locus, SH18, showed sequence similarity with many regions in different chromosomes (1 × 10−149 ≤ E-value ≤ 1 × 10−142), including Pv08 at the 54,381,505–54,382,406 region (Fig. 2). The markers SCARY20 and SCARC08, previously mapped at 1.2 and 7.8 cM away from the Co-4 locus for the TO genotype, respectively (Queiroz et al. 2004), show the best alignment scores (BLASTN E-value = 0.0) on Pv08 at positions 28,034,637–28,034,904 and 7,414,123–7,415,017, respectively (Fig. 2). These markers also aligned with different regions on Pv08 as well as other bean chromosomes (0.0 ≤ E-value ≤ 1 × 10−134). SH18, SCARY20, and SCARC08 were located at genome regions with no predicted coding sequences. The SBB14 marker sequence, however, was found at the position 2,809,493–2,810,488 of Pv08 (BLASTN E-value = 0.0), approximately 240 Kbp away from the Co-4 locus (Fig. 2), in the 5′UTR from the Phvul.008G033800, a predicted amylase gene, and does not have significant similarity to any other region of the bean genome.
Genomic boundaries and structure of the bean anthracnose resistance locus Co-4. a Genome browser representation of the 325 Kbp in the chromosome 8 (Chr08) spanning the Co-4 locus that contains the markers SAS13, PvTA25, and PvSNPCOK-4, as well as the BAC 78L17 sequences. Physical location was determined by BLASTN analysis (threshold E-value ≤ 1 × 10−5) of the marker sequences against the common bean genome v.1.0 (Schmutz et al. 2014; http://www.phytozome.net/). b Predicted transcripts in Co-4 region are shown below their genomic positions in (a). Color codes as indicated in the legend below the figure, represent predicted gene functions. Asterisks above the transcript indicate genes that were analyzed by RT-qPCR
Consistently, the tightly linked PvTA25, PvSNPCOK-4, and SAS13 marker sequences were also found at unique regions of the Pv08 chromosome (Fig. 2). SAS13 is located in the Phvu1.008G028500 gene (BLASTN E-value = 0.0), PvTA25 is 650 bp away from the Phvu1.008G029500 gene (BLASTN E-value = 4 × 10−40), and the PvSNPCOK-4 primers align within the Phvul.008G028400 gene (BLASTN E-value = 1 × 10−7), with a predicted DNA fragment of 724 bp, similar to the one amplified from Black Magic (Fig. 1C). None of these markers aligned at a different genomic location, confirming the single locus segregation data analysis (Table 1; Awale and Kelly 2001; Melotto and Kelly 2001).
Previously, the BAC 78L17 has been physically located at the chromosome Pv08 as revealed by FISH analysis (Melotto et al. 2004). BLASTN analysis of the BAC 78L17 against the common bean genome v1.0 (Schmutz et al. 2014) located this BAC in the 2,345,000–2,464,000 region of Pv08 (BLASTN E-value = 0.0). BLASTN alignment between the previously identified COK-4 gene sequence from SEL 1308 (Melotto et al. 2004), and the bean genome revealed 20 significant hits on Pv08 (E-value ≤ 1 × 10−64 and identity >70 %; Table 2), extending our previous description of the BAC 78L17 region that contains 10 sequences with similarity to COK-4 (Fig. 2). Out of the 20 hits, 18 lie on a genomic region with predicted transcripts encoding a tyrosine kinase domain (Table S4). The COK-4 copies were numbered according to their order of location in the genome (Table 2). Outside the Pv08 chromosome, only one match to the SEL 1308 COK-4 gene was found on chromosome Pv05 (Table 2), in a region with no predicted transcript. Furthermore, none of the genetic markers tightly linked to Co-4 were found on chromosome Pv05; thus, we have not considered it as a possible location for this anthracnose-resistant locus. Thereby, we predict that the Co-4 locus is the most likely to be at the 325-Kbp region (Chr08:2,245,000–2,570,000) adjacent to the Pv08 telomere (Richard et al. 2013) based on genetic linkage (Fig. 1), the genomic location (Fig. 2) of the markers closely linked to Co-4 (SAS13, PvTA25, and PvSNPCOK-4), and the presence of multiple potential COK-4 paralogs in that region (Fig. 2; Table 2).
The Co-4 locus is enriched with putative kinases of the CrRLK1L family
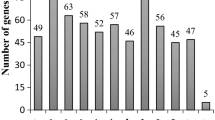
Once the 325-Kbp region on Pv08 (Chr08:2,245,000–2,570,000) was determined as the most likely one to contain the Co-4 locus, we sought to characterize its gene content. Forty-nine transcripts were identified (Fig. 2 and Table S4) with the support of expression data obtained with RNA-seq and EST analyses (Phytozome). Function annotation of the transcripts revealed three putative transcription factors located next to each other in the chromosome (two SRF-type transcription factors and one Myb-like domain), three DSBA-like (disulfide oxidoreductase-like), three COBRA-like, one NB-LRR (Nucleotide Binding–Leucine-Rich Repeat) domain-encoding gene, eleven genes showing various putative functions, and four with unknown functions (Table S4). Twenty-four transcripts in the Co-4 region are predicted to encode protein kinases with significant similarity to the predicted SEL 1308 COK-4 protein (BLASTP E-value ≤ 2 × 10−31; Table S5). Four of the COK-4 gene copies showed the highest similarity (BLASTP E-values = 0.0) to the protein COK-4 from SEL 1308: Phvul.008G028300 (identity = 81.5 %), Phvul.008G028400 (identity = 78.4 %), Phvul.008G028500 (identity = 83.4 %), and Phvul.008G028600 (identity = 84.0 %) (Table S5). All predicted kinases in the Co-4 region showed significant similarity with members of the Arabidopsis CrRLK1L family: FERONIA (FER), ANXUR2 and AT5G39000 (Table S5). In addition, BLASTP analysis of COK-4_SEL1308 against the nonredundant database of NCBI showed high similarity with CrRLK1L family members from different plant species, with FER and ANXUR being also overrepresented (Table S6). Phylogenetic analysis of these proteins showed that COK-4 forms a major clade with serine/threonine kinases from Glycine max, Cicer arietinun, Lotus japonicus, Theobroma cacao, and Malus domestica, as well as Pto-like proteins from three Solanum species and Capsicum chinense (Fig. 3).
The COK-4_SEL 1308-predicted protein clustered with members of the Catharanthus roseus RLK1-like (CrRLK1L) protein family (mainly FERONIA-like and ANXUR-like) from diverse plant species. Phylogenetic analysis was performed with the maximum parsimony method using the MEGA 5.05 software (Tamura et al. 2011). Bootstrap support values are adjacent to the tree nodes
Among the 24 putative kinases in the Co-4 locus, 20 are predicted to encode a single kinase domain protein, and four are predicted to encode both a kinase and a malectin domain (Table S5). Malectin is an endoplasmic reticulum membrane-anchored domain, and is found in proteins of the CrRLK1L family (Kessler et al. 2010), among other protein families. Three of these proteins located toward the right edge of the Co-4 locus (Fig. 2b) showed high similarity with the Arabidopsis CrRLK1L family member, FER (BLASTP E-value = 0.0; Table S5). The fourth putative protein with a kinase and malectin domain, encoded by Phvul.008G030200, is similar to a malectin/receptor-like protein kinase from Arabidopsis with no specific function having been established yet (Table S5).
Phylogeny analysis indicates evolutionary relationship between the kinase proteins encoded by genes in the Co-4 locus and COK-4
A large number of copies of the COK-4 genes in the region of the Co-4 resistance locus indicates that gene duplication events may have taken place in this region during the course of the common bean evolution, resulting in the genetic and phenotypic variations observed among bean lines such as TO and G2333 (Long et al. 2013). Thus, the phylogenetic relationship of proteins similar to COK-4 using the common bean proteome was investigated.
Owing to its highly conserved kinase domain, the COK-4_SEL1308 protein showed significant similarity to protein kinases throughout the common bean genome, including the kinases at the Co-4 locus. Therefore, these first 100 best hits identified by BLASTP (E-value ≤ 2 × 10−37) were used to identify the ones that formed a single clade with COK-4_SEL1308. All of these proteins contain a kinase domain annotated as belonging to the protein superfamily PTKc cd14066 conserved kinase domain (NCBI-conserved domain database), which was considered to perform the phylogeny analysis (Fig. S1). One of the predicted kinases on Co-4 locus (Phvul.008G029800) showed low similarity to the SEL 1308 COK-4 protein, and it was not in the best 100 kinase matches used for the phylogeny analysis. Also, another kinase at the Co-4 locus formed a cluster close to the putative COK-4 paralogs (Fig. S1); however, its encoding gene (Phvul.008G031100.1) was not considered a COK-4 copy as it does not have significant nucleotide similarity to the SEL 1308 COK-4 gene. Interestingly, all 18 COK-4 copies located on the Co-4 locus (Table 2; Table S5) formed a single cluster with COK-4_SEL 1308 (Fig. 4). Four proteins shown to be the closest related to COK-4 form SEL 1308, forming a small sub-clade, which included Phvul.008G028300, Phvul.008G028400, Phvul.008G028500, and Phvul.008G028600 (Fig. 4), confirming the BLASTP results. The four kinases predicted to encode a malectin-kinase protein (Table S5) also formed a single cluster with other RLK proteins from Pv04 (Fig. S1). These results indicate that the kinases present at the Co-4 locus are closer related to each other than they are to other kinases in the bean genome.
The COK-4_SEL 1308-predicted protein clustered with common bean kinases located in the Co-4 genomic region of Pv08. Phylogenetic analysis of predicted amino acid sequence was performed with the maximum parsimony method using the MEGA 5.05 software (Tamura et al. 2011). Bootstrap support values are provided adjacent to nodes. The diagram shown in front of the transcript name represents the single kinase domain (gray rectangles) within the protein. The numbers indicate the total amino acids of each protein. Diagram was adapted from the protein domain view of Phytozome. Only the clade containing the COK-4 is shown (refer to Fig. S1 for the entire tree with the top 100 kinases most similar to COK-4_SEL1308 in the G19833 reference genome)
Genes in the Co-4 locus are transcriptionally regulated during common bean innate immune response
Phylogeny and BLAST analyses indicate that the majority of putative proteins in the Co-4 locus are similar to CrRLK1L proteins as described above. Members of the CrRLK1L family, such as FER, ANXUR, HERCULES, and THESEUS are known to be involved in plant growth and reproduction (Lindner et al. 2012), but recent results have shown that FER, in particular, is involved also in PAMP-triggered immunity (Keinath et al. 2010). Thus, we reasoned that the predicted kinases at the Co-4 locus could be regulated by pathogens other than C. lindemuthianum as originally identified, and play a role in broad immune response. To test this hypothesis, we used the flg22 peptide found at the N-terminus of bacterial flagellin, which is typically used to assess the PTI response in plants, such as Arabidopsis, Lotus japonicus, and common bean (Navarro et al. 2004; Hou et al. 2011; Lopez-Gomez et al. 2011).
First, we determined whether flg22 could induce PTI in G2333 by assessing callose deposition in treated leaves, a hallmark PTI response in plants (Boller and Felix 2009; Hou et al. 2011). In fact, G2333 leaves showed high numbers of callose deposits 12-h post infiltration. The number of callose deposits decreased after 24 h after flg22 treatment; nonetheless, it was still higher than that of the water control (Fig. 5a and b). To further confirm that flg22 can trigger defense responses in G2333, the expressions of two putative Arabidopsis FLS2 orthologs in beans were assessed. The FLS2-like (LRR) (Phvul.005G149200) is predicted to have only the leucine-rich repeat (LRR) domain, while the FLS2-like (RLK) (Phvul.002G196200) has both LRR and kinase domains similar to FLS2 (Zipfel et al. 2004). FLS2-like (RLK) is the protein with the highest similarity (BLASTP E-value = 0.0 and 44 % identity) to the Arabidopsis FLS2 in the bean reference genome. In agreement with the induction of PTI assessed by callose deposition, both FLS2-like (LRR) and FLS2-like (RLK) were significantly induced by flg22. The upregulation of the FLS2-like (RLK) gene lasted until 24 h after flg22 treatment (Fig. 5c).
G2333 responses to the PAMP flg22. a Graph shows the average number of callose deposits per mm−2 of G2333 leaf tissue infiltrated with 1 µM flg22 or water. Results are shown as average of 108–135 images in three independent biological replicates ± standard error. b Representative images (×100 magnification) of aniline blue-stained G2333 leaves at 12 or 24 h post-incubation (hpi) with flg22 or water. c Expression of the indicated genes (x-axis) in G2333 leaves immersed in 5 µM flg22 at 6, 12, and 24 hpi relative to the their expression in water-immersed leaves (control) considered as 1. Data points are average values of at least two biological replicates (n ≥ 6 ± standard error). Asterisks above the bars of all graphs indicate statistical significance calculated with Student’s t test (**p < 0.01, ***p < 0.001)
Next, primers were designed for all genes in the Co-4 locus, including all COK-4 copies; however, gene-specific and/or efficient primers could not be obtained for all of them. Thus, we were able to select four genes, which represented different functions in the locus and for which gene-specific and efficient RT-qPCR primers could be designed, to test their expressions after flg22 treatment. Three of them were found to be modulated by flg22: COK-4-3 (Phvul.008G026900) was significantly induced at 24 hpi, while FER-like (Phvul.008G030800) and the putative transcription factor FUL-like (Phvul.008G027800) showed transient repressions at early time points and returned to basal levels at 24 hpi (Fig. 5c). Finally, the only NB-LRR domain-encoding gene found at the Co-4 locus (Phvul.008G031200) was not responsive to flg22 treatment (Fig. 5c).
To determine whether live bacteria also regulate the expression of these genes, G2333 plants were inoculated with the bacterium P. syringae pv. phaseolicola (Pph). The G2333 seems to be tolerant to Pph, as these plants supported a large bacterial population in their leaf apoplast since the first day after inoculation (Fig. 6a), and yet, no symptoms were observed even after 7 days post inoculation (Fig. 6b). By contrast, the susceptible cultivar Beluga supported high bacterial titers in the apoplast and showed typical halo blight symptoms later in the infection cycle (Fig. 6a, b). Analysis of gene expression in the inoculated G2333 plants revealed repressions of both FLS2-like (LRR) and FLS2-like (RLK) genes as bacterial infection progressed (i.e., 12 and 24 hpi), suggesting a low level of defense response that correlated well with high bacterial titer in the leaves. Similarly, SEL 1308 incompatible response to C. lindemuthianum involved the repression of PTI pathway and downregulation of the FLS2-like (LRR) after fungus infection (Oblessuc et al. 2012). The COK-4-3 gene was also downregulated as early as 6 hpi, returning to normal levels at 24 hpi. In contrast, the FER-like gene was upregulated after Pph infection, also returning to normal levels after 24 hpi (Fig. 6c).
G2333 responses to Pseudomonas syringe pv. phaseolicola (Pph). a G2333 showed tolerance to Pph (NPS3121), with no bacterial growth in the leaf apoplast of fully expanded primary leaves dip inoculated with 1 × 108 CFU/ml. b Halo blight symptoms were observed 7 days after inoculation only in the Beluga genotype. c Expression of the indicated genes (x-axis) in G2333 leaves dipped in 108 CFU/ml of Pph relative to the their expression in mock-inoculated leaves (control) considered as 1. Data points are averages of at least two biological replicates (n ≥ 6 ± standard error). Asterisks above the bars of all graphs indicate statistical significance calculated with Student’s t test (**p < 0.01, ***p < 0.001)
The putative transcription factor FUL-like showed no change in transcript levels in response to Pph, while the NB-LRR domain coding gene was slightly repressed in the initial phase of Pph infection (6 hpi), maintaining normal levels after 12 hpi (Fig. 6c). Altogether, these results suggest that the Co-4 locus is involved in basal immunity as evidenced by the two kinases that are inversely regulated in plants undergoing immune response (i.e., flg22 treatment) or infected with a phytopathogen (i.e., large Pph population in the leaves). In addition, our results suggest that other genes in the Co-4 locus (transcription factors and NB-LRR-resistance gene analogs) might be involved with either resistance or susceptibility as they are only regulated by either flg22 or Pph infection.
Discussion
Plant responses to pathogens implicate in drastic changes in host genes expressions, and protein turnover resulted from pathogen recognition and activation/inactivation of a complex chain of metabolic pathways. Overall, the final outcome of the plant response is resistance or susceptibility to the pathogen (Spoel and Dong 2012). Understanding these molecular mechanisms involved in plant immunity is crucial for crop improvement. In the present study, we have determined the most probable location of the Co-4 locus of common bean, assessed the phylogenetic relationship of the predicted kinases found in the locus, and provided genetic evidence that Co-4 may have a role in basal immunity in addition to the originally assigned function of its resistance to anthracnose.
The genomic structure of Co-4 was first analyzed through the molecular mapping of new markers linked to anthracnose resistance. Linkage analysis showed that the newly developed markers PvTA25 and PvSNPCOK-4, as well as SAS13 are closely linked to each other and to the Co-4 resistance gene. The identified genetic distance between the markers, however, may be overestimated due to inherent restrict recombination frequency observed in small mapping populations (Liu 1998), such as the one used here. Thus, these markers could be physically closer to each other than the genetic linkage analysis predicted. Indeed, PvTA25, PvSNPCOK-4, and SAS13 markers were located in a small interval also covered by the BAC 78L17 in the common bean chromosome Pv08, confirming that they form a unique locus in the genome. In addition, mismatches between the marker primer sequences and the genome of the bean genotype G19833 indicate that these three markers either could not be amplified by PCR in this bean line or would show different allele size for the PCR amplicon, supporting the linkage of these markers to the resistant allele of the Co-4 locus.
The G19833 common bean genotype is resistant to some races of C. lindemuthianum, such as races 521, 3481, 3977 and 3993, but seems not to contain a resistant allele of the Co-4 locus (Kelly and Vallejo 2004; Ferreira et al. 2013). Our results revealed that the newly identified markers PvTA25 and PvSNPCOK-4 amplify the susceptible alleles of the bean line Black Magic showing their transferability across bean genotypes in addition to their being easily assayed with PCR and gel electrophoresis. Thus, these markers may be important tools to be applied in molecular breeding for the development of cultivars containing the resistant allele in the Co-4 locus.
Genetically linked markers together with the recent release of the common bean genome (Schmutz et al. 2014) enabled us to further refine the genomic structure of the Co-4 locus. In addition to the markers PvTA25, PvSNPCOK-4, SAS13 (Young et al. 1998), all publicly available sequences linked to Co-4 were located in the common bean genome including the markers SBB14, SH18 (Awale and Kelly 2001), SCARY20 and SCARC08 (Queiroz et al. 2004), as well as the BAC 78L17 and the COK-4 gene (Melotto and Kelly 2001; Melotto et al. 2004). With the results of this alignment analysis, the most probable region for the locus containing the functional resistance gene was determined to be a 325 Kbp-long sequence at the end of chromosome 8 (Pv08) of the common bean reference genome (G19833 genotype; Schmutz et al. 2014).
Interestingly, this region contains 18 copies of the COK-4 coding sequence originally identified by Melotto and Kelly (2001), extending the physical boundaries of the Co-4 locus considerably beyond the BAC 78L17. That BAC, isolated from the bean cultivar Sprite, was reported to have five COK-4 kinases (Melotto et al. 2004). However, the corresponding region in the G19833 genome contains ten COK-4 kinases confirming the evolutionary complexity of the locus, where the number of putative COK-4 paralogs varies according to the bean genotype. Furthermore, all of the COK-4 coding sequences found on the bean reference genome are transcribed into RNA, as confirmed by RNA-seq and EST mapping analysis (Schmutz et al. 2014), suggesting that they are all active genes. A single COK-4 related sequence was found in another chromosome (Pv05); however, it may not be functional as no COK-4 transcript mapped to Pv05. The putative COK-4 parologs may collectively contribute to Co-4-based resistance, or at least, one of these genes might be the single functional Co-4- resistance gene. These alternatives remain to be experimentally validated.
Previously, COK-4 was regarded as a Pto-like gene, in which all the COK-4 homologs studied formed a cluster with the Pto protein of tomato (Melotto et al. 2004). Surprisingly, the present analysis showed that the a majority (65.2 %) of putative COK-4 kinases have the highest similarity to the RLK FERONIA (FER, At3g51550) of Arabidopsis, in addition to FER-like proteins from other species, including tomato. Other members of the CrRLK1L kinase subfamily, such as ANXUR, were also found to be similar to COK-4. COK-4 seems to be closely related to Pto-like kinases of various Solanum species. This apparent discrepancy between previous and current analyses may be due to the much smaller database available at the time of the first study. In addition, COK-4 may have clustered with Pto-like protein from Solanum ssp. because these plants are not as well studied as Solanum lycopersicum and fully sequenced and/or annotated genomes are still not available. This close phylogenetic relationship among the COK-4 kinases and only two members of the CrRLK1L family, FER and ANXUR, suggests that the COK-4-encoding gene underwent extensive duplication that may or may not have retained the kinase function. Further biochemical analyses are needed to verify the activity of COK-4 proteins in Pv08.
In plants, genes located in the same region of the genome can be involved in the same pathway and be coregulated, forming operon-like gene clusters (Zmasek and Godzik 2011; Boycheva et al. 2014). The Co-4 locus might be one such example where COK-4 paralogs and FER-like genes may have evolved from similar functions, and some other genes in this locus, such as the FUL-like gene, may be involved in the same pathway. The FUL-like (FRUITFULL-like) is a putative MADS box transcription factor, and its ortholog in Arabidopsis (AT5G60910) is an AGAMOUS-like 8 called FRUITFULL (FUL) because of its involvement in the control of flowering time, fruit development, and determinacy (Pabón-Mora et al. 2012). In addition, the soybean MADS-box transcription factor modulates floral organ numbers, petal identity, and sterility (Huang et al. 2014). Here, the common bean FUL-like was co-regulated in the same direction as FER-like during PTI induction, suggesting that these genes may have roles in the same response pathway.
In general, CrRLK1L family members seem to be involved, at least in part, in the modulation of reactive oxygen species (ROS) production to regulate cell growth in different developmental stages and hormone signaling pathways, such as ethylene (ET), jasmonate (JA), and salicylic acid (SA), after cell wall damage perception (Wolf et al. 2012). ROS production and activation of ET, JA, and SA pathways are well-known plant responses to pathogens; thus, CrRLK1L family members are potential membrane receptors that could be active during plant–pathogen interaction. In fact, FER is one member of CrRLK1L family that has been associated with plant immunity so far (Kessler et al. 2010).
The striking similarity between COK-4 and FER prompted us to check whether the Co-4 locus could be involved in the bean innate immunity. In conditions where bean immunity was activated, i.e., flg22 treatment, we observed that expression of the COK-4-3 was significantly upregulated along with both FLS2-like genes. On the other hand, the FER-like gene was strongly repressed during PTI, that is, at the same time points of induction of FLS2-like and high callose deposition after flg22 treatment. These data suggest that both COK-4-3 and FER-like genes play distinct roles in PTI responses. While COK-4-3 may be a positive regulator of PTI, the FER-like gene on the Co-4 locus may be involved in repression of PTI. To further test this hypothesis, the expression of these genes was assessed in bean plants that are tolerant to the bean pathogen Pph (i.e., these plants are symptomless but support high bacterial population in their leaves typical of susceptible interactions). At the same time that the bacterial population was high and FLS2-like genes were repressed in these plants indicating low level of PTI, COK-4-3 was also repressed, and FER-like was upregulated. Taken together, these findings provide strong evidence that both COK-4 and FER-like may be involved in the basal immune response to different pathogens.
COK-4 and FER may have a common evolutionary origin, but they might have assumed different functions by either COK-4 losing the malectin domain or FER gaining that domain. In cases where evolutionary events in eukaryotes that distinguish a protein from its closest ancestor have been studied, it has been found that domain loss is more common than domain gain and the exchange of a domain is rare (Björklund et al. 2005; Zmasek and Godzik 2011). Thereby, COK-4 may be a PTI defense-response activator, while FER-like may act as PTI inhibitor. Additional studies on the evolution of new biochemical functions emerging in the Co-4 locus through the FER-like and COK-4 genes should further the current understanding of the molecular pathways underlying bean immunity against a broad range of pathogens. Nonetheless, our results advanced knowledge toward achieving that goal by establishing the boundaries of the Co-4 locus, providing additional markers for molecular breeding as new tools for employing anthracnose resistance in beans, and reinforcing the role of the putative COK-4 kinases in common bean basal immunity.
Author contribution statement
Performed experiments: CF, PRO, MM. Analyzed data: PRO, MM. Conceived and coordinated the project: MM. Wrote the manuscript: PRO, MM. All authors have read and approved the final version of the manuscript.
References
Awale HE, Kelly JD (2001) Development of SCAR markers linked to Co-4 2 gene in common bean. Annu Rep Bean Improv Coop 44:119–120
Balardin RS, Kelly JD (1998) Interaction between races of Colletotrichum lindemuthianum and gene pool diversity in Phaseolus vulgaris. J Am Soc Hortic Sci 123:1038–1047
Björklund ÅK, Ekman D, Light S, Frey-Skött J, Elofsson A (2005) Domain rearrangements in protein evolution. J Mol Biol 353(4):911–923. doi:10.1016/j.jmb.2005.08.067
Boller T, Felix G (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60:379–406. doi:10.1146/annurev.arplant.57.032905.105346
Borges A, Tsai SM, Caldas DGG (2011) Validation of reference genes for RT-qPCR normalization in common bean during biotic and abiotic stresses. Plant Cell Rep 5:827–838. doi:10.1007/s00299-011-1204-x
Boycheva S, Daviet L, Wolfender JL, Fitzpatrick TB (2014) The rise of operon-like gene clusters in plants. Trends in Plant Sci 7:447–459. doi:10.1016/j.tplants.2014.01.013
Broughton WJ, Hernandez G, Blair M, Beebe S, Gepts P, Vanderleyden J (2003) Beans (Phaseolus spp.)—model food legumes. Plant Soil 252:55–128. doi:10.1023/A:1024146710611
Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18:465–476. doi:10.1105/tpc.105
Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JDG, Felix G, Boller T (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448:497-U412. doi:10.1038/nature05999
Dodds PN, Rathjen JP (2010) Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet 11(8):539–548. doi:10.1038/nrg2812
Ferreira JJ, Campa A, Kelly JD (2013) Organization of genes conferring resistance to anthracnose in common bean. In: Varshney RK, Tuberosa R (eds) Translational genomics for crop breeding, 1st edn. Wiley, Blackwell, pp 151–182
Fouilloux G (1979) New races of bean anthracnose and consequences on our breeding programs. In: Maraitre H, Meyer JA (eds) Disease of tropical food crops. Université Catholique de Louvain la Neuve, Belgium, pp 221–235
Hazen SP, Leroy P, Ward R (2002) AFLP in Triticumaestivum L.: patterns of genetic diversity and genome distribution. Euphytica 125:89–102. doi:10.1023/A:1015760802026
Hou S, Mu R, Ma G, Xu X, Zhang C, Yang Y, Wu D (2011) Pseudomonas syringae pv. phaseolicola effector HopF1 inhibits pathogen-associated molecular pattern-triggered immunity in a RIN4-independent manner in common bean (Phaseolus vulgaris). FEMS Microbiol Lett 323(1):35–43. doi:10.1111/j.1574-6968.2011.02356.x
Huang F, Xu G, Chi Y, Liu H, Xue Q, Zhao T, Gai J, Yu D (2014) A soybean MADS-box protein modulates floral organ numbers, petal identity and sterility. BMC Plant Biol 14(1):89. doi:10.1186/1471-2229-14-89
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444(7117):323–329. doi:10.1038/nature05286
Katagiri F, Thilmony R, He SY (2002) The Arabidopsis thaliana-Pseudomonas syringae interaction. In: Somerville CR, Meyerowitz EM (eds) The Arabidopsis book. American Society of Plant Biologists, Rockville, pp 1–35. doi:10.1199/tab.0039
Keinath NF, Kierszniowska S, Lorek J, Bourdais G, Kessler SA, Shimosato-Asano H, Panstruga R (2010) PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J Biol Chem 285(50):39140–39149. doi:10.1074/jbc.M110.160531
Kelly JD, Vallejo VA (2004) A comprehensive review of the major genes conditioning resistance to anthracnose in common bean. Hortic Sci 39(6):1196–1207
Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, Grossniklaus U (2010) Conserved molecular components for pollen tube reception and fungal invasion. Science 330(6006):968–971. doi:10.1126/science.1195211
Kuć J (1982) Induced immunity to plant disease. Bioscience 32(11):854–860. doi:10.2307/1309008
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181. doi:10.1016/0888-7543(87)90010-3
Lindner H, Müller LM, Boisson-Dernier A, Grossniklaus U (2012) CrRLK1L receptor-like kinases: not just another brick in the wall. Curr Opin Plant Biol 15(6):659–669. doi:10.1016/j.pbi.2012.07.003
Liu BH (1998) Statistical genomics: linkage, mapping and QTL analysis. CRC Press, Cleveland 611
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Long M, VanKuren NW, Chen S, Vibranovski MD (2013) New gene evolution: little did we know. Annu Rev Genet 47:307–333. doi:10.1146/annurev-genet-111212-133301
Lopez-Gomez M, Sandal N, Stougaard J, Boller T (2011) Interplay of flg22-induced defence responses and nodulation in Lotus japonicus. J Exp Bot. doi:10.1093/jxb/err1291
Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Shennan Lu, Marchler GH, Song JS, Thanki N, Yamashita RA, Zhang D, Bryant SH (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res 41(D1):D384-52. doi:10.1093/nar/gks1243
Melotto M, Kelly JD (2001) Fine mapping of the Co-4 locus of common bean reveals a resistance gene candidate, COK-4, that encodes for a protein kinase. Theor Appl Genet 103(4):508–517. doi:10.1007/s001220100609
Melotto M, Balardin RS, Kelly JD (2000) Host-pathogen interaction and variability of Colletotrichum lindemuthianum. In: Prusky D, Freeman S, Dickman MB (eds) Colletotrichum host specificity, pathology, and host-pathogen interaction. APS Press, St Paul, pp 346–361
Melotto M, Coelho MF, Pedrosa-Harand A, Kelly JD, Camargo LEA (2004) The anthracnose resistance locus Co-4 of common bean is located on chromosome 3 and contains putative disease resistance-related genes. Theor Appl Genet 109(4):690–699. doi:10.1007/s00122-004-1697-6
Michelmore RW, Paran J, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregation populations. Proc Natl Acad Sci 88:9828–9832. doi:10.1073/pnas.88.21.9828
Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S, Boller T, Jones JDG (2004) The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol 135:1113–1128. doi:10.1104/pp.103.036749
O’Connell RJ, Thon MR, Hacquard S et al (2012) Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat Genet 44(9):1060–1065. doi:10.1038/ng.2372
Oblessuc PR, Borges A, Chowdhury B, Caldas DGG, Tsai SM, Camargo LEA, Melotto M (2012) Dissecting Phaseolus vulgaris innate immune system against Colletotrichum lindemuthianum infection. PLoS ONE 7(8):e43161. doi:10.1371/journal.pone.0043161
Pabón-Mora N, Ambrose BA, Litt A (2012) Poppy APETALA1/FRUITFULL orthologs control flowering time, branching, perianth identity, and fruit development. Plant Physiol 158(4):1685–1704. doi:10.1104/pp.111.192104
Queiroz VT, Sousa CS, Costa MR, Sanglad DA, Arruda KMA, Souza TLPO, Ragagnin VA, Barros EG, Moreira MA (2004) Development of SCAR markers linked to common bean anthracnose resistance genes Co-4 and Co-6. Annu Rep Bean Improv Coop 47:249–250
Reuter M (2012) Image analysis: dot count. DotCount v1.2. http://reuter.mit.edu/software/dotcount/
Richard MMS, Chen NWG, Thareau V, Pflieger S, Blanchet S, Pedrosa-Harand A, Iwata A, Chavarro C, Jackson SA, Geffroy V (2013) The subtelomerickhipu satellite repeat from Phaseolus vulgaris: lessons learned from the genome analysis of the andean genotype G19833. Front Plant Sci 4:109. doi:10.3389/fpls.2013.00109
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3(6):1101–1108. doi:10.1038/nprot.2008.73
Schmutz J, McClean PE, Mamidi S et al (2014) A reference genome for common bean and genome-wide analysis of dual domestications. Nat Genet 46:707–713. doi:10.1038/ng.3008
Sessa G, Martin GB (2000) Signal recognition and transduction mediated by the tomato Pto kinase: a paradigm of innate immunity in plants. Microb Infec 2(13):1591–1597
Shiu SH, Bleecker AB (2001) Plant receptor-like kinase gene family: diversity, function, and signaling. Sci STKE 113:re22. doi:10.1126/stke.2001.113.re22
Silverio L, Vidigal MC, Vidigal Filho PS, Barelli MAA, Thomazella C, Nunes WMC (2002) Genetic resistance to Colletotrichum lindemuthianum race 2047 in G2333. Annu Rep Bean Improv Coop 45:74–75
Singh SP, Schwartz HF (2010) Breeding common bean for resistance to diseases: a review. Crop Sci 50(6):2199. doi:10.2135/cropsci2009.03.0163
Song WY, Wang GL, Chen L, Kim HS, Pi LY, Gardner J, Wang B, Holsten T, Zhai WX, Zhu LH, Fauquet C, Ronald PC (1995) A receptor kinase-like protein encoded by the rice disease resistance gene Xa21. Science 270:1804–1806. doi:10.1126/science.270.5243.1804
Spoel SH, Dong X (2012) How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol 12(2):89–100. doi:10.1038/nri3141
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Tatusova TA, Madden TL (1999) BLAST 2 sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett 174:247–250. doi:10.1111/j.1574-6968.1999.tb13575.x
Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhour S, McCouch S (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genom Res 11:1441–1452. doi:10.1101/gr.184001 PMID:11483586
Thomma BPHJ, Nürnberger T, Joosten MHAJ (2011) Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23(1):4–15. doi:10.1105/tpc.110.082602
Vanhouten W, MacKenzie S (1999) Construction and characterization of a common bean bacterial artificial chromosome library. Plant Mol Biol 40(6):977–983. doi:10.1023/A:1006234823105
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78. doi:10.1093/jhered/93.1.77
Wolf S, Hématy K, Höfte H (2012) Growth control and cell wall signaling in plants. Annu Rev Plant Biol 63:381–407. doi:10.1146/annurev-arplant-042811-105449
Young RA, Kelly JD (1996) Characterization of genetic resistance to Colletotrichum lindemuthianum in common bean differential cultivars. Plant Dis 80(6):650–654. doi:10.1590/S1516-89132008000500002
Young RA, Melotto M, Nodari RO, Kelly JD (1998) Marker assisted dissection of the oligogenic anthracnose resistance in common bean cultivar G2333. Theor Appl Genet 96:87–94. doi:10.1007/s001220050713
Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428:764–767. doi:10.1038/nature02485
Zmasek CM, Godzik A (2011) Strong functional patterns in the evolution of eukaryotic genomes revealed by the reconstruction of ancestral protein domain repertoires. Genom Biol 12(1):R4. doi:10.1186/gb-2011-12-1-r4
Acknowledgments
The authors thank Dr. L.E.A. Camargo for hosting CF and MM in his lab during the screening process for AFLP markers; Dr. J.D. Kelly for common bean seeds and the Colletotrichum lindemuthianum isolate; Dr. David Guttman for the Pseudomonas phaseolicola strain. The authors also thank CAPES/Science Without Borders for the post-doctoral fellowship awarded to PRO (award #9773-13-4), and FAPESP for the Undergraduate Research Assistantship to CF (award #01/11218-0). This study was funded by FAPESP—Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (Grant #00/09049-2) to MM.
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical standards
All experiments described in this manuscript comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. E. Mather.
Electronic supplementary material
Below is the link to the electronic supplementary material.
122_2015_2500_MOESM1_ESM.pdf
Fig. S1 Phylogenetic analysis of the top 100 protein kinases with the highest similarity to the predicted COK-4_SEL1308 protein. The top 100 hits were obtained from BLASTP analysis (threshold E-value ≤ 1 x 10−20 and identity > 30 %) using COK-4_SEL1308 as query against the common bean proteome database available at Phytozome. The phylogenetic tree was obtained with the maximum parsimony method using the MEGA 5.05 software (Tamura et al. 2011). Bootstrap support values are adjacent to the tree nodes. Co-4 locus-associated kinases formed a single cluster (red box), and kinase/malectin proteins formed another sub-cluster (blue box). (PDF 34 kb)
Rights and permissions
About this article
Cite this article
Oblessuc, P.R., Francisco, C. & Melotto, M. The Co-4 locus on chromosome Pv08 contains a unique cluster of 18 COK-4 genes and is regulated by immune response in common bean. Theor Appl Genet 128, 1193–1208 (2015). https://doi.org/10.1007/s00122-015-2500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-015-2500-6