Abstract
Dehydrin defines a complex family of intrinsically disordered proteins with potential adaptive value with regard to freeze-induced cell dehydration. Search within an expressed sequence tags library from cDNAs of cold-acclimated crowns of alfalfa (Medicago sativa spp. sativa L.) identified transcripts putatively encoding K3-type dehydrins. Analysis of full-length coding sequences unveiled two highly homologous sequence variants, K3-A and K3-B. An increase in the frequency of genotypes yielding positive genomic amplification of the K3-dehydrin variants in response to selection for superior tolerance to freezing and the induction of their expression at low temperature strongly support a link with cold adaptation. The presence of multiple allelic forms within single genotypes and independent segregation indicate that the two K3 dehydrin variants are encoded by distinct genes located at unlinked loci. The co-inheritance of the K3-A dehydrin with a Y2K4 dehydrin restriction fragment length polymorphism with a demonstrated impact on freezing tolerance suggests the presence of a genome domain where these functionally related genes are located. These results provide additional evidence that dehydrin play important roles with regard to tolerance to subfreezing temperatures. They also underscore the value of recurrent selection to help identify variants within a large multigene family in allopolyploid species like alfalfa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insufficient tolerance to subfreezing temperatures affects winter survival and reduces long-term persistence of alfalfa (M. sativa spp. sativa L.) stands. Therefore, improvement of cold hardiness remains a key objective of alfalfa-breeding programs in regions experiencing harsh winter conditions. Identification of germplasm with superior adaptation to cold is typically performed in field nurseries but this process is costly and unreliable due to the unpredictable occurrence of test winters (Limin and Fowler 1991). This partly explains why conventional selection approaches have not been very effective to improve winter hardiness in cultivars of high agronomic value in the past (Volenec et al. 2002). We recently applied an indoor screening method to develop a series of populations recurrently selected for superior tolerance to freezing (TF populations; Castonguay et al. 2009). Bulk segregant analysis (BSA) of DNA polymorphisms between TF populations identified genomic regions that were the target of selection and that are potentially linked to superior cold adaptation (Castonguay et al. 2010).
Dehydrin are stress- or desiccation-related proteins that are part of the late embryogenesis abundant (LEA) D-11 gene family. They are characterized by a consensus sequence (EKKGIMDKIKEKLPG) known as the K-segment and can contain accessory motifs like consensus Y-segment (DEYGNP) at the N-terminus region and serine-rich tracks, the S-segment (Rorat 2006). Dehydrin constitute a complex gene family that can be subdivided in five principal classes based on different combinations of the characteristic segments: Y n SK n , K n , K n S, SK n and Y n K n (Close 1996; Allagulova et al. 2003). Search for polymorphisms for a cold-inducible Y2K4 dehydrin among alfalfa TF populations led to the identification of a restriction fragment length polymorphism (RFLP) that was significantly associated with freezing tolerance potential (Rémus-Borel et al. 2010). Whether other members of the dehydrin family of genes responded to selection pressure for superior freezing tolerance should be investigated based on their reported involvement in cold adaptation (Rorat 2006).
A search for sequences with the conserved K-segment within an expressed sequence tag (EST) library from cold-acclimated crowns of alfalfa (M. sativa spp. sativa L.) identified several transcripts putatively encoding dehydrins of different types. Among these, ESTs were found to encode K3-type dehydrins with partial homology to the cold-acclimation specific gene cas18 from M. sativa spp. falcata L. (Wolfraim et al. 1993). In the present study, we characterized the full-length coding sequences of the two K3 dehydrins and documented their response to selection pressure for superior tolerance to freezing. The analysis of their cold-induced expression was also performed to assess a potential association with low-temperature adaptation. This work is part of a long-term effort towards the development of functional markers for the improvement of freezing tolerance in alfalfa.
Materials and methods
Plant materials
Genotypes of alfalfa (M. sativa spp. sativa L.), cultivar Apica (ATF0) and population (ATF5) obtained after five cycles of recurrent selection for superior tolerance to freezing (TF) within that cultivar (Castonguay et al. 2009) were established under environmentally controlled conditions as described in Rémus-Borel et al. (2010). Briefly, plants were started from seeds and grown 6 weeks in a growth chamber set to 22/17 °C (day/night) temperatures, 600–800 μmol photons m−2 s−1 photosynthetic photon flux density (PPFD) during a 16-h photoperiod. Environmental conditions were subsequently changed to 8-h photoperiod, a constant temperature of 2 °C and 150 μmol photons m−2 s−1 PPFD for acclimation to low temperature. After 2 weeks of acclimation, plants were transferred to a freezer at −2 °C in the dark for an additional period of 2 weeks to promote the second stage of hardening at subzero temperatures. In addition, progenies obtained by inter crossing ten ATF0 genotypes with (D+ cross) and ten ATF0 genotypes without (D− cross) a dehydrin RFLP that increases in frequency in response to selection for superior tolerance to freezing as described in Rémus-Borel et al. (2010) were grown and cold-acclimated under the same environmentally controlled conditions.
DNA extraction
Genomic DNA was extracted from 0.3 g fresh weight (FW) of leaves of individual genotypes from each of the cultivar Apica (ATF0) and population ATF5 and the D− and D+ crosses. Total genomic DNA was extracted using the CTAB procedure of Rogers and Bendich (1988) and used for PCR amplification after treatment with RNAse (RNase A, Sigma-Aldrich, Oakville, ON, Canada).
Double-stranded DNA (dsDNA) was quantitated using a 96-well microplate fluorescence assay with SYBR Green I as the DNA-binding dye. DNA quantification with SYBR Green I is not affected by contaminants in the mixture and has been shown to accurately predict DNA over a broad range of concentrations (Leggate et al. 2006). For quantification, 5 μl of DNA samples was mixed with 19 μl of TE buffer [1 mM Tris–HCl (pH 8.0), 100 μM EDTA] and 1 μl of a 100× dilution of SYBR Green I 10,000× concentrate in DMSO (Lonza, Rockland, ME, USA). For each sample, the reaction mix was prepared in duplicates, pipetted into microplate wells (twin.tec real-time PCR plates, Eppendorf Canada, Mississauga, ON, Canada) and incubated for 3 min at 30 °C. SYBR green I fluorescence was measured with a Mastercycler® ep realplex system (Eppendorf Canada, Mississauga, ON, Canada) by setting the EndPoint measurement in the PCR program and choosing the SYBR green filter. Concentrations of dsDNA were determined using a calibration curve established with a 6-point series of serial dilutions ranging from 0 to 40 ng ml−1 of purified lambda phage DNA (Roche Applied Science, Laval, QC, Canada) in TE (TE buffer 100× concentrate, Sigma-Aldrich, Oakville, ON, Canada). Measurements were made in triplicates by mixing 5 μl of the DNA standard dilutions with 20 μl of the 100× dilution of SYBR green I. Fluorescence intensity was linear over the whole range with a R 2 value of ≈1.0 and a mean coefficient of variation lower than 3 %. Electrophoresis of 125 ng of DNA from randomly chosen samples was performed on ethidium bromide-stained gels. Visual assessment of DNA amounts by comparison with the molecular weight marker II (Roche Diagnostics, Canada) of known concentration confirmed the accuracy and repeatability of the SYBR green I method of quantification.
Primer design, PCR amplification and gel electrophoresis of amplified products
Nucleotide sequences encoding K3 dehydrins were initially identified in an alfalfa EST library (Serge Laberge, unpublished data) prepared from cDNAs from cold-acclimated crowns (pooled RNA extracts from 50 genotypes) of the population ATF2 obtained after two cycles of recurrent selection for superior tolerance to freezing within the cultivar Apica (Castonguay et al. 2009). These sequences were used to design primers (Fig. 1; Table 1) with the Oligo Explorer software, version 1.1.0 (T. Kuulasma, University of Kuopio, Kuopio, Finland). PCR reactions were performed in a total volume of 25 μl in 0.2 ml PCR strips containing 2.5 μl of 10× PCR buffer, 1 μl each of 5 μM primers, 0.5 μl of 10 mM dNTP (Roche Diagnostics, Laval, QC, Canada), 0.5 μl of 5 Prime Taq polymerase 5 U μl−1 (Inter Medico, Markham, ON, Canada) and 1 μl of 10 ng μl−1 genomic DNA (10 ng). The conditions for PCR were as follows: an initial denaturing step was performed at 94 °C for 3 min followed by 35 cycles at 94 °C for 1 min, a 1-min annealing at 63 °C for the two dehydrins, an extension at 72 °C for 1 min and a final extension at 72 °C for 7 min. All the reactions were performed on an Eppendorf Mastercycler ep System (Eppendorf Canada, Mississauga, ON, Canada). Upon completing the PCR cycles, 20 μl of each reaction was run for 2–3 h at 70 V on a 2 % agarose gel stained with ethidium bromide. DNA fragments were visualized using a UVP BioDoc-It system (UVP, Upland, CA, USA).
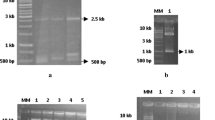
a Alignment of full-length coding sequences for the K3-A and K3-B dehydrin sequence variants identified in Medicago sativa spp. sativa. These sequences are deposited in GenBank under the accession numbers JX460852 and JX460853. K-segments are shown in shaded areas. The location of primers used for the amplification of full-length sequences and RT-qPCR amplification of the K3 dehydrins are shown in dotted boxes and their sequence is provided in Table 1. Predicted length of the RT-qPCR amplicons is indicated above the K3-A sequence and below the K3-B sequence. The number of amino acids and nucleotides (bp) for the full-length open reading frame is indicated to the right of the sequences. b Amino acid alignment of the two M. sativa K3 dehydrin sequence variants with K3-type dehydrins from other species. K-segments are shown in light gray shaded areas, and conserved residues are indicated in dark gray. GenBank accession numbers for each dehydrins are: AAB18204.1 (WCOR726, Danyluk and Sarhan, unpublished data), BAC01112.1 (Wdh13, Ohno et al. 2003), AAU06816.1 (QRDHN3, Sunderlikova et al., unpublished data), CAM98306.1 (Dhn3 type 1, Vornam et al. 2011), CAM98307.1 (DHN3 type 2, Vornam et al. 2011) and AAA21185.1 (CAS18, Wolfraim et al. 1993)
Cloning and sequencing of amplified fragments
DNA fragments were recovered from agarose gels using the QIAquick gel extraction kit (QIAGEN Inc., Mississauga, ON, Canada) according to the manufacturer’s recommendations. Purified DNA was cloned into the pGEM®-T Easy Vector (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Positive transformants were recovered, grown in liquid medium and plasmids purified using the QIAprep Spin Miniprep Kit (QIAGEN Inc., Mississauga, ON, Canada). Plasmid preparations were sent for bi-directional sequencing using M13 forward and reverse primers.
RNA extraction and cDNA synthesis
RNA was purified from individual genotypes or pooled samples from each of the cultivar Apica (ATF0), the population ATF5 and the D− and D+ crosses. Crowns with buds (5-cm transition zone between shoots and roots) were separated from roots and a 0.5-g FW sample was ground to a fine powder in liquid N2. Total RNA was extracted using a CTAB procedure as described by Azevedo et al. (2003). Total RNA was dissolved in TE [10 mM Tris–HCl (pH 7.4), 1 mM EDTA] and subsequently stored at −80 °C. Total RNA was quantified using both the Experion™ RNA StdSens microcapillary chip (Bio-Rad, Mississauga, ON, Canada) and the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Nepean, ON, Canada). The integrity and purity of the RNA was determined based on visual inspection of the fluorescence trace and the RNA quality indicator (RQI) provided by the Experion™ software (Bio-Rad, Mississauga, ON, Canada). All samples had RQI values >8 and any residual genomic DNA was removed by a treatment with DNaseI (Invitrogen, Burlington, ON, Canada) prior to cDNA synthesis. First-strand cDNA was synthesized from 1 μg of total RNA and oligo(dT)18 primers using the Transcriptor First Strand cDNA synthesis Kit (Roche Applied Science, Laval, QC, Canada) following the manufacturer’s instructions. A minimum of two independent cDNA synthesis reactions were performed for each sample and used as technical replicates in subsequent RT-qPCR analyses.
Real-time PCR
RT-qPCR analysis of gene expression was performed according to the MIQE guidelines (Bustin et al. 2009) using four independent biological replicates/pots (10 plants pot−1) and two technical replicates of each PCR reaction. Assays were carried out in 96-well plates (twin.tec real-time PCR plates, Eppendorf Canada, Mississauga, ON, Canada) in a Mastercycler® ep realplex system (Eppendorf Canada, Mississauga, ON, Canada) using the QuantiTect® SYBR Green PCR kit (QIAGEN, Toronto, ON, Canada). The 10-μl reaction mixture contained 3 μl of first-strand cDNA and 0.5 μM of each of the forward and reverse primers. The thermocycler program was set to: 15-min activation at 95 °C followed by 40 cycles of 15 s at 95 °C, 15 s at annealing temperature; 1-min extension at 72 °C. The annealing temperature was set to 60 °C and 65 °C for the two dehydrins and the two reference genes respectively. Real-time PCR was carried out in duplicates and control samples without template or with RNA alone without reverse transcription were included as checks for potential contamination with genomic DNA. Amplified products were electrophoresed on agarose gels, purified, cloned and confirmed by sequencing as described above. PCR efficiency was calculated from the linear regression of a sevenfold dilution of PCR products using the following equation: Efficiency % = (10(−1/slope) − 1) × 100. Efficiency was between 98 and 102 % for all genes. The threshold cycle (C q) values at which the PCR product fluorescence rises over the background fluorescence was determined by the instrument software which was set to default parameters. Data were analyzed with the qBasePLUS software version 1.57 (Biogazelle, Ghent, Belgium). Samples were normalized according to Vandesompele et al. (2002) using the geometric mean of two reference genes (Table 1) with a cut-off V value lower than 0.15. These reference genes were selected by screening several candidate genes for stable expression under non-acclimated and cold-acclimated conditions using the geNorm program included in the qBasePLUS software (Castonguay et al. in preparation). Inter-run calibrators were included on each plate to account for plate-to-plate variation. Normalized relative expression levels were calculated using the \( 2^{{ - \Updelta \Updelta C_{q} }} \) or comparative C q method based on the differences in C q between the target and the reference genes and corrected for PCR efficiency (Hellemans et al. 2007).
Western blot analysis
Soluble proteins from crowns were extracted in a 50-mM HEPES buffer at pH 7.0 and separated by one dimensional SDS-PAGE analysis prior to immunoblotting. Buffer-soluble protein concentrations were determined with a NanoDrop™ 1000 spectrophotometer (Thermo Fischer Scientific, Nepean, ON, Canada). Equal amounts of total soluble proteins were loaded on a Criterion™ TGX Any kD™ precast gel (Bio-Rad Laboratories, Mississauga, ON, Canada). Proteins were subsequently transferred electrophoretically onto Immobilon-P™ polyvinylidene fluoride (PVDF) membranes (Sigma-Aldrich, Oakville, ON, Canada) and blocked with TBST buffer (10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.05 % Tween-20) containing 5 % skim milk for 60 min at room temperature. Membranes were incubated with a 1:1,000 dilution of rabbit anti-dehydrin polyclonal antibody (PLA-100, Stressgen Bioreagents Corp., Victoria, BC, Canada) in TBST for 2 h. After primary antibody incubation, membranes were immersed in TBST containing the secondary goat anti-rabbit IgG polyclonal antibody (dilution 1:1,500) conjugated to alkaline phosphatase (Bio-Rad Laboratories, Mississauga, ON, Canada). Secondary antibodies were detected using 5-bromo-4-chromo-3-indolyl phosphate and nitroblue tetrazolium substrate solution.
Bioinformatic analyses
BLASTn and BLASTp sequence identity searches were performed at the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov). Chromatograms from the sequencing of the different fragments were edited and assembled using BioEdit software version 7.1.3.0 (Hall 1999). Amino acid sequences were aligned using the CLUSTALW tool included in BioEdit and refined manually. VecScreen, an online contaminant vector screening software developed by NCBI (http://www.ncbi.nlm.nih.gov/VecScreen/VecScreen.html) was used to remove vector sequence. Sequence data from this article have been deposited in the GenBank data library under the following names and associated accession numbers (in parentheses): K3-A (JX460852) and K3-B (JX460853).
Data analysis
A total of 145 genotypes for each of the ATF0 and the ATF5 population were scored for the presence of the two K3 sequence variants (K3-A and K3-B). The sample set included 45 genotypes used in Rémus-Borel et al. (2010) and Castonguay et al. (2012b) and a group of 100 genotypes that was sampled for DNA and for RNA extraction from cold-acclimated crowns. Genotypes showing a strong amplification of the K3 dehydrins were deemed positive. A separate group of 100 genotypes was also sampled for DNA and for protein extraction from cold-acclimated crowns. These samples were used for Western blot analyses but were not included in the analysis of the frequency of occurrence of the two K3 dehydrin variants in the genome. Chi-square (χ 2) tests to assess difference in the distribution of frequencies of K3 dehydrins between the ATF0 and ATF5 populations and the Cochran–Mantel–Haenszel statistics for independent assortment of pairwise comparisons of dehydrin sequence variants were performed with the FREQ procedure of the Statistical Analysis System (2006). Statistical comparisons of relative gene expression between treatments are reported independently for each gene based on 95 % confidence intervals calculated by the qBasePLUS software.
Results
Amino acid sequence of K3 dehydrins
Two groups of homologous transcripts encoding K3-type dehydrins were identified within an EST library and used to design primers (Table 1). Following amplification, full-length sequences were cloned and the analysis of nucleotide sequences revealed the presence of two sequence variants of, respectively, 372 bp (K3-A; accession no. JX460852) and 354 bp (K3-B; accession no. JX460853) that are not interrupted by introns. The full-length sequence of the K3-A encodes a putative 124-residue polypeptide with predicted molecular mass of 12.2 kDa and an isoelectric point of 7.2 (Fig. 1a). The shorter K3-B sequence encodes a putative 118-residue polypeptide with predicted molecular mass of 11.7 kDa and a more basic isoelectric point of 8.1. Sequence analysis of several cloned fragments within two ATF0 genotypes indicated the presence of multiple allelic forms within each of the K3 dehydrin groups (Table 2; Supplementary Fig. 1 and 2). In addition, more than four alleles were detected for both K3 sequences in one of the genotypes.
The two K3 dehydrin sequences contain three copies of the K motif and lack the Y and S segments often present in other dehydrins. The three K-segments of the “A” group of K3 dehydrins share a conserved KIKEKIPG sequence of amino acids at the end of that signature motif. The residues that precede that sequence do, however, vary with the replacement of N by E in position 2, K by R or its deletion in position 3 and A by V in position 5 of the K-segments. The “B” group of K3 dehydrins is highly homologous to the “A” group of sequences with, however, unique features. The most striking difference is the deletion of a TGSCTG stretch of six residues at the C terminus. Differences between the K1 and K3 segments of the “A” and “B” groups are also observed with the exchange of a K for an R residue in position three of the K1 segment as well as the replacement of E by K, K by Q and I by L in position 2, 9 and 12 of the K3 segment, respectively.
The M. sativa K3 dehydrins were aligned with homologous sequences found in other species (Fig. 1b). The length of the K3 dehydrin was very similar among species and varied from 100 up to 150 amino acid residues. In spite of frequent conservative substitutions of amino acids, the K-segments are highly conserved both in size and structure. Except for the M. falcata sequence, we note that the spacing between the K-segments is of the same order of magnitude and that each sequence ends with a histidine residue. The unique presence of a SCTGHG stretch of residues at the C terminus of the K3-A group of M. sativa and M. falcata sequences and its conspicuous absence from the other sequences is also noteworthy.
K3 dehydrins respond to selection for freezing tolerance
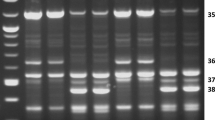
Amplification of full-length K3-A dehydrin revealed marked differences in intensity between pools of genotypes of the initial population ATF0 and population ATF5 (Fig. 2a, pools). Genotypic assessment of K3-A amplification in a group of 45 genotypes in each population showed a marked increase in the number of genotypes with a strong amplification from 3 positive scores in ATF0 up to 16 in the ATF5. Chi-square (χ 2) analysis of 145 genotypes in each population confirmed a significant increase in the frequency of occurrence of the K3-A sequence in response to selection for superior freezing tolerance (Table 3). The most intense signals in Fig. 2a were found to occur in genotypes identified by black arrows that were previously scored as being positive for a Y2K4 dehydrin RFLP linked to superior freezing tolerance in alfalfa (Rémus-Borel et al. 2010). An association between strong amplification of the K3-A dehydrin and the RFLP is further supported by observations made with pools of genotypes from the D+ or D− crosses that were, respectively, obtained by crossing genotypes with (+) or without (−) the Y2K4 dehydrin RFLP (Fig. 2a, pools).
Amplification of the full-length coding sequences of a K3-A and b K3-B dehydrin sequence variants using K3 fullf as a common forward primer and the K3-Ar or K3-Br primers for the K3-A and K3-B variants, respectively. Amplifications were performed with 45 genotypes within each of the ATF0 and ATF5 populations, and with pooled DNA samples (≈45 genotypes) from the ATF0 and ATF5 populations and with pooled samples (≈20 genotypes) from crosses between ATF0 genotypes with (D+) and without (D−) a dehydrin polymorphism. N indicates the number of genotypes with a positive amplification signal for the corresponding K3 fragment. Length of the amplified fragments is indicated on the left of a and b. Downward arrows in a indicate genotypes that were previously scored positive for a dehydrin RFLP with a demonstrated impact on freezing tolerance (Rémus-Borel et al. 2010)
In comparison with the K3-A results, there was a lesser intensification of the full-length amplification signal of the K3-B dehydrin in pools of genotypes from the ATF5 population as compared with ATF0 (Fig. 2b, pools). Analysis of genotypic frequency in the group of 45 genotypes revealed a higher initial number of positive scores in ATF0 and a smaller increase in the number of individuals with a strong signal in ATF5 (7 vs. 13 for K3-A). Nevertheless, the proportion of positive genotype for K3-B is significantly higher in ATF5 than in ATF0 when the 145 genotypes are included in the Chi-square (χ 2) analysis (Table 3). No association was apparent between a strong amplification of K3-B and the Y2K4 dehydrin RFLP (black arrows in Fig. 2a). This is consistent with the lack of difference in the intensity of amplification between pools of genotypes from the D− and D+ crosses (Fig. 2b, pools). Independent assortment of the K3-A and K3-B sequences is supported by a non-statistically significant correlation between their respective genotypic distribution (Table 4). A highly significant correlation between K3-A and the Y2K4 RFLP supports a co-inheritance of these two sequences. On the other hand, the non-significant correlation between K3-B and the Y2K4 RFLP indicates a lack of association between these two genes.
Cold-induced expression of K3 dehydrins
Using RT-qPCR we assessed the cold induction of K3 dehydrins in M. sativa and the impact of the observed differences in genotypic frequency between populations on the level of cold-induced transcripts. The two K3 dehydrins were strongly and significantly up-regulated in cold-acclimated crowns of alfalfa (CA vs. NA treatments in Fig. 3a and b). For both dehydrins, there was a clear tendency for a higher expression in ATF5 than in ATF0 although this difference was not statistically significant. The observation that K3-A transcripts did not accumulate in cold-acclimated crowns of D− progenies is in stark contrast with the strong cold-induced expression in D+ progenies (Fig. 3c). Transcripts of the K3-B dehydrin were markedly up-regulated in cold-acclimated plants of ATF0 and ATF5 and showed similar relative levels of cold-induced expression in the D− and D+ crosses (Fig. 3d). In ATF0 and ATF5 populations, significant differences in the expression of the K3-A dehydrin were observed between genotypes yielding a positive (+) or negative (−) genomic amplification (Fig. 4a). Variation in the expression of the K3-B gene was linked to the intensity of the amplification of its encoding gene but was not related to the amplification signal for the K3-A dehydrin (Fig. 4b).
RT-qPCR assessment of the expression of the K3-A and K3-B dehydrins in non-acclimated (NA) and cold-acclimated (CA) plants from the ATF0 and ATF5 populations (a, b) and the D+ and D− crosses (c, d). The average of relative expression from four pots (10 plants pot−1) for each combination of population and cold acclimation treatments is presented. Treatments with different letters are significantly different at P ≤ 0.05
RT-qPCR determination of the expression of the K3-A (a) and K3-B (b) dehydrin sequence variants in cold-acclimated genotypes from the ATF0 and ATF5 populations. Genotypes were selected on the basis of positive or negative genomic amplification of the K3-A dehydrin. Relative levels of gene expression are shown in the top panels. Asterisk indicates significant difference at P ≤ 0.05 between the group of genotypes with positive K3-A amplification when compared to the group with negative amplification. Genomic amplification results for the K3-A and the K3-B dehydrins is provided in bottom panels. Arrows to the right of bottom panels indicate the length of the fragments
Detection of K3 dehydrin polypeptides
Western blot hybridization with a polyclonal dehydrin antibody revealed the complexity of this large multigene family in alfalfa. The striking difference in the detection of dehydrin polypeptides between non-acclimated (NA) and cold-acclimated (CA) samples indicates that several members of this gene family are cold inducible in alfalfa (Fig. 5a). The presence of an additional peptide of the expected molecular weight (MW) in pooled extracts from genotypes with a strong amplification of the K3-A gene suggests an association with the accumulation of this gene product (Fig. 5b). The low MW polypeptide putatively corresponding to the K3-A dehydrin was unambiguously detected in protein extracts from the D+ cross and did not appear to be present in extracts from the D− cross. The relationship between genomic amplification of K3-A and the accumulation of its encoded polypeptide is further confirmed by the consistent detection of an additional polypeptide of low MW in genotypes of ATF0 and ATF5 with a positive amplification (Fig. 5c). Conversely, no additional polypeptide was found in genotypes with a negative amplification. The detection of a polypeptide corresponding to the putative K3-B encoded product differed between genotypes with a negative and positive amplification of the K3-A gene. Whereas its presence was associated to the strength of the K3-B gene amplification in genotypes with a negative K3-A amplification, a polypeptide was detected in all genotypes with a positive K3-A amplification regardless of the intensity of the K3-B genomic amplification signal. The putative K3-B polypeptide detected in genotypes with a positive K3-A amplification does, however, appear to have a slightly lower MW than the one detected in genotypes with a negative K3-A amplification (Fig. 5c).
Western blot hybridization of a dehydrin polyclonal antibody with soluble proteins extracts from a pools of non-acclimated (NA) and cold-acclimated (CA) genotypes (n = 50) from ATF0 and ATF5 populations: b pools of cold-acclimated ATF0 and ATF5 genotypes (n = 10) with positive or negative genomic amplification of the K3-A dehydrin and pools of cold-acclimated genotypes (n = 20) from the D+ and D− crosses; c cold-acclimated ATF0 and ATF5 genotypes with positive or negative genomic amplification of the K3-A dehydrin. The location of a low MW dehydrin detected in genotypes with a positive K3-A signal or in the D+ cross is indicated by a black arrow in b and c. Molecular weight standards in kDa are indicated on the left of the Western blots in a–c. Genomic amplification of the K3-A and the K3-B dehydrins in ATF0 and ATF5 genotypes are provided at bottom of c. MW standards in bp are provided at the left while the length of the amplified fragments is indicated on the right
Discussion
Sequence features of alfalfa K3 dehydrins
An initial search of an EST library from cold-acclimated crowns of alfalfa led us to the identification of transcripts encoding K3-type dehydrins that were assigned to two groups (K3-A and K3-B) of sequence variants. Analysis of full-length coding sequences indicated the absence of intron and revealed hydrophilic peptides with three repeats of the obligate K-segment as well as a lack of the facultative S- and Y-segments. The three K-segments of the alfalfa K3-A dehydrin and two K-segments of the K3-B variant share a DKIKEKIPG motif that only differs from the DKIKEKLPG terminal sequence of the highly conserved K-segment of dehydrins (Rorat 2006) by the conservative replacement of leucine by isoleucine. There were, however, several departures from the consensus sequence at the beginning of the 15-amino acid K-segment. Conservative (R) and non-conservative substitutions (N/E) of charged K residues may have implications on the folding of the predicted α-helices. Several studies suggest a role of dehydrins in membrane and protein stabilization in the dehydrated state through interactions with the K-segments (Kovacs et al. 2008; Koag et al. 2009; Hughes and Graether 2011). Protonable histidine residues that are flanking the K-segments of the K3-A and K3-B dehydrins may be involved in reversible interactions with negatively charged lipids in a pH-dependent manner as recently proposed by Eriksson et al. (2011). These authors also presented evidence that phosphorylation of the numerous threonine (T) sites located in the intervening Φ segments of alfalfa K3 dehydrins may also be equally determinant by preventing the formation of lipid vesicles aggregates in freeze-desiccated cells.
The unusual presence of a cysteine at the C-terminus of the K3-A variant is also noteworthy considering that dehydrins are typically devoid of cysteine and tryptophan (Close 1997). This single cysteine residue is part of a conserved “TGS/GCTG” stretch of residues found at the C terminus of dehydrins of several legume species including Y2K4 dehydrins from Medicago truncatula (Pennycooke et al. 2008) and M. sativa spp. sativa (Rémus-Borel et al. 2010), a Y2K3 dehydrin from the very winter hardy forage legume Galega orientalis (accession no. ADT80777), a Y2K2 dehydrin from Pisum sativum (Grosselindemann et al. 1998), a Y2K dehydrin from Vicia monantha (accession no AB5006695.1) and a K3 dehydrin from M. sativa spp. falcata (Wolfraim et al. 1993). The deletion of this particular segment is a distinctive feature of the K3-B variant. This sequence modification may have important functional impacts considering that disulfide bridges between thiol groups of cysteines are involved in crosslinking of proteins or may contribute to the determination of the tertiary structure of a protein when it occurs within a polypeptide. Yakubov et al. (2005) reported the presence of two cysteine residues in a cold-inducible K5 dehydrin from Pistacia vera L. Prediction of a non-disulfide-bonding state for these cysteine residues led to the conclusion that they were unlikely to contribute to the three dimensional conformation of the protein. However, a role in protein–protein interactions following post-translational modification of cysteine residues remains a possibility.
K3 dehydrins are closely associated with cold tolerance
K3 dehydrins are not frequently reported in the literature but have been identified in species able to develop tolerance to subfreezing temperatures after a cold acclimation period. In wheat (Triticum aestivum L.), the Wdhn13 and WCOR726 genes encode small K3 dehydrins that share 88 % identity in their amino acid sequence (Ohno et al. 2003). Vornam et al. (2011) recently described two variant forms of a K3 dehydrin in sessile oak (Quercus petraea [Matt.] Liebl.). Although there is still no demonstrated in vitro function, evidence suggests that K n and Y2K n dehydrins may exert important roles with regard to adaptation to low temperature (Rorat 2006). The fact that K3 sequences from distant species code for short hydrophilic polypeptides of nearly identical MW (12–13 kDa) and that their K-segments are similarly spaced may be relevant to their physiological function. There are indeed indications that spacing of K-segments may be geometrically important for interactions with specific membrane domains (Eriksson et al. 2011). In that respect it is noteworthy that the K3-type dehydrin which differs the most in size from the K3-A and K3-B dehydrin variants is the cas18 sequence (17.6 kDa) isolated from a diploid population of the closely related M. sativa spp. falcata. Although the K-segments of cas18 are highly conserved, its Φ segments are significantly different due to atypical motifs not present in the other K3 dehydrins. Based on this observation, the alfalfa K3 dehydrins described in the current study are clearly different from the cas18 gene (Wolfraim et al. 1993).
The significant increase in the number of genotypes positive for K3-A and for K3-B in response to recurrent selection for superior freezing tolerance clearly suggests an association with this trait. Moreover, the accumulation of K3-A and K3-B transcripts and their encoded products was strongly up-regulated at low temperatures. Cold-induced accumulation of transcripts of the two K3 dehydrins in the ATF5 population did not statistically differ from that of ATF0. However, a tendency for superior levels of expression in ATF5 and markedly higher levels of transcripts in genotypes showing positive gene amplification support an impact of recurrent selection on the expression of these genes. Whether K3 dehydrins are landmarks for regions of the genome under selection pressure or are partly responsible for the observed increase in tolerance to freezing in TF populations remains to be elucidated. In that regard, the report by Holková et al. (2009) that cold-induced expression of the K3 gene Wdhn13 is highly correlated to variation in freezing tolerance among wheat cultivars is particularly interesting. On the other hand, Vornam et al. (2011) noted few differences in K3 gene diversity between populations of sessile oak from different altitude. They did not, however, assess whether the presence of specific allelic forms could be associated with altitudinal provenance nor compare cold-induced expression of K3 dehydrins between these populations.
The alfalfa K3 sequence variants are encoded by different genes
The identification, within genotypes, of multiple allelic forms of the K3-A and K3-B variants constitutes evidence that these two highly homologous K3 dehydrin sequences are encoded by two distinct genes (Supplementary Fig. 1). In addition, the detection, based on a limited sampling of cloned sequences, of more than four allelic variants within a genotype of tetraploid M. sativa suggests the presence of multiple copies of the K3-A and K3-B encoding genes. Superior levels of expression of dehydrins in arctic-adapted species of the legume Oxytropis as compared with temperate species was related to tandem duplication (Archambault and Strömvik 2011). A parallel was recently drawn between the observation by Zhang et al. (2011) of superior accumulations of cold-induced Y2K4 dehydrin transcripts in cold hardy M. sativa spp. falcata than in cold-sensitive M. truncatula and highly contrasted genomic amplification signals for these genes between these two species (Castonguay et al. 2012a). This, along with marked genotypic variations in the amplification signals for a Y2K4 dehydrin variant (Castonguay et al. 2012b) and the K3-A dehydrin raises the possibility that copy number variation may play a role in adaptation to subfreezing temperatures. In Populus trichocarpa (Torr. & Gray), dehydrins were found to occur as duplicated blocks distributed throughout the genome likely as a result of segmental and tandem duplication (Liu et al. 2012). The independent segregation of the K3-A and K3-B genes (Table 3) and the unrelated genotypic variation of the levels of both genes transcripts and encoded polypeptides (Figs. 4, 5c) provide additional evidence for the occurrence of two genes located at distinct loci.
K3-A co-segregates with a Y2K4 variant that affects freezing tolerance
Amplification with genotypes from ATF0 and ATF5 revealed a highly significant association between the presence of the K3-A dehydrin and a Y2K4 dehydrin RFLP with a demonstrated impact on freezing tolerance (Rémus-Borel et al. 2010). The marked enrichment of the K3-A genomic amplification signal in pools of genotypes with the RFLP (D+ cross) along with the absence of cold-induced expression in plants without the RFLP (D− cross) further support the co-inheritance of the K3-A gene with the Y2K4 dehydrin RFLP. Chromosome blocks that vary in size with the extent of linkage disequilibrium are the genomic unit of genetic transmission (Bingham 1998.). Investigations on the nature of heterosis in alfalfa led to the conclusion that the accumulation over generations of favorable alleles on chromosome blocks rather than allelic interactions at a single locus was responsible for genetic gains in heterotic crosses (Woodfield and Bingham 1995). Chromosomal domains with groups of co-expressed genes or with genes that are functionally related have been recently described (Zhan et al. 2006; Michalak 2008; Liu and Han 2009). Zhan et al. (2006) noted an over representation of housekeeping and stress-related genes that could share a common regulatory environment within these clusters. In wheat and barley, K n dehydrins were located on the homologous group 6 chromosomes with the exception of the K3-type WDHN13 that was encoded by genes located on the group 7 chromosomes (Ohno et al. 2003).
Differences in transcriptional regulation may also influence the function and expression patterns of dehydrin genes (Wisniewski et al. 2006). Specific motifs of cis-acting regulatory elements like (DRE)/-C repeats are often found in promoter regions of cold-inducible genes, and their organization affects the molecular processes of abiotic stress responses (Yamaguchi-Shinozaki and Shinozaki 2005). Transcript initiation sites can also vary according to the developmental stages of the plant, as described by Basset et al. (2009) in peach, leading to products of different sizes. These authors also found that conformational transitions associated with decreasing temperature, in the sequences surrounding the TATA box, can contribute to the regulation of expression of cold-inducible dehydrin genes. The analysis of the upstream sequences of both K3 dehydrin variants could thus be interesting.
In spite of evidence that the K3-A and K3-B genes are located at different loci both variants responded positively to recurrent selection for superior tolerance to freezing and were strongly up-regulated at low temperatures. This raises the question whether concomitant selection for both variants could have a synergistic impact on cold tolerance of alfalfa. Puhakainen et al. (2004) noted a significant increase in freezing tolerance of Arabidopsis plants overexpressing two dehydrin genes when compared with single dehydrin transformants. Based on these considerations, search for other adaptive genes co-located at the K3-A and the K3-B loci is certainly warranted.
References
Allagulova CR, Gimalov FR, Shakirova FM, Vakhitov VA (2003) The plant dehydrins: structure and putative functions. Biochemistry (Moscow) 68:945–951
Archambault A, Strömvik M (2011) PR-10, defensin and cold dehydrin genes are among those over expressed in Oxytropis (Fabaceae) species adapted to the arctic. Funct Integr Genomics 11:497–505
Azevedo H, Lino-Neto T, Tavares R (2003) An improved method for high-quality RNA isolation from needles of adult maritime pine trees. Plant Mol Biol Rep 21:333–338
Basset CL, Wisniewski ME, Artlip TS, Richart G, Norelli JL, Farrell RE Jr (2009) Comparative expression and transcript initiation of three peach dehydrin genes. Planta 230:107–118
Bingham ET (1998) Role of chromosome blocks in heterosis and estimates of dominance and overdominance. In: Lamkey KR, Staub JE (eds) Concepts and breeding of heterosis in crop plants. Crop Science Society of America, Madison, pp 71–88
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Castonguay Y, Michaud R, Nadeau P, Bertrand A (2009) An indoor screening method for improvement of freezing tolerance in alfalfa. Crop Sci 49:809–818
Castonguay Y, Cloutier J, Bertrand A, Michaud R, Laberge S (2010) SRAP polymorphisms associated with superior freezing tolerance in alfalfa (Medicago sativa spp. sativa). Theor Appl Genet 120:1611–1619
Castonguay Y, Dubé M-P, Cloutier J, Bertrand A, Michaud R, Laberge S (2012a) Molecular physiology and breeding at the crossroads of cold hardiness improvement. Physiol Plant. doi:10.1111/j.1399-3054.2012.01624.x
Castonguay Y, Dubé M-P, Cloutier J, Michaud R, Bertrand A, Laberge S (2012b) Intron-length polymorphism identifies a Y2K4 dehydrin variant linked to superior freezing tolerance in alfalfa. Theor Appl Genet 124:809–819
Close TJ (1996) Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiol Plant 97:795–803
Close TJ (1997) Dehydrins: a commonality in the response of plants to dehydration and low temperature. Physiol Plant 100:291–296
Eriksson SK, Kutzer M, Procek J, Gröbner G, Harryson P (2011) Tunable membrane binding of the intrinsically disordered dehydrin Lti30, a cold-induced plant stress protein. Plant Cell Online 23:2391–2404
Grosselindemann E, Robertson M, Wilmer JA, Chandler PM (1998) Genetic variation in pea (Pisum) dehydrins: sequence elements responsible for length differences between dehydrin alleles and between dehydrin loci in Pisum sativum L. Theor Appl Genet 96:1186–1192
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19
Holková L, Prášil IT, Bradáčová M, Vítámvás P, Chloupek O (2009) Screening for frost tolerance in wheat using the expression of dehydrin genes Wcs120 and Wdhn13 at 17°C. Plant Breed 128:420–422
Hughes S, Graether SP (2011) Cryoprotective mechanism of a small intrinsically disordered dehydrin protein. Protein Sci 20:42–50
Koag M-C, Wilkens S, Fenton RD, Resnik J, Vo E, Close TJ (2009) The K-segment of maize DHN1 mediates binding to anionic phospholipid vesicles and concomitant structural changes. Plant Physiol 150:1503–1514
Kovacs D, Kalmar E, Torok Z, Tompa P (2008) Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol 147:381–390
Leggate J, Allain R, Isaac L, Blais B (2006) Microplate fluorescence assay for the quantification of double stranded DNA using SYBR Green I dye. Biotechnol Lett 28:1587–1594
Limin AE, Fowler DB (1991) Breeding for cold hardiness in winter wheat: problems, progress and alien gene expression. Field Crops Res 27:201–218
Liu X, Han B (2009) Evolutionary conservation of neighbouring gene pairs in plants. Gene 437:71–79
Liu C-C, Li C-M, Liu B-G, Ge S-J, Dong X-M, Li W, Zhu H-Y, Wang B-C, Yang C-P (2012) Genome-wide identification and characterization of a dehydrin gene family in poplar (Populus trichocarpa). Plant Mol Biol Rep. doi:10.1007/s11105-011-0395-1
Michalak P (2008) Coexpression, coregulation, and cofunctionality of neighboring genes in eukaryotic genomes. Genomics 91:243–248
Ohno R, Takumi S, Nakamura C (2003) Kinetics of transcript and protein accumulation of a low-molecular-weight wheat LEA D-11 dehydrin in response to low temperature. J Plant Physiol 160:193–200
Pennycooke JC, Cheng H, Stockinger EJ (2008) Comparative genomic sequence and expression analyses of Medicago truncatula and alfalfa subspecies falcata COLD-ACCLIMATION-SPECIFIC genes. Plant Physiol 146:1242–1254
Puhakainen T, Hess MW, Makela P, Svensson J, Heino P, Palva ET (2004) Overexpression of multiple dehydrin genes enhances tolerance to freezing stress in Arabidopsis. Plant Mol Biol 54:743–753
Rémus-Borel W, Castonguay Y, Cloutier J, Michaud R, Bertrand A, Desgagnés R, Laberge S (2010) Dehydrin variants associated with superior freezing tolerance in alfalfa (Medicago sativa L.). Theor Appl Genet 120:1163–1174
Rogers SO, Bendich AJ (1988) Extraction of DNA from plant tissues. In: Gelvin S, Schilperoort RA (eds) Plant molecular biology manual A6. Kluwer, Dordrecht, pp 1–10
Rorat T (2006) Plant dehydrins—tissue location, structure and function. Cell Mol Biol Lett 11:536–556
Statistical Analysis System (2006) Version 9.1. SAS Institute Cary, NC
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:research0034.1–research0034.11
Volenec JJ, Cunningham SM, Haagenson DM, Berg WK, Joern BC, Wiersma DW (2002) Physiological genetics of alfalfa improvement: past failures, future prospects. Field Crop Res 75:97–110
Vornam B, Gailing O, Derory J, Plomion C, Kremer A, Finkeldey R (2011) Characterisation and natural variation of a dehydrin gene in Quercus petraea (Matt.) Liebl. Plant Biol 13:881–887
Wisniewski ME, Basset CL, Renaut J, Farrell R Jr, Tworkoski T, Artlip TS (2006) Differential regulation of two dehydrin genes from peach (Prunus persica) by photoperiod, low temperature and water deficit. Tree Physiol 26:575–584
Wolfraim LA, Langis R, Tyson H, Dhindsa RS (1993) cDNA sequence, expression, and transcript stability of a cold acclimation-specific gene, cas18, of alfalfa (Medicago falcata) cells. Plant Physiol 101:1275–1282
Woodfield DR, Bingham ET (1995) Improvement in two-allele autotetraploid populations of alfalfa explained by accumulation of favorable alleles. Crop Sci 35:988–994
Yakubov B, Barazani O, Shachack A, Rowland LJ, Shoseyov O, Golan-Goldhirsh A (2005) Cloning and expression of a dehydrin-like protein from Pistacia vera L. Trees Struct Funct 19:224–230
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10:88–94
Zhan S, Horrocks J, Lukens LN (2006) Islands of co-expressed neighbouring genes in Arabidopsis thaliana suggest higher-order chromosome domains. Plant J 45:347–357
Zhang L-L, Zhao M-G, Tian Q-Y, Zhang W-H (2011) Comparative studies on tolerance of Medicago truncatula and Medicago falcata to freezing. Planta 234:445–457
Acknowledgments
The authors would like to thank Dr. Serge Laberge for giving access to the EST library from cold acclimated alfalfa. They would also like to acknowledge the contribution of Mr. Réjean Desgagné and Mr. David Gagné for their contribution to the development of the EST collection and database. The participation of Marie-Pier Dubé to this project was supported by a NSERC visiting fellowship in Canadian federal government laboratories. This project was supported by the competitive grant program of Agriculture and Agri-Food Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Varshney.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dubé, MP., Castonguay, Y., Cloutier, J. et al. Characterization of two novel cold-inducible K3 dehydrin genes from alfalfa (Medicago sativa spp. sativa L.). Theor Appl Genet 126, 823–835 (2013). https://doi.org/10.1007/s00122-012-2020-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-012-2020-6