Abstract
Background
Abdominal lymphatic malformations (LM) are relatively rare findings in the differential diagnosis of focal abdominal lesions; however, they represent a challenge especially in younger patients. The aim of this review article is to provide up-to-date information about the different kinds of LM manifestations. In addition, related syndromes and typical imaging features to facilitate the diagnosis are discussed.
Results
The clinical presentation of abdominal LM is unspecific, whereby most are asymptomatic and comprise incidental findings of thin-walled cystic masses anywhere in the abdomen. The fluid in the cystic masses may be proteinaceous, contain blood, or be infected. Radiological imaging features overlap with other cystic diseases; hallmark in LM is a lack of a solid component and exclusive enhancement of the walls and septa.
Conclusion
In cystic abdominal masses in early childhood or young adults, abdominal LM must be taken into account by the radiologist. Newly defined entities in this spectrum of diseases are central conducting lymphatic anomaly (CCLA) and generalized lymphatic anomaly (GLA).
Zusammenfassung
Hintergrund
Abdominelle lymphatische Malformationen (LM) stellen relativ seltene Befunde in der Differenzialdiagnose fokaler abdomineller Läsionen dar. Dennoch sind sie gerade bei Patienten in einer jungen Altersgruppe diagnostisch eine Herausforderung. Das Ziel dieses Übersichtsartikels ist es, aktuellste Informationen über die unterschiedlichen Arten der Manifestation von LM zu geben. Darüber hinaus werden die in diesem Zusammenhang auftretenden Syndrome sowie typischen Merkmale der Bildgebung behandelt.
Resultate
Klinisch treten abdominelle LM meist asymptomatisch auf. In der Bildgebung werden häufig Zufallsbefunde von dünnwandig begrenzten zystischen Raumforderungen im gesamten Abdomen auffällig. Die Flüssigkeit in den zystischen Raumforderungen kann proteinreich sein, Blut enthalten oder Zeichen der Infektion aufweisen. Die radiologische Präsentation überschneidet sich dabei häufig mit anderen zystischen Erkrankungen. Das typische Zeichen der LM ist das Fehlen von soliden Anteilen und die ausschließliche Kontrastmittelaufnahme der zystischen Wände und Septen.
Schlussfolgerung
Differenzialdiagnostisch müssen Radiologen bei zystischen abdominellen Raumforderungen v. a. bei Kindern und jungen Erwachsenen eine LM in Betracht ziehen. Die kürzlich neu definierten Entitäten dieses Krankheitsspektrums sind die sog. „central conducting lymphatic anomaly“ (CCLA) und die generalisierte lymphatische Anomalie (GLA).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Abdominal lymphatic malformations (LM) are predominantly cystic abdominal masses. These are rare, accounting for about 5% of all lymphatic malformations [11]. However, abdominal LM comprise 6% of benign masses in childhood [18]; thus, it is justified to take them into account as a differential diagnosis, especially in the younger age group. Besides describing the typical presentation in imaging, this overview article aims to describe relevant differential diagnoses to these entities and describe newly defined subtypes.
Presentation
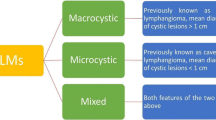
Abdominal LM consist of dysplastic, thin-walled endothelial channels containing lymphatic fluid, which are separated by thin fibrous septa. They are formed of an endothelial lining that develops around the 12th gestational week from the mesenteric root and bifurcation of the femoral vein with the sciatic vein in the groin [21]. According to the 2014 updated classification of the International Society for the Study of Vascular Anomalies (ISSVA), LM are subclassified according to the size of the cystic spaces into microcystic, macrocystic, or mixed types [20]. Before this interdisciplinary classification, nomenclature was inconsistent, with varying terms for the same condition, including cystic hygroma, lymphangioma, lymphangiomatosis, or even “hemangioma.” Different medical subspecialties described different classifications and the disease was diagnosed in children and adults, adding to the overall confusing misnomers [6].
As lymphatic channels are ubiquitous in the abdomen, they are localized either in the peritoneal or retroperitoneal space, or in solid organs. The extent of the lesion seems to imply “infiltrative growth” in some cases, which is not true, as there is almost no proliferation of the endothelial lining histologically. The effect of active infiltration is mimicked by the inborn extent of the LM, which can involve otherwise separate organ systems. The most common location is the mesentery.
Abdominal LM may increase in size at an early age—without substantial proliferation—through increasing fluid collection, and can gain substantial volume (Fig. 1). The lymphatic fluid contained within can be serous, sanguineous after common bleeding into the cystic spaces, or chylous if fluid collection has a connection to the lymphatic fluid of the bowel.
Symptoms and signs
Most abdominal LM are asymptomatic and comprise incidental findings in imaging. Symptoms of abdominal lymphatic malformations may derive from the mass effect causing local compression of the surrounding organs or bowel, and consist of abdominal distension, local pain, nausea, bowel obstruction, constipation, or diarrhea. Obstruction of the central conducting lymphatic channels in central conducting lymphatic anomaly (CCLA) can lead to retention of the lymphatic flow with lymphedema of the extremities or pubic/genital area, with or without lymph leakage through the skin and symptoms of protein-losing enteropathy. As is common in LM in other locations, sudden enlargement and even painfulness can occur due to a response to immunologic stimuli (e. g., respiratory or gastrointestinal infection) or bleeding into the lesion, which becomes harder to palpate, painful, and more echogenic in ultrasound; at a later stage with fluid-fluid levels due to separation of the corpuscular blood components from the liquid content. Another typical complication is bacterial superinfection of the cystic lesions, which may pose a threat to the patient if the infection becomes systemic. A typical germ in infected abdominal LM is Salmonella species, due to its ability to disseminate via the lymphatic vessels [5].
Histology
Biopsy of abdominal LM is difficult, as the solid endothelial walls are very thin. However, aspiration of yellow, serous fluid or chyle is highly suggestive of an LM when combined with a laboratory analysis of the eosinophilic liquid. In immunochemistry, the lining of the cystic spaces in LM stain with CD-31 and CD-34 as endothelial markers. A highly specific additional marker is podoplanin (D2-40), which seems to be specific for lymphatic endothelium [13].
Imaging features in ultrasound
First suspicion of an abdominal LM in imaging is based primarily on ultrasound, which is easy to perform and readily available in the pediatric age group without radiation exposure. Nowadays, the first signs of an abdominal LM are frequently identified in fetal grayscale ultrasound as a cystic abdominal mass [14]. The walls are echogenic and thin, whereas the contained fluid is anechogenic. Microcystic LM can appear more echogenic, comparable to a more solid lesion, but multiple small identifiable cysts are usually always part of the lesion (Fig. 2). In color-coded duplex ultrasound, the lesion is hypovascular; however, its common extension along major vessels, often encasing them, may lead to the false impression of vascularity of the lesion.
After bleeding or infection, the liquid content may be more echogenic or even contain fluid-fluid levels [16]. After bleeding into the cysts, a retracting residual solid-appearing thrombotic mass may be present, but this is completely avascular and should not be mistaken for a solid component of a tumor, which shows signs of perfusion [7].
Cross-sectional imaging
Even if MRI remains the standard for diagnosis or differential diagnosis of abdominal LM, CT scans of the abdomen may readily display the cystic lesions with thin walls and septa without a solid component. However, the septa are less conspicuous than in T2-weighted MRI. The surrounding bowel is displaced. The advantages of fast CT scanners are very few motion artifacts (can possibly be performed without sedation), and less bowel and pulsation artifacts, particularly if supplemented with intravenous and oral contrast. A CT scan may be helpful for detecting fine calcium deposits in the differential diagnosis to other cystic abdominal lesions, because LM rarely contain any substantial calcifications. However, in contrast to superficial LM, in abdominal LM, small, more linear, thin calcifications along the septa have been described in CT [1].
Contrast-enhanced MRI with its superior tissue contrast is the most reliable modality for detection and differential diagnosis of abdominal LM. As slow-flow lesions, LMs do not demonstrate any perfusion of the cystic lesions in dynamic contrast-enhanced MR angiography, even if vessels may be interspersed or encased along the cystic walls at low frequency. The contrast medium enhancement is restricted to the thin cystic walls and septa of the lesions, and may be tiny (Fig. 3). However, in superinfected cysts the enhancement of the walls can be substantial. LMs show extremely high signal intensity in T2- and T2-weighted fat-saturated imaging, whereas the contained fluid normally shows intensity comparable to water in all sequences. The signal of the cystic fluid may be different to water depending on the content after bleeding or infection, or chyle or proteinaceous components, again with possible fluid-fluid levels. A fat-rich liquid component in the cysts, especially a chylous content, may lead to signal loss in fat-saturated imaging or in-phase versus opposed-phase imaging [4, 22].
a Coronal, T2-weighted, fat-saturated image of the abdomen depicts thin-walled, very hyperintense abdominal mass with septa. No solid component is seen. b Same patient, axial T1-weighted image after contrast demonstrates hypointense fluid contained within and only tiny enhancement of the cystic walls
Microcystic LM may represent a challenge in T1-weighted imaging after gadolinium administration, as they seem to enhance almost completely due to the multiple very small cyst-walls and septa. Contrary to a solid tumor, the cystic nature of the lesion—even if it consists of multiple tiny cysts—is demonstrated in T2-weighted fat-sat imaging with very high signal intensity. The imaging hallmark is the absence of solid parts in the lesion.
Special forms
A combination of multifocal macrocystic or microcystic LM of solid organs involving the abdomen in combination with intraosseous LM is represented by generalized lymphatic anomaly (GLA) as well as its more aggressive form, Gorham–Stout disease (GSD), with progressive osteolysis and loss of cortical bone [15, 19]. These entities consist of a multitude of cystic LM manifestations in various osseous and periosseous locations, including ribs, spine (mostly cervical), pelvis, and long bones, together with multiple cystic LM in the thorax and/or abdomen including solid organs. In the abdomen, beside mesenteric LM, solid organs such as spleen and liver are involved, with multiple thin-walled cysts representing LM (Fig. 4). This finding is more often found in GLA than in GSD. New data suggest a viable medical treatment option in these diseases to be the mTOR inhibitor sirolimus, rendering correct diagnosis crucial [2].
Another newly defined LM in children involving the abdomen is CCLA, which consists of an inadequate clearing and reflux of lymphatic flow by dysmotility, stenosis, or aplasia of the central abdominal and/or abdominal main lymphatic drainage ducts [8, 19]. This insufficient drainage due to inborn errors of the lymph ducts leads to lymphostasis, hypertension, and backflow in various organ systems depending on the site of the blockage. Thoracic hypertension may lead to pulmonary lymphostasis and recurrent chylous pleural effusions, where an abdominal blockage (e. g., dys-/aplasia of the cisterna chyli) may lead to protein-losing enteropathy, chylous ascites, abdominal lymphatic cysts, and/or backflow and retention of lymphatic fluid in one or both extremities (lymphedema of the leg) or pubic area, resulting in lymphatic oozing through the skin. Intranodal lymphangiography with lipiodol injection through punctured lymph nodes in the groin helps to diagnose and treat this severe condition.
Differential diagnosis
Age at diagnosis is a major help in the differential diagnosis, as abdominal LM are diagnosed from newborns to school-age children and are very rarely seen in adults. Every cystic abdominal tumor-like lesion is to be taken into account as a differential diagnosis [3].
Compartmentalized ascites or other fluid collections such as abscesses, seromas, bilomas, urinomas, or lymphoceles are common findings and may mimic LM as a polycystic mass in the peritoneum. However, these liquids tend to be less compartmentalized, form no round cysts, be located between bowel loops without grossly dislocating them, and have a tendency to collect according to gravity, in the paracolic gutters and Morrison pouch.
In the pediatric age group, mesothelial or enteric duplication cysts are a potential differential diagnosis; here, the more circumscribed nature of the lesions and the location are taken into account. However, aspiration of fluid, together with laboratory and even cytological tests, may be necessary. Neonatal ovarian cysts may mimic abdominal LM, even with different signal intensities of the contained fluid. The intimate relation to the stretched, folliculate parenchyma of the residual ovaries is a clue too diagnosis here. In repeated imaging (preferably ultrasound), spontaneous resolution of ovarian cysts can be documented. Enteric duplication cysts, which are intestinal wall duplications lined with epithelium and contain smooth muscle cells, are typically located along the antimesenteric border. They are usually unilocular, without internal septa or encased mesenteric vessels, and appear more thick-walled as they contain all enteric layers [12]. All cystic neoplasms (especially cystic teratomas or cystic hepatoblastomas) are an important differential diagnosis to abdominal LM. Helpful criteria in the differential diagnosis are a solid part of the lesion and thicker, more asymmetric cyst walls, in part with marked vascularity. This is a feature shared with inflammatory cysts such as hydatoid cysts (liver, mesentery). Tumors or tumor-like lesions show calcifications far more often than LM.
Abdominal solid organ cysts or polycystic disease (liver, kidney, pancreas) can be a very difficult differential diagnosis to LM, as there is no typical morphological clue to separate these entities apart from the typical distribution pattern. Choledochal cysts may be difficult to differentiate from abdominal LM in the pediatric age group. However, the clinical presentation (jaundice, pain, and mass effect in the first years of life versus pancreatitis in older children) may be completely different, as LM do not lead to jaundice or pancreatitis.
Splenic LM are a typical imaging finding in patients with GLA; further evaluation should include MRI of the spine, pelvis, and large bones.
Therapy
As abdominal LM tend to increase in size [9,10,11], many specialists in interdisciplinary vascular anomaly centers prefer early treatment after diagnosis, even if some asymptomatic LM may involute spontaneously [14]. Surgical resection is prone to technical and anatomical difficulties, and often remains incomplete, with an ongoing discussion regarding whether there is recurrence or simply a residual lesion which tends to increase in size. In macrocystic abdominal LM, sclerotherapy (Fig. 5) is currently the first-choice treatment modality, with direct puncture via a needle, insertion of catheters with multiple sideholes, aspiration, and sclerotherapy with different agents as picibanil (OK-432), doxycycline, bleomycin, pure or gelified ethanol, polidocanol, sodium tetradecyl sulfate (STS), and others [17].
Practical conclusion
-
Abdominal lymphatic malformations (LM) are rare and represent a diagnostic challenge.
-
Since most LM are asymptomatic, they are often only detected as incidental findings.
-
Abdominal LM consist of dysplastic, thin-walled endothelial channels containing lymphatic fluid.
-
In radiological images, LM appear as cystic masses, although the absence of solid components and exclusive uptake of contrast medium by the cystic walls and septa are characteristic.
-
Standard treatment for macrocystic abdominal LM is sclerotherapy.
-
Particularly in children and young adults with corresponding findings should abdominal LM be considered in the differential diagnosis.
Literatur
Abbott RM, Levy AD, Aguilera NS et al (2004) From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics 24:1137–1163
Adams DM, Trenor CC 3rd, Hammill AM et al (2016) Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics 137:e20153257
Arraiza M, Metser U, Vajpeyi R et al (2015) Primary cystic peritoneal masses and mimickers: spectrum of diseases with pathologic correlation. Abdom Imaging 40:875–906
Ayyappan AP, Jhaveri KS, Haider MA (2011) Radiological assessment of mesenteric and retroperitoneal cysts in adults: is there a role for chemical shift MRI? Clin Imaging 35:127–132
Chinai N, Sun Y, Steffen C (2010) Salmonella enteritidis-infected intra-abdominal lymphangioma. Anz J Surg 80:102–103
Faul JL, Berry GJ, Colby TV et al (2000) Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med 161:1037–1046
Francavilla ML, White CL, Oliveri B et al (2017) Intraabdominal lymphatic malformations: pearls and pitfalls of diagnosis and differential diagnoses in pediatric patients. Ajr Am J Roentgenol 208:637–649
Goyal P, Alomari AI, Kozakewich HP et al (2016) Imaging features of kaposiform lymphangiomatosis. Pediatr Radiol 46:1282–1290
Hancock BJ, St-Vil D, Luks FI et al (1992) Complications of lymphangiomas in children. J Pediatr Surg 27:220–224
Kosir MA, Sonnino RE, Gauderer MW (1991) Pediatric abdominal lymphangiomas: a plea for early recognition. J Pediatr Surg 26:1309–1313
Lal A, Gupta P, Singhal M et al (2016) Abdominal lymphatic malformation: spectrum of imaging findings. Indian J Radiol Imaging 26:423–428
Macpherson RI (1993) Gastrointestinal tract duplications: clinical, pathologic, etiologic, and radiologic considerations. Radiographics 13:1063–1080
North PE (2010) Pediatric vascular tumors and malformations. Surg Pathol Clin 3:455–494
Oliveira C, Sacher P, Meuli M (2010) Management of prenatally diagnosed abdominal lymphatic malformations. Eur J Pediatr Surg 20:302–306
Ozeki M, Fujino A, Matsuoka K et al (2016) Clinical features and prognosis of generalized lymphatic anomaly, kaposiform lymphangiomatosis, and gorham-stout disease. Pediatr Blood Cancer 63:832–838
Paltiel HJ, Burrows PE, Kozakewich HP et al (2000) Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology 214:747–754
Russell KW, Rollins MD, Feola GP et al (2014) Sclerotherapy for intra-abdominal lymphatic malformations in children. Eur J Pediatr Surg 24:317–321
Tasnadi G (1993) Epidemiology and etiology of congenital vascular malformations. Semin Vasc Surg 6:200–203
Trenor CC 3rd, Chaudry G (2014) Complex lymphatic anomalies. Semin Pediatr Surg 23:186–190
Wassef M, Blei F, Adams D et al (2015) Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics 136:e203–214
Wilson SR, Bohrer S, Losada R et al (2006) Retroperitoneal lymphangioma: an unusual location and presentation. J Pediatr Surg 41:603–605
Yoo E, Kim MJ, Kim KW et al (2006) A case of mesenteric cystic lymphangioma: fat saturation and chemical shift MR imaging. J Magn Reson Imaging 23:77–80
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
W.A. Wohlgemuth, L.M. Dendl, R. Brill, F. Stangl, D. Stoevesandt, and A. Schreyer declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Rights and permissions
About this article
Cite this article
Wohlgemuth, W.A., Brill, R., Dendl, L.M. et al. Abdominal lymphatic malformations. Radiologe 58 (Suppl 1), 29–33 (2018). https://doi.org/10.1007/s00117-017-0337-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00117-017-0337-5
Keywords
- Differential diagnosis
- Central conducting lymphatic anomaly
- Lymphatic malformation
- Imaging features
- Cystic masses