Abstract
Powdery mildew (PMD) of soybean [Glycine max (L.) Merr.] is caused by the fungus Microsphaera diffusa. Severe infection of PMD on susceptible varieties often causes premature defoliation and chlorosis of the leaves, which can result in considerable yield losses under favorable environmental conditions for disease development in the field. A total of 334 F7-derived recombinant inbred lines (RILs) from a cross of a PMD susceptible soybean cultivar Wyandot and PMD-resistant PI 567301B were used for genetic mapping of PMD resistance in PI 567301B and for development of molecular markers tightly linked to the gene. The result of the PMD screening for each line in the field was in agreement with that in the greenhouse test. The genetic map containing the PMD resistance gene was constructed in a 3.3 cM interval flanked by two simple sequence repeat (SSR) markers on chromosome 16. The PMD resistance gene was mapped at the same location with SSR marker BARCSOYSSR_16_1291, indicating that there was no recombination between the 334 RILs and this marker. In addition, a single nucleotide polymorphism (SNP) marker developed by high-resolution melting curve analysis and a cleaved amplified polymorphic sequence (CAPS) marker with Rsa1 recognition site were used for the genetic mapping. These two markers were also mapped to the same genomic location with the PMD resistance gene. We validated three tightly linked markers to the PMD resistance gene using 38 BC6F2 lines and corresponding BC6F2:3 families. The three marker genotypes of the backcross lines predicted the observed PMD phenotypes of the lines with complete accuracy. We have mapped a putatively novel single dominant PMD resistance gene in PI 567301B and developed three new molecular markers closely linked to the gene. Molecular markers developed from this study may be used for high-throughput marker-assisted breeding for PMD resistance with the gene from PI 567301B.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Powdery mildew (PMD) of soybean is a common disease, caused by the fungus Microsphaera diffusa, in many soybean growing regions. Symptoms of PMD can be easily detected by white, powdery patches that form on all plant parts, especially on the upper surface of leaves. Green and yellow islands, defoliation, chlorosis, veinal necrosis or a mixture of these symptoms on leaves can appear depending on the soybean cultivar (Grau 2006; Mulrooney 2009). This disease usually occurs and develops under cool air temperature (approximately 18–24 °C), but disease development and progression may stop when temperatures are greater than 30 °C (Phillips 1984; Grau 2006).
Under favorable environmental conditions, such as continuous cool temperatures between flowering and maturity, PMD can cause significant yield losses in soybean. The yield reductions caused by PMD have been reported by comparing yield of plots sprayed and non-sprayed with a fungicide or comparing yield between resistant and susceptible cultivars with the occurrence of PMD wherein up to 35 % yield loss has been recorded on susceptible cultivars (Dunleavy 1980; Phillips 1984). Recently, PMD has affected all soybean growing regions in Brazil, causing yield losses ranging from 30 to 40 % (Gonçalves et al. 2002). Yorinori and Hiromoto (1998) and Silva and Seganfredo (1999) reported that the epidemics of PMD during 1996–97 in soybean in a large area of Brazil (from the Central West region to the Rio Grande do Sul state) resulted in average yield losses of 15 and 20 % in susceptible cultivars, with extremes ranging from 50 to 60 %. Also, yield of late-planted soybean can be reduced more than that of early-planted crops by PMD. Sometimes co-infection of Microsphaera diffusa and other pathogens may occur (Grau 2006).
Inheritance of host plant resistance to PMD in soybean has been reported to be controlled by three alleles consisting of Rmd, Rmd-c, and rmd at the Rmd locus (Lohnes and Bernard 1992). Adult plant resistance to PMD is controlled by the gene Rmd (Mignucci and Lim 1980), while the Rmd-c derived from cultivar CNS provides resistance to PMD for the entire life cycle of soybean plants (Lohnes and Bernard 1992). Gonçalves et al. (2002) reported that adult-plant resistance was controlled by one major dominant gene through two evaluations, one 20 days after planting and the other after flowering. Recently, PMD resistance in PI 243540 was reported to be controlled by a single dominant gene that provided resistance at all plant growth stages (Kang and Mian 2010).
In a previous study, the Rmd gene was reported to be linked to Rps2 and Rj2 at distance 2.3 and 1.9 cM on classical linkage group (LG) 19 corresponding to LG J or chromosome 16 using 246 F2-derived F3 lines (Lohnes et al. 1993). The Rps2 gene is associated with Phytophthora root and stem rot (caused by Phytophthora sojae) resistance, and the Rj2 confers non-nodulation response with certain strains of Bradyrhizobia japonicum (Kirchner) Buchanan (Caldwell 1966; Kilen et al. 1974). Polzin et al. (1994) also mapped the Rmd gene between Rj2 and Rps2 genes on LG J at distances of 1.8 and 2.0 cM from the genes, respectively. Recently, the PMD resistance gene Rmd_PI243540 from PI 243540 was mapped between simple sequence repeat (SSR) marker Sat_224 and single nucleotide polymorphism (SNP) marker BARC-021875-04228 with a distance of 9.6 and 1.3 cM from the flanking markers, respectively (Kang and Mian 2010). The map position of the Rmd gene reported by Polzin et al. (1994) was more than 5–6 cM away from the map position of the PMD resistance gene from PI 243540 with reference to the 2003 composite (SoyBase) map (Kang and Mian 2010). The soybean aphid (Aphis glycines Matsumura) resistant PI 567301B (Jun et al. 2012) also showed lifelong resistance to PMD in greenhouse and field environments under high disease pressures (M.A.R. Mian, personal observation, 2006).
Abundant molecular markers, such as SNP and SSRs, have been developed from the Williams 82 whole-genome shotgun sequence (Hyten et al. 2010; Song et al. 2010), and these markers have been used in breeding programs (Kim et al. 2010; Vuong et al. 2010; Jun et al. 2012). Specifically, SNP markers that are tightly linked to or within genes of interest can be used for high-throughput marker-assisted selection (MAS). The objectives of this study were to (1) map the PMD resistance gene in PI 567301B and (2) develop SNP markers tightly linked to the gene that will be useful for high-throughput marker-assisted breeding for introgression of the gene into commercial cultivars.
Materials and methods
Plant materials and PMD resistance assays
A population of 334 F7 and F7-derived recombinant inbred lines (RILs) obtained from a cross of ‘Wyandot’ × PI 567301B by single seed descent (SSD) and the two parental lines were used for genetic mapping of PMD resistance gene. Wyandot is a maturity group (MG) II soybean cultivar that is highly susceptible to PMD. By contrast, PI 567301B is a MG IV accession from China with PMD resistance for all growth stages. Eleven F1 plants obtained from the cross and the two parents were grown to maturity in a greenhouse at the Ohio Agricultural Research and Development Center (OARDC) during the winter 2006. Each plant was inoculated with spores of M. diffusa at the Stage V1 by brushing with PMD-infected soybean leaves from a susceptible plant source maintained in the greenhouse. The greenhouse was maintained at approximately 24/20 °C day/night temperatures and the plants were kept under 15 h light daily. While the susceptible parent (Wyandot) showed symptoms of PMD within 2 weeks of inoculation, none of the 11 F1 plants and the resistant parent (PI 567301B) displayed any PMD symptom during the entire life cycle (data not shown). The seeds from the 11 PMD-resistant F1 plants were used to develop the RILs used in this study by SSD method in a greenhouse at OARDC.
In the summer of 2009, a field test by natural infection of PMD was performed using the 334 F7 RILs of the Wyandot × PI 567301B population in a field at OARDC. Each of the 334 F7 RILs was represented by a single plant. Ten replications of each parent were placed among the RILs at random. Seeds were planted with a 10-cm spacing between seeds within a row and 76-cm spacing between 8-m long rows. The sources of PMD inoculums were provided by transplanting greenhouse grown PMD-infected soybean plants around the field. Six weeks after planting, the evaluation of PMD resistance was performed by inspecting each F7 and parent plants separately. A plant with no PMD colonies on any leaf was regarded as resistant, while a plant with one or more PMD colonies on leaves was rated as susceptible. The seeds from each F7 plant were hand harvested separately. In the fall of 2009, the PMD phenotypes of the RILs were retested on the 334 F7-derived families and two parents in a USDA greenhouse at OARDC, Wooster, OH, USA. In this test, four seedlings per family were grown in plastic pots 10.16 cm in diameter and 8.89 cm deep. Each plant was inoculated with spores of M. diffusa at the Stage V1 by brushing with PMD-infected soybean leaves from a susceptible plant source maintained in the greenhouse (Kang and Mian 2010). Two parents (each with ten pots) were also included as checks for reference points for classification of RILs as resistant or susceptible. After inoculation, the disease reaction of leaves to PMD was evaluated at 4 weeks with the same criteria used in the field screening. The greenhouse was maintained at approximately 24/20 °C day/night temperatures and the plants were kept under 15 h light daily.
Before inoculation, young leaf fragments from the four seedlings of each RIL at Stage V1 were collected and pooled in a 2-ml tube and were immediately frozen in liquid nitrogen. The frozen leaf tissues were lyophilized in a freeze dryer (SP Industries inc., Stone Ridge, NY, USA) for 2 days and then were ground into fine powder using a Mixer Mill (Retsch, Model MM301, Hannover, Germany). The genomic DNA was extracted using a slightly modified CTAB protocol (Saghai-Maroof et al. 1984).
For confirmation of inheritance of the PMD resistance gene in an early generation, a PMD-resistant BC6F1 plant from backcross between ‘Wyandot’ × PI 567301B was grown to maturity in the greenhouse and 100 BC6F2 seeds were harvested from the plant during the winter of 2010. The 90 BC6F2 seedlings from the F2 seeds were grown in the greenhouse during the spring of 2011 and screened for PMD resistance using the same greenhouse protocol described above.
For the validation of markers for potential marker-assisted selection, the seedlings were then transplanted in the field in the summer of 2011 to grow them to maturity for harvesting seeds. Sixteen PMD resistant (R1-16) and 22 PMD susceptible (S1-22) BC6F2 near isogenic lines (NILs) that produced more than 60 seeds each were used for validation of markers and segregation in the corresponding BC6F2:3 families. The 38 BC6F2:3 families or NILs and the two parents ‘Wyandot’ and PI 567301B were planted in the greenhouse in October, 2011. Two susceptible and four resistant genotypes were also included as checks, which were obtained from the National Plant Germplasm System (NPGS) (http://www.ars-grin.gov/npgs/). The susceptible checks were Corsoy (PI 548540) and Harosoy (PI 548573) previously reported as susceptible to PMD (Dunleavy 1980). The resistant checks were Wayne (PI 548628) (Dunleavy 1980), CNS (PI 548445) (Lohnes and Bernard 1992), Blackhawk (PI 548516) (Lohnes and Bernard 1992) and PI 243540 (Kang and Mian 2010). Six seedlings per accession were grown in a pot 19.2 cm in diameter and 17.3 cm deep, and DNA from each line was extracted from pooled young unfolded trifoliolate leaves of 6 seedlings at the V2-stage. The PMD resistance assay and DNA extraction were performed using the same protocols described above. Segregation of the resistant NILs (families) was further confirmed by testing more (27–56) progenies per family.
Bulked-segregant analysis (BSA) and SSR analysis
Bulked segregant analysis (BSA) was performed to identify SSR markers potentially linked to the PMD resistance (Michelmore et al. 1991). Two resistant bulks were formed from a total of 16 resistant RILs with no disease symptom in both field and greenhouse tests. Each resistant bulk was constructed by pooling an equal amount of DNA from eight RILs. Similarly, two susceptible bulks were made using equal amounts of DNA from eight highly susceptible RILs each.
Ten SSR markers positioned every 10 cM on chromosome 16 according to the Soybean Consensus Map 4.0 (Hyten et al. 2010) were selected for BSA and used to screen for polymorphisms using the contrasting DNA bulks (resistant and susceptible), two parents, one resistant and one susceptible RIL. Two SSR markers (Satt431 and Sat_394) on chromosome 16 were identified to be linked to the PMD resistance gene in this population using the BSA approach. After finding the potential gene location, four SSR markers polymorphic between two parents on chromosome 16 (Sat_396, Satt431, Sat_394, and Sat_393) were screened on randomly selected 94 RILs of the mapping population. A genetic linkage map of the PMD resistance gene and the four SSR markers was constructed and then additional 36 SSR markers located between Satt431 and Sat_394 on chromosome 16 were screened for fine mapping of the gene with the set of 334 RILs. The primer sequences and location of the SSR markers were obtained from BARCSOYSSR_1.0 soybean SSR database (Song et al. 2010) and the Williams 82 soybean genomic sequences (http://www.phytozome.net).
PCR amplifications were performed in 20 μl reactions containing 50 ng of template DNA, 1× PCR buffer, 2.5 mM Mg2+, 200 μM dNTP, 100 nM of forward and reverse primers, and 1.0 U of Taq DNA polymerase (GenScript USA Inc., Piscataway, NJ, USA). The PCR cycles consisted of initial denaturation at 95 °C for 5 min, followed by 32 cycles of 20 s denaturation at 94 °C, 20 s annealing temperature between 50 and 65 °C depending on the optimum annealing temperature for each primer pair, and 20 s extension at 72 °C. The PCR reaction was finished with a final 10 min extension at 72 °C on a thermalcycler (Techne Inc., model TC-512, Burlington, NJ, USA). The PCR products were analyzed in 4 % 3:1 agarose gel (RPI corp., Mount Prospect, IL, USA).
SNP identification
A total of 31 primer pairs were designed to identify SNPs using the sequences from 4 candidate resistance genes (Glyma16g34070, Glyma16g34090, Glyma16g34110, and Glyma16g34120), which were annotated in approximately 126 kb (36,751,400-36,877,300 bp) between BARCSOYSSR_16_1291 and BARCSOYSSR_16_1298 on chromosome 16 in the Williams 82 soybean genomic sequences (http://www.phytozome.net) (Table 1). Additional 13 primer pairs were also designed from about 100 kb genomic regions (36,706,260–36,805,470 bp) spanning BARCSOYSSR_16_1291 marker on chromosome 16 based on the Williams 82 sequences. Designing of sequencing primer pairs was performed using BatchPrimer3 software (http://probes.pw.usda.gov/batchprimer3/index.html).
All primer pairs were amplified using a gradient PCR program (55–67 °C) to determine the optimum annealing temperature. The PCR products with a single amplicon each on 4 % 3:1 agarose gels were directly purified using the mixture of Exonuclease I and Shrimp Alkaline Phosphatase (ExoSAP) (Affymetrix Inc. USB® Products, Cleveland, OH, USA), which were conducted in 12 μl reactions containing 10 μl PCR product, 2.0 U of Exonuclease I, and 1.8 U of Shrimp Alkaline Phosphatase. Incubation was performed at 37 °C for 1 h and 72 °C for 15 min. All PCR products were sequenced in both directions of forward and reverse using 3′ BigDye-labeled dideoxynucleotide triphosphates labeling chemistry and ABI Prism 3100 genetic analyzer (Life Technologies Corp., Carlsbad, CA, USA), and the sequences from each sample were aligned using CodonCode Aligner 3.5.1 demo version (http://www.codoncode.com/aligner/) and Clustal W multiple alignment of BioEdit Sequence Alignment Editor 7.0.9 software (Hall 1999).
Development of SNP and CAPS markers
High-resolution melting (HRM) curve analysis using unlabeled oligonucleotide probes specific to SNP sites was performed with three SNPs obtained from three different PCR amplicons, and only one SNP (PMSNP_1) successfully generated different melting curves between two parents. For SNP genotyping using 334 RILs, the SimpleProbe 5′-FLQ- ATG GTG GCA CTG TAA CTG ATA GAC T-Phosphate (TIB MOLBIOL, Adelphia, NJ, USA) labeled with fluorescein at the 5′ end and blocked with phosphate at the 3′ end was designed using the LightCycler® Probe Design Software 2.0 (Roche Diagnostics, IN, USA), which was perfectly matched to the susceptible allele in Wyandot. The forward 5′-CAA TCC GAT TTC AAC CCA AT-3′ and reverse 5′-TCC TCG AAA CCA GTA GAC TTG C-3′ primers used in the previous melting curve analysis with unlabeled probe were utilized for PCR, representing a 129-bp fragment.
PCR amplifications were performed in 384-well plates with a total volume of 3 μl per well containing 25 ng of template DNA, 0.2 μM of limiting primer, 1.0 μM of excess primer, 0.2 μM of SimpleProbe, 3.0 mM MgCl2, and 0.5× of LightCycler 480 Genotyping Master mix using the Roche LightCycler® 480 System (Roche Diagnostics, IN, USA). The PCR cycles consisted of initial denaturation at 95 °C for 10 min, followed by 55 cycles of 20 s denaturation at 95 °C, 20 s annealing at 61 °C, and 20 s extension at 72 °C. A final melting cycle was conducted by increasing the temperature to 95 °C for 1 min, lowering the temperature to 50 °C for 2 min, and raising the temperature up to 75 °C acquiring fluorescence continuously. Lastly, the PCR and melting cycle were finished with a final cool down to 40 °C. The data analysis for grouping of melting curves was performed by the LightCycler® Data Analysis software (Roche Diagnostics, IN, USA).
The conversion of SNP sites into cleaved amplified polymorphic sequence (CAPS) markers was performed using dCAPS Finder 2.0 program (http://helix.wustl.edu/dcaps/dcaps.html). About 15 μl PCR product was digested with 2.5 U of Rsa1 in a volume of 17 μl, and incubated at 37 °C for 4 h. The restriction digested PCR products were resolved on 4 % 3:1 agarose gels to detect the polymorphism.
Statistical and linkage analysis
Association test between PMD resistance and marker genotype was performed by single factor analysis of variance (single factor ANOVA) at the 0.05 significance level using the PROC GLM procedure of SAS (SAS Institute, 2002). Chi-square tests for goodness-of-fit were performed to identify if the segregation ratio of alleles at each locus fit the theoretically expected ratio with a significance threshold of P = 0.05. The linkage map was constructed with the Maximum Likelihood mapping function using JoinMap 4.0 (Van Ooijen 2006). A logarithm of the odds (LOD) score of 3.0 as a threshold and a maximum genetic distance of 50 cM were used to assign all markers to chromosomes (or linkage groups). The candidate resistance genes were predicted and annotated from Phytozome soybean database (http://www.phytozome.net/soybean.php).
Results
Evaluation of the PMD resistance in the mapping population
Among the 90 BC6F2 plants screened in the greenhouse, 67 were PMD resistant while 23 were PMD susceptible, showing a tight fit (X 2 = 0.01, P = 0.938) for the 3 resistant:1 susceptible segregation of a single dominant gene trait. Of the 334 RILs, 178 were susceptible to the disease and 156 revealed resistant reactions (Table 2). The segregation of resistant and susceptible plants in the mapping population showed a good fit to the expected 1 resistant:1 susceptible ratio (X 2 = 1.45, P = 0.229) for a single gene trait in F7 RILs. The field and greenhouse PMD phenotypes of each RIL were in agreement (data not shown).
Molecular marker analysis and genetic map
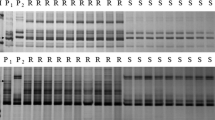
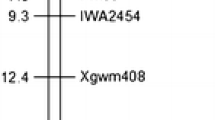
The initial linkage map spanned 25.7 cM and the PMD resistance gene in PI 567301B was mapped between the markers Satt431 and Sat_394 located at a distance of 4.7 and 10.5 cM from the gene, respectively (Fig. 1a). Of the 36 SSRs screened between Satt431 and Sat_394, 9 SSRs were polymorphic between the parents and four (BARCSOYSSR_16_1236, BARCSOYSSR_16_1272, BARCSOYSSR_16_1291, and BARCSOYSSR_16_1298) were used for mapping (Table 2; Fig. 1b). Data from all 4 SSR markers fit the 1:1 ratio expected for highly advanced RIL population at the 0.05 significance level, representing no segregation distortion near the PMD resistance gene region in this population (Table 2). There were highly significant associations (P < 0.0001) between each SSR marker and the PMD resistance (Table 2). The final linkage map containing the PMD resistance gene spanned 3.3 cM with markers BARCSOYSSR_16_1236 and BARCSOYSSR_16_1298 positioned at the two ends (Fig. 1b). The PMD resistance gene was mapped at the same location with SSR marker BARCSOYSSR_16_1291, indicating that all 334 RILs co-segregated with this marker.
To develop SNP markers tightly linked to the PMD resistance in PI 567301B, 31 primer pairs designed between BARCSOYSSR_16_1291 and BARCSOYSSR_16_1298 on chromosome 16, and 13 primers designed from about 100 kb genomic regions spanning BARCSOYSSR_16_1291 were used for direct sequencing of the two parents. From these 44 primers, a total of 13 SNPs were identified, 11 of which were identified on 2 candidate disease resistance genes Glyma16g34090 and Glyma16g34120 (Table 3). The high-resolution melting analysis was used to find tightly linked SNP markers to the PMD resistance gene. However, only 3 of 13 SNPs identified from the direct sequencing were useful to design the unlabeled probes for HRM analysis; PMSNP_1, PMSNP_12, and PMSNP_13. The rest of the SNPs could not be utilized due to: (1) the sequence duplication near the SNP site (PMSNP_6) and (2) concentration of SNPs in a narrow region (PMSNP_2–5 and PMSNP_7–11), which interfered with design of primer pairs and probes for the HRM analysis. Of the 3 SNPs, only PMSNP_1 revealed different melting temperature between the two parents: 61.5 and 68.5 °C for PI 567301B and Wyandot, respectively. The PMSNP_1 was mapped to the same location with the PMD resistance gene and BARCSOYSSR_16_1291 (Fig. 1b).
One CAPS marker was developed from the PMSNP_6 which could not be used for the HRM analysis. The CAPS marker (PMCAPS) with Rsa1 recognition site was used for the genetic mapping using the whole set of 334 RILs and showed complete co-segregation with the PMD resistance gene, PMSNP_1, and BARCSOYSSR_16_1291 (Fig. 1b).
Marker validation using backcross lines
The three molecular markers that mapped to the same location with the PMD resistance gene were used for the marker validation using 16 resistant and 22 susceptible NILs (BC6F2 and corresponding BC6F2:3 families), Wyandot, PI 567301B, and 6 PIs previously reported as resistant or susceptible to PMD (Table 4). In the BC6F2:3 families, three (NIL_R5, R6 and R9) of the 16 PMD resistant NILs showed homozygous-resistant genotypes for all three markers and also all six seedlings in each family had resistant phenotypes (Table 4). One resistant NIL (NIL_16) showed no segregation for phenotype among the six seedlings, but had heterozygous marker genotypes for all three markers. The remaining 12 PMD-resistant NILs each had heterozygous phenotypes as well as heterozygous marker genotypes. The progenies of the 22 susceptible NILs all showed susceptible reactions to PMD and homozygous susceptible genotypes for the three markers (Table 4). The phenotypic segregation of the 16 resistant NILs was further tested on more progenies (27–56) per family that confirmed the earlier results from the six seedlings per line, except NIL_R16 (data not shown). For NIL_R16, 48 additional seedlings were tested for PMD that showed segregation in PMD phenotypes (31 resistant:17 susceptible). Thus, the PMD reactions of 38 NILs in the greenhouse tests were in complete agreement with the 3 markers (PMSNP_1, BARCSOYSSR_16_1291, and PMCAPS) genotypes.
However, the three marker genotypes of the PMD-resistant check sources, except PI 548628, did not match with genotypes of PI 567301B (Table 4). Specially, PI 548445(CNS) that is known to provide a lifelong PMD resistance like PI 567301B showed completely different genotypes for the three markers compared to the marker genotypes of PI 567301B (Table 4). Two susceptible sources, PI 548540 and PI 548573, possessed the same marker genotypes as Wyandot, the susceptible parent (Table 4).
Discussion
We successfully performed genetic mapping to develop molecular markers tightly linked to PMD resistance using 334 RILs of a ‘Wyandot’ × PI 567301B population. The reaction of the PMD for each line in the field test was in agreement with that in the greenhouse screening despite different inoculation methods and environmental conditions, which indicates that environmental factors have a relatively low impact on the expression of PMD resistance in this population.
The disease symptoms of PMD were well developed and severely infected plants were visibly stunted and revealed other symptoms such as chlorosis or yellowing of the leaves. These results indicate that heavy infection of the PMD disease could result in considerable yield losses on susceptible cultivars, like Wyandot, under favorable environmental conditions for disease development in the field. In other crops, PMD has become one of the most severe diseases causing serious yield losses up to 50 %. Many studies have been performed to identify and dissect the PMD resistance genes: Blumeria graminis D.C. (Speer) f. sp. avenae Em. Marchal in oat (Avena sativa L.) (Yu and Herrmann 2006), Erysiphe pisi D. C. (Ep) in pea (Pisum sativum L.) (Pavan et al. 2011), Blumeria graminis f. sp tritici (Bgt) in common wheat (Triticum aestivum L.) (Ma et al. 2011). Specially, mlo PMD resistance, which is caused by the loss-of-function alleles of plant-specific MLO genes such as Arabidopsis AtMLO2, barley Mlo, tomato SlMlo1, has been discovered in several species, and their mutants have been used in plant breeding providing effective mlo-mediated PMD resistance under field conditions (Buschges et al. 1997; Bai et al. 2008; Pavan et al. 2011). Three molecular markers tightly linked to the PMD resistance gene were developed in the present study. Of these markers, PMSNP_1 can be used to perform a high-throughput genotyping for the selection of the PMD-resistant plants using melting curve analysis, while BARCSOYSSR_16_1291 can be screened by agarose gel electrophoresis method. The PMCAPS marker SNP was derived from the exon sequence of a candidate gene, thus it has the potential to be a functional marker, if it has real functions on the expression of the PMD disease. We consider the map position of the PMD gene identified in our study to be highly accurate because of the large population used (334 RILs) and dense marker coverage used in mapping the gene. When compared with the locations of PMD resistance genes reported recently, the position of PMD gene in PI 567301B is approximately 6 and 2 cM away from the Rmd_PI243540 from PI 243540 (Kang and Mian 2010) and the Rmd gene from CNS cultivar (Polzin et al. 1994), respectively, based on the Soybean Consensus Map 4.0 (Hyten et al. 2010) and BARCSOYSSR_1.0 soybean SSR database (Song et al. 2010). Such small differences in map positions alone do not indicate the existence of different PMD-resistant genes in the three resistant sources. However, in the validation test with three markers, two PMD resistant sources, PI 243540 and cultivar CNS, revealed different genotypes than PI 567301B in spite of having the same phenotype. These marker data suggest that the three soybean lines may carry different genes for PMD resistance. However, allelism tests using segregating populations from pairwise crosses of these three PMD- resistant soybeans would be helpful to reveal the allelic relationships among the PMD genes in question. Alternatively, further fine mapping of the PMD genes in each of the three sources may also resolve the allelic relationships. If they carry different resistance genes, pyramiding of multiple genes can provide more durable PMD resistance.
A cluster of disease resistance genes or quantitative trait loci (QTL) to several soybean pathogens has been mapped near the region of PMD resistance gene identified in this study. The gene Rps2 conferring resistance to Phytophthora sojae (Polzin et al. 1994); soybean cyst nematode resistance QTL SCN1-2 and SCN5-2 (Concibido et al. 1994, 1997) from Soybase (www.soybase.org/); three brown stem rot resistance genes Rbs 1 , Rbs 2 , and Rbs 3 (Bachman et al. 2001) were mapped to this region on chromosome 16. In addition, the multiple resistance gene analogs—(RGAs), RGA_1a, RGA_1b, and RGA_1c, have been mapped to this region spanning PMD resistance gene in PI 567301B on the Soybean Consensus Map 4.0 (Hyten et al. 2010). Thus, the presence of the multiple RGAs might be associated with the resistance to various soybean pathogens (Kanazin et al. 1996; Bachman et al. 2001). Richter and Ronald (2000) suggested that the clustered gene families of disease resistance genes could be created through the duplication of ancestor resistance genes. Interestingly, at least four candidate resistance genes (Glyma16g34030, Glyma16g34070, Glyma16g34090, and Glyma16g34110) were annotated in the region close to the PMD resistance gene in PI 567301B on chromosome 16 from the Williams 82 sequence, encoding a Toll-interleukin receptor (TIR)-nucleotide-binding site (NBS)-Leucine rich repeat (LRR) disease resistance protein. In barley, three distinct Mlo-associated NBS-LRR resistance gene families were reported, which can provide resistance to the PMD of barley, caused by Erysiphe graminis f. sp. hordei (Wei et al. 1999). Therefore, additional studies to investigate relationship between candidate resistance genes and PMD resistance will be needed.
In summary, we have mapped a potentially novel single dominant gene for PMD resistance in PI 567301B and developed three molecular markers tightly linked to the gene. These markers showed perfect co-segregation in a marker validation test of near isogenic lines. One SNP marker, PMSNP_1, can be used to perform a high-throughput genotyping for the selection of the PMD-resistant plants using melting curve analysis on the LightCycler 480® real time PCR platform. Therefore, this study provides speedy and reliable marker-assisted selection tools for breeding for PMD resistance using the gene from PI 567301B. We have also developed backcross (BC6) lines by transferring the gene to a soybean cultivar adapted to northeastern USA. The molecular markers developed in this research are publicly available to interested soybean breeders and researchers.
References
Bachman MS, Tamulonis JP, Nickell CD, Bent AF (2001) Molecular markers linked to brown stem rot resistance genes, Rbs1 and Rbs2, in soybean. Crop Sci 41:527–535
Bai Y, Pavan S, Zheng Z, Zappel NF, Reinstädler A, Lotti C, De Giovanni C, Ricciardi L, Lindhout P, Visser R, Theres K, Panstruga R (2008) Naturally occurring broad-spectrum powdery mildew resistance in a Central American tomato accession is caused by loss of Mlo function. Mol Plant Microbe Interact 21:30–39
Buschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Topsch S, Vos P, Salamini F, Schulze-Lefert P (1997) The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 88:695–705
Caldwell BE (1966) Inheritance of a strain-specific ineffective nodulation in soybeans. Crop Sci 6:427–428
Concibido VC, Denny RL, Boutin SR, Hautea R, Orf JH, Young ND (1994) DNA marker analysis of loci underlying resistance to soybean cyst nematode (Heterodera glycines Ichinohe). Crop Sci 34:240–246
Concibido VC, Lange DA, Denny RL, Orf JH, Young ND (1997) Genome mapping of soybean cyst nematode resistance genes in ‘Peking’, PI 90763, and PI 88788 using DNA markers. Crop Sci 37:258–264
Dunleavy JM (1980) Yield losses in soybeans induced by powdery mildew. Plant Dis 64:291–292
Gonçalves ECP, Mauro AOD, Centurion MAPC (2002) Genetics of resistance to powdery mildew (Microsphaera diffusa) in Brazilian soybean populations. Genet Mol Biol 25:339–342
Grau C (2006) Powdery Mildew of Soybean. Department of Plant Pathology, Madison, WI. http://www.planthealth.info/pdf_docs/powdery_mildew_WI.pdf. Accessed 14 Dec 2011
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hyten DL, Choi IY, Song Q, Specht JE, Carter TE, Shoemaker RC, Hwang EY, Matukumalli LK, Cregan PB (2010) A high density integrated genetic linkage map of soybean and the development of a 1536 universal soy linkage panel for QTL mapping. Crop Sci 50:960–968
Jun TH, Rouf Mian MA, Michel AP (2012) Genetic mapping revealed two loci for soybean aphid resistance in PI 567301B. Theor Appl Genet 124:13–22
Kanazin V, Marek LF, Shoemaker RC (1996) Resistance gene analogs are conserved and clustered in soybean. Proc Natl Acad Sci USA 93:11746–11750
Kang ST, Mian MA (2010) Genetic map of the powdery mildew resistance gene in soybean PI 243540. Genome 53:400–405
Kilen TC, Hartwig EE, Keeling BL (1974) Inheritance of a second major gene for resistance to phytophthora rot in soybeans. Crop Sci 14:260–262
Kim KS, Hill CB, Hartman GL, Hyten DL, Hudson ME, Diers BW (2010) Fine mapping of the soybean aphid-resistance gene Rag2 in soybean PI 200538. Theor Appl Genet 121:599–610
Lohnes DG, Bernard RL (1992) Inheritance of resistance to powdery mildew in soybeans. Plant Dis 76:964–965
Lohnes DG, Wagner RE, Bernard RL (1993) Soybean genes Rj2, Rmd, and Rps2 in linkage group 19. J Hered 84:109–111
Ma H, Kong Z, Fu B, Li N, Zhang L, Jia H, Ma Z (2011) Identification and mapping of a new powdery mildew resistance gene on chromosome 6D of common wheat. Theor Appl Genet 123:1099–1106
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Mignucci JS, Lim SM (1980) Powdery mildew (Microspraera diffusa) development on soybeans with adult-plant resistance. Phytopathology 70:919–921
Mulrooney B (2009) Weekly Crop Update: Soybean Disease Update. University of Delaware Cooperative Extension. http://agdev.anr.udel.edu/weeklycropupdate/?p=1550. Accessed 14 Dec 2011
Pavan S, Schiavulli A, Appiano M, Marcotrigiano AR, Cillo F, Visser RG, Bai Y, Lotti C, Ricciardi L (2011) Pea powdery mildew er1 resistance is associated to loss-of-function mutations at a MLO homologous locus. Theor Appl Genet 123:1425–1431
Phillips DV (1984) Stability of Microsphaera dijfusa and the effect of powdery mildew on yield of soybean. Plant Dis 68:953–956
Polzin KM, Lohnes DG, Nickell CD, Shoemaker RC (1994) Integration of Rps2, Rmd, and Rj2 into linkage group J of the soybean molecular map. J Hered 85:300–303
Richter TE, Ronald PC (2000) The evolution of disease resistance genes. Plant Mol Biol 42:195–204
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
SAS Institute (2002) The SAS system for Windows. Release 9.00. SAS Institute Inc., Cary, NC, USA
Silva OC, Seganfredo R (1999) Quantificação de danos ocasionados pordoenças de final de ciclo e oídio, em duas variedades de soja. In: CONGRESSO BRASILEIRO DE SOJA, Embrapa Soja, Londrina, Brasil, p 460
Song Q, Jia G, Zhu Y, Grant D, Nelson RT, Hwang EY, Hyten DL, Cregan PB (2010) Abundance of SSR motifs and development of candidate polymorphic SSR markers (BARCSOYSSR_1.0) in soybean. Crop Sci 50:1950–1960
Van Ooijen JW (2006) JoinMap 4. Software for the calculation of genetic linkage maps in experimental populations. B. V. Kyazma, Wageningen
Vuong TD, Sleper DA, Shannon JG, Nguyen HT (2010) Novel quantitative trait loci for broad-based resistance to soybean cyst nematode (Heterodera glycines Ichinohe) in soybean PI 567516C. Theor Appl Genet 121:1253–1266
Wei F, Gobelman-Werner K, Morroll SM, Kurth J, Mao L, Wing R, Leister D, Schulze-Lefert P, Wise RP (1999) The Mla (Powdery Mildew) resistance cluster is associated with three NBS-LRR gene families and suppressed recombination within a 240-kb DNA interval on chromosome 5S (1HS) of barley. Genetics 153:1929–1948
Yorinori JT, Hiromoto DM (1998) Determinação de perdas em soja causadas por doenças fúngicas. In: EMBRAPA—Centro Nacional de Pesquisa de Soja, vol 118. Londrina, pp 112–114
Yu J, Herrmann M (2006) Inheritance and mapping of a powdery mildew resistance gene introgressed from Avena macrostachya in cultivated oat. Theor Appl Genet 113:429–437
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Ray.
Trade and manufacturers’ names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by the USDA implies no approval of the product to the exclusion of others that may also be suitable.
Rights and permissions
About this article
Cite this article
Jun, TH., Mian, M.A.R., Kang, ST. et al. Genetic mapping of the powdery mildew resistance gene in soybean PI 567301B. Theor Appl Genet 125, 1159–1168 (2012). https://doi.org/10.1007/s00122-012-1902-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-012-1902-y