Abstract
Lymphatic malformation (LM) is the currently preferred term for what was previously known as lymphangioma. Retroperitoneal LMs are extremely rare, benign, cystic masses that arise from lymphatic vessels. They can be challenging to diagnose because they resemble other retroperitoneal cystic tumors. The development of treatment strategies for rare diseases, including retroperitoneal LM, requires the acquisition of new knowledge to enhance our understanding of the disease progression. Therefore, we present an update regarding fundamental and advanced issues associated with retroperitoneal LM. This review describes the epidemiology, histopathology, biomedicine, clinical manifestations, radiological features, differential diagnosis, and management of this lesion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the term lymphangioma can be found in both previous and present literature, it can be confusing [1, 2], and the currently preferred term for this disease is lymphatic malformation (LM) [3, 4]. Recent literature has provided evidence to suggest that this entity represents a true malformation of the lymphatic system instead of being a tumor. The International Society for the Study of Vascular Anomalies (ISSVA) has adopted a basic classification system for differentiating vascular tumors from vascular malformations [5], in which LMs are local or diffuse soft-tissue lesions that are classified as slow-flow malformations. Three types of LM have been described based on clinical and diagnostic imaging results: macrocystic, microcystic, and mixed (Figs. 1, 2) [6].
Etiology
LMs are benign proliferations that typically manifest as fluid-filled cysts. LMs appear to arise from the isolation of localized lymphatic tissues that are unable to communicate with the normal lymphatic system of the body [7] and are thought to be congenital [8]. Although some acquired etiologies, such as fibrosis, trauma, and tumors, have also been associated with LMs, these relationships are not clear [9]. The spread of lymphatic lesions is primarily caused by the excessive stretching of fluid follicles [10]. However, LM development has also been hypothesized to occur due to proliferation [11]. Some authors have hypothesized that LMs represent an anomaly of lymphatic system development but concluded that they also have proliferative potential because of the identification of intrusive germ seeding in the peripheral tissues of resected lesions [12, 13]. Recently, low-level hyperplasia has been demonstrated by fertility markers [14, 15]. Therefore, LMs (in addition to arterial or venous malformations) may grow and regenerate due to retained proliferative abilities [16, 17].
Epidemiology
LMs are uncommon malformations of the lymph vessels [9]. LMs typically occur in children [18] and are commonly located in the head and neck region, but they can occur in any location throughout the body [19,20,21]. Usually, tumors present in the retroperitoneum are mostly malignant tumors [22], and cystic masses that develop in the retroperitoneal space are rare [23, 24]. Intra-abdominal LM represents 3–9.2% of all LMs [25, 26]. In the abdomen, LMs occur most commonly in the mesentery, omentum, and mesocolon [27]. Retroperitoneal LMs are very rare, accounting for fewer than 1% of all LM cases [28, 29].
Histopathology
Specimens of LMs are characterized by vascular channels of various sizes with an impaired endothelial lining, although cubic endothelial regions may also be observed (Fig. 3) [28, 30]. The smallest channels are only lined by the endothelium, whereas the larger channels may exhibit an irregular and dissimilar smooth muscle layer. The wall thickness can be variable, and many of the lumens are empty [31], although some lumens contain pale proteins, lymphocyte clusters, blood, blood clots, or hemosiderin (Figs. 3, 4, and 5) [2, 32]. These lumen contents may be the results of spontaneous bleeding, trauma, surgery, or connection to the venous system [33, 34].
LM in a 26-year-old male patient admitted to the hospital with a fever of unknown cause. A transverse ultrasound image of the abdomen (A) showed a cystic mass sized 17 × 20 × 36 cm, which occupies most of the left abdomen. The cystic mass had a multilobed shape and a thin wall, with many thin septa inside the mass. Photograph (B) showed a gross LM specimen, with multiple cysts and intersecting septae. Photomicrographs (× 50 and × 200; hematoxylin–eosin stain) (C, D) showed multiple, large, irregular, cystic luminal spaces. The cystic spaces were lined by a single layer of benign, flattened endothelium. The stroma showed a dense lymphocytic infiltration, with the formation of lymphoid follicles, and a dense fibro-cellular layer supporting the cystic spaces
Cavernous LM in a 23-year-old female patient with dull epigastric pain. Comprehensive clinical and laboratory testing ruled out a pancreatic pseudocyst. Her condition did not improve, so she underwent laparoscopic surgery to remove the lesion. Axial CT image (A) showed a cystic lesion (asterisk) in the epigastric retroperitoneal space. Micrograph using H and E stains at × 100 magnification (B) showed spaces (Ls) lined by flat endothelial cells (arrowheads)
LM in a young male patient who complained of dull pain in the left abdomen for 14 days. Axial contrast-enhanced CT image (A) showed a large cystic mass in the left abdomen with multiple thin septa. Microscopic (B, × 200; C, × 400; hematoxylin–eosin stain) images showed multiple, large, irregular, cystic luminal spaces. The cystic spaces were lined by a single layer of benign, flattened endothelium. The stroma showed a dense lymphocytic infiltration and the formation of lymphoid follicles. The cystic spaces are supported by a dense fibro-cellular layer
Macrocystic LMs typically feature a single, thick-walled compartment containing fibrous muscle tissue, a few smooth muscle cells, and an interstitial matrix. The endothelium is usually absent. Superstructure studies of small vascular channels have revealed endothelial cells that are collapsed with an incomplete basal layer and fibrils that bind the basal cells with the underlying connective tissue [35,36,37].
Lymph endothelial cells can be identified using several antibodies, such as anti-Proxl and anti-vascular endothelial growth factor receptor (VEGFR)-3, which are superior to the anti-podoplanin antibody D2-40 or anti-lymphatic vessel endothelial hyaluronan receptor (LYVE-1) antibodies. In particular, large circuit channels often appear to be partially stained, either without all the antibodies or without D2-40 and LYVE-1 antibodies. Arteries and veins do not feature endothelial cells. The response to anti-CD31 antibodies tends to be unsystematic, and CD34 is often faint or absent [38,39,40]. Miettinen and Wang also noted the detection of Proxl in vascular malformations associated with veins and the lymphatic system [35, 41].
Molecular biology
Recently, DNA analysis studies of LM tissue have identified pre-zygotic somatic mutations as an underlying cause of LMs [42, 43]. Novel approaches to DNA sequencing have facilitated the detection of post-zygotic somatic mutations that have clarified the causes of LMs [44,45,46,47]. Unlike genetic mutations, which occur in every cell, somatic mutations can have local effects at many different anatomic locations, often resulting in mosaic patterns [48].
A functional somatic mutation in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) affects the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway in the LM tissue. Activated PIK3CA promotes cell proliferation, growth, angiogenesis, and protein synthesis [49, 50]. This molecular pathway is correlated with tissue overgrowth and the persistence of malformed lesions [51, 52]. The increased understanding of the role played by this pathway in the development of LMs has introduced the potential of molecular medicine-based treatments for patients with LMs [42] and the development of novel treatment options [43].
Genetics of LMs
Hereditary LMs are caused by inadequate lymphatic drainage, defects, or hyperplasia in lymphatic vessels. The first locus identified for hereditary LMs was mapped to chromosome 5q35, and the pathogenic gene was eventually identified as FLT4, which encodes VEGFR-3 [53, 54].
Hereditary LMs associated with VEGFR-3 mutations are typically characterized as type-I, which are early-onset, often occurring at birth or shortly after birth. Hereditary type-II LMs are late-onset, with little infiltration, altered phenotypes, and other characteristics, including pigmentation disorders, visceral prolapse, cleft palate, yellow nails, and congenital heart problems. Type-II LMs are thought to be caused by mutations in the gene encoding MFH1 on chromosome 16q24.3 [54, 55].
Physiological studies of LMs have been aided by remarkable progress in the overall understanding of the factors that regulate the development of the lymphatic and vascular systems. Furthermore, technical advances, such as immunohistochemistry staining using excellent markers, such as glucose transporter 1 (GLUT1), D2-40, and PROX1, have facilitated the identification of vascular malformations [41, 56]. Staining against VEGFR-3 can be used to distinguish lymphatic vessels from arteries and veins [56, 57].
Genetic factors play important roles in the pathogenesis of LMs. Recently, significant advances have been made toward identifying the genetic and molecular factors associated with a variety of vascular malformations, and several genes associated with LM development have been identified [58, 59].
Clinical manifestations
The clinical presentation of LMs depends on the anatomic location, size, and characteristics of the lesion. Most patients are asymptomatic, and LMs are often detected incidentally during imaging evaluations or surgery for other indications [60, 61]. However, a minority of LMs can cause symptoms and present as a palpable abdominal mass that compress adjacent structures or as an internal cystic hemorrhage, causing abdominal pain, intestinal or ureteric obstruction, and hematuria [62, 63]. When LMs are combined with other vascular malformations, such as venous malformation or capillary malformation, symptoms may vary depending on the number of involved blood vessels [64, 65].
Imaging findings
Ultrasound is often used as the initial diagnostic tool for the evaluation of cystic abdominal masses and can be used to identify LM properties [66]. LMs are categorized as macrocystic when individual malformed channels are larger than 10 mm, whereas LMs are categorized as microcystic when the individual channels are smaller than 10 mm, and both sizes can be present together. Macrocystic LMs present as multilobular cystic lesions, whereas microcystic lesions are ambiguous and hyperechoic due to multiple interfaces between small follicular walls. Microcystic forms typically manifest with more infiltration and internal bleeding tendency. Mixed lesions include both cystic and solid components, which are related to the size of the cyst and their shape on ultrasound (Figs. 3, 6, and 7) [5, 67]. Color Doppler ultrasound may show vascular channels inside the septum, including normal veins and arteries, which can be confirmed by spectral Doppler analysis (Fig. 8) [68]. In cases of hemorrhagic or inflammatory complications, fluid–fluid levels can be observed in the follicles [69, 70].
Macrocystic LM in a 33-year-old male patient who presented with mild abdominal pain in the left abdomen. Grayscale ultrasound (A, B) and post-contrast CT (C, D) images showed a multilobed cystic lesion in the retroperitoneal space in front of the left kidney. The fluid compartments (asterisks), the inner septa of the lesion (arrows). The lesion was diagnosed by histopathology
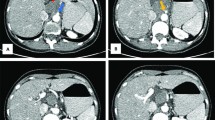
Macrocystic LM was detected incidentally in a 26-year-old patient. Grayscale (A) and color Doppler ultrasound (B) images showed a large well-defined multiseptated cystic lesion at the posterior right kidney of retroperitoneal space. Contrast-enhanced CT images with the arterial (C) and delayed (D) phases showed a cystic lesion that is adjacent to the right kidney and does not enhance after contrast injection. The fluid compartments (asterisks), the inner septa of the lesion (arrows)
Mixed LM in a 55-year-old female patient presented with a right retroperitoneal cystic mass. She had no remarkable history. Color Doppler (A) and grayscale (B) ultrasound images showed a large mixed cystic mass in the right retroperitoneal space with multiple vascularly signaled walls. The lesion was surgically removed because of suspected malignancy. Pathology confirmed the lesion to be mixed LM
On computed tomography (CT), most LMs appear as homogeneous cystic components, but some may appear heterogeneous due to the presence of proteinaceous, fluid, blood, or fat components within the lesions [13]. Cystic LMs are typically well-defined, multicystic, and may show mild enhancement of the septa or the wall after intravenous contrast agent administration [66]. They may also form unilocular or multilocular cystic masses, which may not be limited to a particular abdominal compartment and may displace intra-abdominal organs and vessels (Figs. 4, 5, 6, 7) [71, 72].
On magnetic resonance imaging (MRI), the lesions present a multilobular septal form [73]. They have iso-intensity to hypointensity on T1-weighted images and hyperintensity on T2-weighted and short tau inversion recovery (STIR) images, as these cystic lesions can be of various sizes and are often filled with fluid (Fig. 9, 10) [74, 75]. Internal fluid–fluid levels can be observed. To detect blood components in the lesion can use basic sequences combined with magnetic sensitive sequences. Similarly, for fat component detection, fat suppression imaging, or chemical shift imaging can be used. Pure LMs do not enhance inside after contrast agent administration and consist entirely of fluid storage spaces that are unconnected to the venous system. Contrast agents can enhance the peripheral walls and septa of the lesion [76, 77], and the enhancement of capsules and walls is especially apparent in macrocystic LMs. Microcystic LMs do not enhance significantly. Surrounding lymphoedema may also be observed [13, 78].
Axial T2-weighted (A), sagittal T2-weighted fat-suppressed (B), coronal T1-weighted (C) images of a 43-year-old woman who was detected incidentally during pelvic examination show a small well-defined, unilocular, thin-walled cystic lesion adjacent to left psoas muscle. The lesion appeared as homogeneous hyperintense on the T2-weighted image and homogeneous hypointense on the T1-weighted image. The lesion was treated conservatively and was likely to be diagnosed as retroperitoneal LM. During subsequent follow-ups, no growth was observed
Macrocystic LM in a 37-year-old woman presenting with periumbilical abdominal pain. Axial T2-weighted (A), T1-weighted (B), and DWI (C) images of the pelvis showed a well-defined multilocular cystic lesion in the left pelvis with mass effect on the adjacent organs (asterisks). It appears heterogeneously hyperintense on the T2-weighted image, hypointense on the T1-weighted image, and no diffusion restriction. The lesion was resected by laparoscopic surgery. Histopathology confirmed as macrocystic LM. This lesion initially was misdiagnosed as a left ovarian cyst
Differential diagnosis
The retroperitoneal space is the region between the peritoneum and the posterior parietal wall of the abdominal cavity, which extends from the pelvic floor to the diaphragm [79]. Masses of retroperitoneal origin include a heterogeneous and diverse group of lesions [80]. Retroperitoneal masses can be classified as solid or cystic, depending on their radiological appearance [81]. Solid lesions can be categorized into four groups according to the origin: germ cell, neural, mesenchymal, and lymphoproliferative [80, 81].
Retroperitoneal cysts can be classified into two predominant types. The first type is epithelial cysts arising from the major retroperitoneal organs (kidney, pancreas, colon, duodenum, adrenal glands). The latter arises from the retroperitoneal space but outside the major organs. Among cystic lesions, the most common are LMs, mesothelial cyst, enterogenous cysts, urogenital cyst, or cystic neoplasms [81, 82].
Many of the retroperitoneal masses are malignancies, approximately 75% of which have mesenchymal origins [22]. The differential diagnosis of a cystic lesion in the retroperitoneum can be malignant or benign, which is important for treatment plans [22, 80]. Identifying the site of origin of any mass (compartment or organ) is important in determining the differential diagnosis.
Cystic lesions can be classified as neoplastic and non-neoplastic lesions (Tables 1, 2) [83, 84]. Neoplastic cystic lesions include lymphangiomatosis (Fig. 11), lymphangioleiomyoma, lymphangioleiomyomatosis, cystic teratoma (Fig. 12), epidermoid cyst, mucinous cystadenoma (Fig. 13), cystic mesothelioma (Fig. 14), Müllerian cyst (Fig. 15), tailgut cyst (Fig. 16), bronchogenic cyst (Fig. 17), perianal mucinous carcinoma, pseudomyxoma retroperitonei, cystic lesions of retroperitoneal organs (Figs. 18), cystic lesions from peritoneal organs extending to retroperitoneal space (Figs. 19, 20), and cystic degeneration in solid lesions. Non-neoplastic cystic lesions include pancreatic pseudocyst (Fig. 21), nonpancreatic pseudocyst, lymphocele (Fig. 22), hematoma, and urinoma (Fig. 23) [83,84,85,86]. Other rare non-neoplastic diseases include retroperitoneal cystic fibrosis, extramedullary hematopoiesis, and non-Langerhans histiocytosis [87].
Generalized lymphangiomatosis in a 16-year-old male with a large mass over the left lower back area growing progressively for 3 months and pain in the right pelvic and bilateral lower back radiating into lower limbs. Non-enhanced axial (A) and coronal CT (B) images showing multilocular cystic lesions within bilateral psoas muscles, left extensor muscles and left lower back subcutaneous region (asterisks). There was an osteolytic of the spine with a sclerotic margin (arrows). MRI coronal STIR (C) and axial T1W Vibe Dixon Gado (D) show multilobar fluid signal lesion (asterisks) with moderately thickened septa with post-gadolinium enhancement. Sagittal T2W image (E) shows hyperintense lesions over lumbosacral vertebrae (arrows) extending into the spinal canal that results in nerve root compression of cauda equina and cystic lesions are also observed in the lower abdominal region anterior to the sacrum (asterisks). Cytopathological examination (F) of yellowish fluid obtained by ultrasound-guided fine-needle aspiration of the psoas muscle revealed a homogeneous population of small and round lymphocytes with some interstitial histiocytes. Some centrosomes, centroblasts, and plasma cells were also observed. All showed a heterogeneous population of mature lymphocytes and chylomicrons with no suspect malignant cells, consistent with the diagnosis of generalized lymphangiomatosis (Giemsa stain, at 40 × magnification). The patient was treated conservatively and the patient was symptom-free after 1 month
Retroperitoneal mature cystic teratoma in a 22-year-old patient attending with right hypochondria and a history of left ovary dermatome cysts removal. Axial (A) and coronal (B) CT images show a multilobular cystic lesion (asterisk) with fat components inside (arrow). The lesion is a large retroperitoneal mass covered in the middle of the liver and right kidney with 120 × 80 × 65 mm. It is composed of both cystic and solid elements, but no calcification density was found. MRI gives images similar to CT Scan with a large multilobed cystic mass with mild enhanced smooth wall and thin septations and little non-enhanced solid contents. The cystic has a hemorrhagic heterogeneous signal intensity in sites with many different phases. MRI of a tumor in the axial plane on T2 TSE FS (C) and in the coronal plane on T2 Haste (D) show a multilocular lesion with the signal intensity of greasy components (asterisk). Wall and septum of the tumor show enhancement (arrow). (E) The surgical specimen after Kocher's laparotomy demonstrated a multilobed cystic mass well-circumscribed with smooth borders and rubbery consistency. Cut sample of the mass showed multilocular cystic spaces, whitish-gray walls, scattered yellowish adipose tissue collections, and mucus secretions. The cyst wall was up to 4 mm thick. (F) A cross-sectional micrograph of a part of the lesion shows that the lesion includes stratified squamous epithelium (orange arrowhead), cartilaginous tissue (red arrowhead), and muscle tissue (blue arrowhead). The diagnosis of mature cystic teratoma was made up as final with no mark of malignancy or immature elements. The patient then had a 2-week follow-up with no development of ailment nor significant complications
Retroperitoneal primary mucinous adenocarcinoma in a 23-year-old female patient presenting with progressive abdominal distension and weight loss within 4 months. She had no significant past medical histology or family history of the disease. Laboratory data showed high levels of the carcinoembryonic antigen and carbohydrate antigen. MRI (A–F) revealed a large multilobed cystic mass in the abdominal cavity with enlarged lymph nodes along the aorta. The wall and septum of the lesion are strongly enhanced after using contrast agents (asterisks). Lesions located in the retroperitoneal space were confirmed by surgery. Pathology confirmed the diagnosis as mucinous adenocarcinoma. The patient was stable thereafter and no recurrence was observed after 1 year of follow-up
Retroperitoneal cystic mesothelioma in a 53-year-old woman presenting with mild right upper quadrant tenderness. Axial CT images in the late arterial (A) and delayed (B) phases after contrast administration show a single-lobed cystic mass in the right abdomen (asterisks). The mass did not show enhancement and displaced intestinal loops, contacts, and compresses the liver parenchyma (arrows) and gallbladder. A simple hepatic cyst was diagnosed initially and laparoscopic surgery was subsequently performed to avoid the risk of rupture. However, no intraperitoneal masses were seen and a large multiloculated retroperitoneal cyst was detected, in contact with the right adrenal gland and colon. This mass was completely aspirated and excised. The lesion was confirmed by histopathology
A retroperitoneal Müllerian cyst was detected incidentally in a 57-year-old asymptomatic woman. A Axial CT image shows a cyst in the retroperitoneal region of the left iliac bundle (asterisk). A laparoscopy with cystectomy was performed. B Histological image shows an inner cystic lining of ciliated epithelium of Mullerian type (arrow) and some lymphocytic aggregates and smooth muscle cells in the stroma
Retroperitoneal tailgut cyst in a 48-year-old female with a history of dull discomfort in the lower abdomen and constipation. MRI axial T2W image (A) showed a well-defined hyperintense cystic mass with a thin wall in presacral space (asterisk). The rectum and uterus were compressed and anteriorly displaced. The photomicrograph of the histologic specimen (B) showed the wall of the cyst that was lined with ciliated epithelium (arrow)
Retroperitoneal bronchogenic cyst in a 70-year-old male patient who was admitted to the hospital because of abdominal pain in the epigastrium. Abdominal ultrasound (A) shows a large well-defined cystic mass in the right epigastrium adjacent to the abdominal aorta (asterisk). A CT survey was performed immediately to rule out abdominal aortic rupture. Axial (B–D), coronal (E), and sagittal (F) CT images revealed a retroperitoneal thin-walled cystic mass with regular margins, located between the spine, vena cava, abdominal aorta, adjacent to the liver and diaphragm. After intravenous contrast injection, no internal septa or nodules were seen (asterisks). Laparoscopic surgery was performed; however, complete resection of the cyst was not achieved due to its adhesion to the aorta and inferior vena cava. Histopathological examination revealed a cystic lesion lined with respiratory epithelium confirming the diagnosis of a bronchogenic cyst
Cystic Wilms tumor arising from the right kidney mimicked retroperitoneal LM in a 3-year-old boy. Contrast-enhanced CT images in the corticomedullary phase (A) and delayed (B) phase showed a large cystic mass (asterisks) in the right retroperitoneal abdomen with thin enhanced septa that attached to the right kidney (arrows). Wilms tumor was confirmed by surgery and histopathology
Coronal (A) and sagittal CT (B) images of a 47-year-old woman showed a mostly intra-abdominal ovarian cyst (asterisks). Often, very large tumors can be difficult to distinguish whether they are in the peritoneal or retroperitoneal space. However, this cystic mass showed connection to the left ovary (arrow). An ovarian cyst tumor was confirmed by surgery
Esophageal duplication cyst mimicking retroperitoneal LM in a 31-year-old man presenting with abdominal pain. Abdominal grayscale (A) and color Doppler (B) ultrasound incidentally revealed a cystic structure in contact with the left hepatic posterior surface (asterisks). The CT images (C-F) showed a fluid-dense structure in the epigastrium adjacent to the esophagus (asterisks) with location and appearance most consistent with an esophageal duplication cyst
Pancreatic pseudocyst mimicking LM in a 55-year-old woman presenting with 3-day dull epigastric pain. She had a history of chronic pancreatitis. Ultrasound image (A) showed a cystic structure in the epigastrium with echogenic fluid (asterisk). CT image (B) showed a cystic structure in the epigastrium slightly to the right (asterisk) and the lesion contacted with the pancreas (arrow). The patient was treated conservatively. During follow-up, the cyst size gradually decreased in size and stabilized. With history and image studies, the final diagnosis was confirmed as a pancreatic pseudocyst
Intraperitoneal and retroperitoneal lymphocele mimicking LM in a 46-year-old female patient with a history of pelvic trauma 7 days ago and presenting to abdominal dull pain. CT images (A–D) showed hypodense cystic structures in the right mesenteric, retroperitoneal, and hypogastric regions (asterisks). The cystic masses appeared with smooth lobulated contours and regular walls did not show contrast enhancement after intravenous contrast. The patient was not followed up because she refused to be treated at the hospital. However, based on imaging and trauma condition, the most likely diagnosis was lymphocele. Peritoneal inclusion cyst (peritoneal pseudocyst) should also be included in the differential diagnosis
Urinoma in a 55-year-old female patient presented for general check-ups with a history of blunt trauma to the left kidney 3 months ago. Axial contrast-enhanced CT images (A, B) showed a well-defined collection of fluid (asterisks) in the retroperitoneal space adjacent to the left ureter. No contrast extravasation from blood vessels or ureters was seen. The lesion was stable and decreased in size in the next follow-ups. Based on the trauma history and imaging studies, the final diagnosis was urinoma. In many cases, urinomas can mimic LM if there is no well-documented clinical history
Management
The treatment of choice for retroperitoneal LMs is complete surgical resection, in most cases, including asymptomatic cases, due to the risk of future complications. However, aspiration and the injection of sclerosing agents have also been recommended [88, 89]. Indications for the treatment of LMs depend on the degree of disfigurement, malformation size, evidence of chronic lymph fluid leakage, and the frequency of inflammatory episodes. During the surgical treatment of LM lesions, injury to important adjacent structures should be avoided, particularly because LM lesions are benign [90, 91].
The identification of disordered genetic pathways in LM tissue has encouraged clinicians to use a variety of treatments for LMs associated with the PIK3CA mutation and tissue overgrowth. Rapamycin can inhibit a component of the PI3K/AKT1 pathway called mammalian target of rapamycin (mTOR) [92]. Rapamycin is a macrolide antibiotic that affects various pathological processes that require the activation of mTOR and is often used to suppress immunity during organ transplantations or autoimmune lymphoproliferative syndrome [93, 94].
Recently, a phase II clinical trial demonstrated that the empirical use of rapamycin in patients with complex LMs was safe and reduced the incidence of cellulitis, days of treatment, and infection. Side effects include gastrointestinal disturbances, lipid metabolism disorders, and blood and bone marrow abnormalities [95, 96]. The response to treatment included pain relief and decreased bleeding, although a complete response was infrequent. The complete lack of response is unclear, and future studies remain necessary to determine the optimal therapy for patients with LMs. Corticosteroids can help reduce inflammation associated with LMs [97, 98].
Basic and clinical research is currently being performed to enhance the reliability of non-surgical treatments, which will hopefully result in increased options for the medical and biological therapy of LMs. As this develops, the methods supporting the standardization will produce more than expected results [94,95,96,97,98,99].
Conclusion
Retroperitoneal LMs are rare clinical entities in adults. Because most LMs are asymptomatic, they are often only detected as incidental findings. The differentiation of cystic LMs from other cystic growths using imaging studies alone is often challenging. Ultrasound is the modality of choice for the initial assessment of LMs, followed by CT scans and MRI to delineate the lesion extension. Surgery is the most recommended option to provide both a definitive diagnosis and treatment. LMs should be considered in children and young adults who present with retroperitoneal cystic masses.
References
Morrison SC, Reid JR. Continuing problems with classifications of vascular malformations. Pediatr Radiol. 2007;37:609. https://doi.org/10.1007/s00247-007-0442-0.
Ramashankar PC, Shah NK, Giraddi G. Lymphatic malformations: a dilemma in diagnosis and management. Contemp Clin Dent. 2014;5(1):119–22. https://doi.org/10.4103/0976-237X.128689.
Khunger N. Lymphatic malformations: current status. J Cutan Aesthet Surg. 2010;3(3):137–8. https://doi.org/10.4103/0974-2077.74487.
Miceli A, Stewart KM. Lymphangioma. [Updated 2020 Aug 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470333/
ISSVA Classification of Vascular Anomalies © 2018 International Society for the Study of Vascular Anomalies Available at “issva.org/classification” Accessed 12 Nov 2020
Ahlawat S, Fayad LM, Durand DJ, Puttgen K, Tekes A. International society for the study of vascular anomalies classification of soft tissue vascular anomalies: survey-based assessment of musculoskeletal radiologists’ use in clinical practice. Curr Probl Diagn Radiol. 2019;48(1):10–6. https://doi.org/10.1067/j.cpradiol.2017.10.003.
Mirza B, Ijaz L, Saleem M, Sharif M, Sheikh A. Cystic hygroma: an overview. J Cutan Aesthet Surg. 2010;3(3):139–44. https://doi.org/10.4103/0974-2077.74488.
Poroes F, Petermann D, Andrejevic-Blant S, Labgaa I, Di Mare L. Pediatric cystic lymphangioma of the retroperitoneum: a case report and review of the literature. Med (Baltimore). 2020;99(28): e20827. https://doi.org/10.1097/MD.0000000000020827.
Minocha PK, Roop L, Persad R. Cases of atypical lymphangiomas in children. Case Rep Pediatr. 2014;2014: 626198. https://doi.org/10.1155/2014/626198.
Kudur MH, Hulmani M. Extensive and invasive lymphangioma circumscriptum in a young female: a rare case report and review of the literature. Indian Dermatol Online J. 2013;4(3):199–201. https://doi.org/10.4103/2229-5178.115516.
Heredea R, Cimpean AM, Cerbu S, Popoiu CM, Jitariu AA, Raica M. New approach to rare pediatric multicystic mesenteric lymphangioma; would it guide the development of targeted therapy? Front Pediatr. 2018;6:223. https://doi.org/10.3389/fped.2018.00223.
Goetsch E. Hygroma colli cysticum and hygroma axillare: pathologic and clinical study and report of twelve cases. Arch Surg. 1938;36:394–479.
Mulliken JB, Burrows PE, Fishman SJ. Mulliken and young’s vascular anomalies: hemangiomas and malformations. USA: OUP; 2013. p. 327–507.
Kolay SK, Parwani R, Wanjari S, Singhal P. Oral lymphangiomas—clinical and histopathological relations: an immunohistochemically analyzed case series of varied clinical presentations. J Oral Maxillofac Pathol. 2018;22(Suppl 1):S108–11. https://doi.org/10.4103/jomfp.JOMFP_157_17.
Motahhary P, Sarrafpour B, Abdirad A. Bilateral symmetrical lymphangiomas of the gingiva: case report. Diagn Pathol. 2006;1:9. https://doi.org/10.1186/1746-1596-1-9.
Usha V, Sivasankari T, Jeelani S, Asokan GS, Parthiban J. Lymphangioma of the tongue—a case report and review of literature. J Clin Diagn Res. 2014;8(9):ZD12–4. https://doi.org/10.7860/JCDR/2014/9890.4792.
Cox JA, Bartlett E, Lee EI. Vascular malformations: a review. Semin Plast Surg. 2014;28(2):58–63. https://doi.org/10.1055/s-0034-1376263.
Akaike G, Nozaki T, Makidono A, Saida Y, Hirabayashi T, Suzuki K. A case of lymphatic malformation/lymphangioma of the scrotum. Acta Radiol Short Rep. 2012. https://doi.org/10.1258/arsr.2012.120021.
Al-Shaikh SA, Mubarak AM, Harb ZF. Splenic lymphangioma in an adult. Saudi Med J. 2017;38(11):1148–52. https://doi.org/10.15537/smj.2017.11.20625.
Cheng J, Liu B, Farjat AE, Routh J. National characteristics of lymphatic malformations in children: inpatient estimates and trends in the United States, 2000 to 2009. J Pediatr Hematol Oncol. 2018;40(3):221–3. https://doi.org/10.1097/MPH.0000000000001078.
Grasso DL, Pelizzo G, Zocconi E, Schleef J. Lymphangiomas of the head and neck in children. Acta Otorhinolaryngol Ital. 2008;28(1):17–20 (PMID: 18533550; PMCID: PMC2640069).
Mota MMDS, Bezerra ROF, Garcia MRT. Practical approach to primary retroperitoneal masses in adults. Radiol Bras. 2018;51(6):391–400. https://doi.org/10.1590/0100-3984.2017.0179.
Alzaraa A, Mousa H, Dickens P, Allen J, Benhamida A. Idiopathic benign retroperitoneal cyst: a case report. J Med Case Rep. 2008;2:43. https://doi.org/10.1186/1752-1947-2-43.
Morotti A, Busso M, ConsiglioBarozzino M, Cinardo P, Angelino V, Familiari U, Veltri A, Guerrasio A. Detection and management of retroperitoneal cystic lesions: a case report and review of the literature. Oncol Lett. 2017;14(2):1602–8. https://doi.org/10.3892/ol.2017.6323.
Gümüştaş OG, Sanal M, Güner O, Tümay V. Retroperitoneal cystic lymphangioma: a diagnostic and surgical challenge. Case Rep Pediatr. 2013. https://doi.org/10.1155/2013/292053 (Epub 2013 Feb 28. PMID: 23533897; PMCID: PMC3600274).
Kopicky L, Humenansky K, Gitzelmann C, Gulati R. Intraabdominal cystic lymphangioma. J Ped Surg Case Rep. 2017;2017(26):32–4. https://doi.org/10.1016/j.epsc.2017.08.010.
GuachilemaRibadeneira A, Monard ÁRT, Endara MC, Garcia CG, Sandoval MO, Cárdenas DA, et al. Intra-abdominal cystic lymphangioma of the mesocolon sigmoids: a rare entity in adult patient woman. J Surg Case Rep. 2020. https://doi.org/10.1093/jscr/rjaa031.
Tripathi M, Parshad S, Karwasra RK, Gupta A, Srivastva S, Sarwal A. Retroperitoneal lymphangioma in an adult: a case report of a rare clinical entity. Case Rep Surg. 2015;2015: 732531. https://doi.org/10.1155/2015/732531.
Suhani AL, Ali S, Thomas S. Giant retroperitoneal lymphangioma: a rare entity. Indian J Surg. 2014;76(5):402–4. https://doi.org/10.1007/s12262-013-0989-y.
Jones IS. Lymphangiomas of the ocular adnexa: an analysis of 62 cases. Trans Am Ophthalmol Soc. 1959;57:602–65 (PMID: 16693588; PMCID: PMC1316348).
Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular endothelial cell biology: an Update. Int J Mol Sci. 2019;20(18):4411. https://doi.org/10.3390/ijms20184411.
Ha J, Yu YC, Lannigan F. A review of the management of lymphangiomas. Curr Pediatr Rev. 2014;10(3):238–48. https://doi.org/10.2174/1573396309666131209210751.
Shah GH, Deshpande MD. Lymphatic malformation in adult patient: a rare case. J Maxillofac Oral Surg. 2010;9(3):284–8. https://doi.org/10.1007/s12663-010-0082-z.
Ganesh C, Sangeetha GS, Narayanan V, Umamaheswari TN. Lymphangioma circumscriptum in an adult: an unusual oral presentation. J Clin Imaging Sci. 2013;3:44. https://doi.org/10.4103/2156-7514.120779.PMID:24228212;PMCID:PMC3823387.
Flucke U, Karanian M, Broek RWT, Thway K. Soft tissue special issue: perivascular and vascular tumors of the head and neck. Head Neck Pathol. 2020;14(1):21–32. https://doi.org/10.1007/s12105-020-01129-z.
Galambos C, Nodit L. Identification of lymphatic endothelium in pediatric vascular tumors and malformations. Pediatr Dev Pathol. 2005;8(2):181–9. https://doi.org/10.1007/s10024-004-8104-9.
Cai X, Zhang W, Chen G, Li RF, Sun YF, Zhao YF. Mesenchymal status of lymphatic endothelial cell: enlightening treatment of lymphatic malformation. Int J Clin Exp Med. 2015;8(8):12239–51 (PMID: 26550134; PMCID: PMC4612819).
Castro EC, Galambos C. Prox-1 and VEGFR3 antibodies are superior to D2–40 in identifying endothelial cells of lymphatic malformations–a proposal of a new immunohistochemical panel to differentiate lymphatic from other vascular malformations. Pediatr Dev Pathol. 2009;12(3):187–94. https://doi.org/10.2350/08-05-0471.1.
Abe N, Ohtake T, Saito K, Kumamoto K, Sugino T, Takenoshita S. Clinicopathological significance of lymphangiogenesis detected by immunohistochemistry using D2–40 monoclonal antibody in breast cancer. Fukushima J Med Sci. 2016;62(1):57–63. https://doi.org/10.5387/fms.2015-10.
Adamczyk LA, Gordon K, Kholová I, Meijer-Jorna LB, Telinius N, Gallagher PJ, et al. Lymph vessels: the forgotten second circulation in health and disease. Virchows Arch. 2016;469(1):3–17. https://doi.org/10.1007/s00428-016-1945-6.
Miettinen M, Wang ZF. Prox1 transcription factor as a marker for vascular tumors-evaluation of 314 vascular endothelial and 1086 nonvascular tumors. Am J Surg Pathol. 2012;36(3):351–9. https://doi.org/10.1097/PAS.0b013e318236c312.
Perkins JA. New frontiers in our understanding of lymphatic malformations of the head and neck: natural history and basic research. Otolaryngol Clin North Am. 2018;51(1):147–58. https://doi.org/10.1016/j.otc.2017.09.002.
Padia R, Zenner K, Bly R, Bennett J, Bull C, Perkins J. Clinical application of molecular genetics in lymphatic malformations. Laryngoscope Investig Otolaryngol. 2019;4(1):170–3. https://doi.org/10.1002/lio2.241.
Wood DE, White JR, Georgiadis A, Van Emburgh B, Parpart-Li S, Mitchell J, et al. A machine learning approach for somatic mutation discovery. Sci Transl Med. 2018;10(457):eaar7939. https://doi.org/10.1126/scitranslmed.aar7939.
Bessi L, Viailly PJ, Bohers E, Ruminy P, Maingonnat C, Bertrand P, et al. Somatic mutations of cell-free circulating DNA detected by targeted next-generation sequencing and digital droplet PCR in classical hodgkin lymphoma. Leuk Lymphoma. 2019;60(2):498–502. https://doi.org/10.1080/10428194.2018.1492123.
Gao J, Chang MT, Johnsen HC, Gao SP, Sylvester BE, Sumer SO, et al. 3D clusters of somatic mutations in cancer reveal numerous rare mutations as functional targets. Genome Med. 2017;9(1):4. https://doi.org/10.1186/s13073-016-0393-x.
North PE, Sander T. Vascular tumors and developmental malformations: pathogenic mechanisms and molecular diagnosis. New York: Springer-Verlag; 2016. p. 1–149.
Freed D, Stevens EL, Pevsner J. Somatic mosaicism in the human genome. Genes (Basel). 2014;5(4):1064–94. https://doi.org/10.3390/genes5041064.
Blesinger H, Kaulfuß S, Aung T, Schwoch S, Prantl L, Rößler J, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations. PLoS ONE. 2018;13(7): e0200343. https://doi.org/10.1371/journal.pone.0200343.
Castillo SD, Tzouanacou E, Zaw-Thin M, Berenjeno IM, Parker VE, Chivite I, et al. Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans. Sci Transl Med. 2016;8(332):332ra43. https://doi.org/10.1126/scitranslmed.aad9982.
Le Cras TD, Goines J, Lakes N, Pastura P, Hammill AM, Adams DM, et al. Constitutively active PIK3CA mutations are expressed by lymphatic and vascular endothelial cells in capillary lymphatic venous malformation. Angiogenesis. 2020;23(3):425–42. https://doi.org/10.1007/s10456-020-09722-0.
Keppler-Noreuil KM, Parker VE, Darling TN, Martinez-Agosto JA. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway and therapeutic strategies. Am J Med Genet C Semin Med Genet. 2016;172(4):402–21. https://doi.org/10.1002/ajmg.c.31531.
Melikhan-Revzin S, Kurolap A, Dagan E, Mory A, Gershoni-Baruch R. A novel missense mutation in FLT4 causes autosomal recessive hereditary lymphedema. Lymphat Res Biol. 2015;13(2):107–11. https://doi.org/10.1089/lrb.2014.0044.
Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet. 2000;67(2):295–301. https://doi.org/10.1086/303019.
Kaser-Eichberger A, Schroedl F, Bieler L, Trost A, Bogner B, Runge C, et al. Expression of lymphatic markers in the adult rat spinal cord. Front Cell Neurosci. 2016;10:23. https://doi.org/10.3389/fncel.2016.00023.
Ghalamkarpour A, Morlot S, Raas-Rothschild A, Utkus A, Mulliken JB, Boon LM, et al. Hereditary lymphedema type I associated with VEGFR3 mutation: the first de novo case and atypical presentations. Clin Genet. 2006;70(4):330–5. https://doi.org/10.1111/j.1399-0004.2006.00687.x.
Miettinen M, Wang ZF, Paetau A, Tan SH, Dobi A, Srivastava S, et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol. 2011;35(3):432–41. https://doi.org/10.1097/PAS.0b013e318206b67b.
Wetzel-Strong SE, Detter MR, Marchuk DA. The pathobiology of vascular malformations: insights from human and model organism genetics. J Pathol. 2017;241(2):281–93. https://doi.org/10.1002/path.4844.
Wang QK. Update on the molecular genetics of vascular anomalies. Lymphat Res Biol. 2005;3(4):226–33. https://doi.org/10.1089/lrb.2005.3.226.
Lal A, Gupta P, Singhal M, Sinha SK, Lal S, Rana S, et al. Abdominal lymphatic malformation: Spectrum of imaging findings. Indian J Radiol Imaging. 2016;26(4):423–8. https://doi.org/10.4103/0971-3026.195777.
Li Q, Ji D, Tu KS, Dou CW, Yao YM. Clinical analysis of intraperitoneal lymphangioma. Chin Med J (Engl). 2015;128(22):3043–9. https://doi.org/10.4103/0366-6999.169061.
Perez A, Perez MEC, Yuga AC, Viray BAG. Splenic lymphangioma in adulthood: a case report. Int J Surg Case Rep. 2020;67:250–3. https://doi.org/10.1016/j.ijscr.2020.01.061.
Méndez-Gallart R, Solar-Boga A, Gómez-Tellado M, Somoza-Argibay I. Giant mesenteric cystic lymphangioma in an infant presenting with acute bowel obstruction. Can J Surg. 2009;52(3):E42–3 (PMID: 19503641; PMCID: PMC2689734).
Hitzerd E, van Hamont D, Pijnenborg JM. Mesenteric lymphangioma mimicking a cystic ovarian mass on imaging. BMJ Case Rep. 2016. https://doi.org/10.1136/bcr-2015-213727.
Carqueja IM, Sousa J, Mansilha A. Vascular malformations: classification, diagnosis and treatment. Int Angiol. 2018;37(2):127–42. https://doi.org/10.23736/S0392-9590.18.03961-5.
Aslan A, Büyükkaya R, Tan S, Erdoğan C, Hakyemez B. Efficacy of ultrasonography in lymphatic malformations: diagnosis, treatment and follow-up: a case report. Med Ultrason. 2013;15(3):244–6. https://doi.org/10.11152/mu.2013.2066.153.aa1rb2.
Li J, Zhong W, Geng X, Liu X, Zhang X, Wang Y, et al. Ultrasonographic diagnosis, classification, and treatment of cervical lymphatic malformation in paediatric patients: a retrospective study. BMC Pediatr. 2020;20(1):441. https://doi.org/10.1186/s12887-020-02337-w.
Loberant N, Chernihovski A, Goldfeld M, Sweed Y, Vais M, Tzilman B, Cohen I. Role of doppler sonography in the diagnosis of cystic lymphangioma of the scrotum. J Clin Ultrasound. 2002;30(6):384–7. https://doi.org/10.1002/jcu.10080.
Dubois J, Garel L, Grignon A, David M, Laberge L, Filiatrault D, et al. Imaging of hemangiomas and vascular malformations in children. Acad Radiol. 1998;5(5):390–400. https://doi.org/10.1016/S1076-6332(98)80158-X.
Paltiel HJ, Burrows PE, Kozakewich HPW, Zurakowski D, Mulliken JB. Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology. 2000;214(3):747–54. https://doi.org/10.1148/radiology.214.3.r00mr21747.
Hyodoh H, Hori M, Akiba H, Tamakawa M, Hyodoh K, Hareyama M. Peripheral vascular malformations: imaging, treatment approaches, and therapeutic issues. Radiographics. 2005;25(Suppl 1):S159–71. https://doi.org/10.1148/rg.25si055509.
Madani H, Farrant J, Chhaya N, Anwar I, Marmery H, Platts A, et al. Peripheral limb vascular malformations: an update of appropriate imaging and treatment options of a challenging condition. Br J Radiol. 2015;88(1047):20140406. https://doi.org/10.1259/bjr.20140406.
Chaker K, Sellami A, Ouanes Y, Zehani A, Jallouli W, Ben Chehida MA, et al. Retroperitoneal cystic lymphangioma in an adult: a case report. Urol Case Rep. 2018;18:33–4. https://doi.org/10.1016/j.eucr.2018.02.019.
Flors L, Leiva-Salinas C, Maged IM, Norton PT, Matsumoto AH, Angle JF, et al. MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics. 2011;31(5):1321–40. https://doi.org/10.1148/rg.315105213 (discussion 1340-1).
Moukaddam H, Pollak J, Haims AH. MRI characteristics and classification of peripheral vascular malformations and tumors. Skeletal Radiol. 2009;38(6):535–47. https://doi.org/10.1007/s00256-008-0609-2.
Nosher JL, Murillo PG, Liszewski M, Gendel V, Gribbin CE. Vascular anomalies: a pictorial review of nomenclature, diagnosis and treatment. World J Radiol. 2014;6(9):677–92. https://doi.org/10.4329/wjr.v6.i9.677.
Ohgiya Y, Hashimoto T, Gokan T, Watanabe S, Kuroda M, Hirose M, et al. Dynamic MRI for distinguishing high-flow from low-flow peripheral vascular malformations. AJR Am J Roentgenol. 2005;185(5):1131–7. https://doi.org/10.2214/AJR.04.1508.
Bashir U, Shah S, Jeph S, O’Keeffe M, Khosa F. Magnetic resonance (MR) imaging of vascular malformations. Pol J Radiol. 2017;82:731–41. https://doi.org/10.12659/PJR.903491.
Coffin A, Boulay-Coletta I, Sebbag-Sfez D, Zins M. Radioanatomy of the retroperitoneal space. Diagn Interv Imaging. 2015;96(2):171–86. https://doi.org/10.1016/j.diii.2014.06.015.
Brennan C, Kajal D, Khalili K, Ghai S. Solid malignant retroperitoneal masses-a pictorial review. Insights Imaging. 2014;5(1):53–65. https://doi.org/10.1007/s13244-013-0294-0.
Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40(6):1887–903. https://doi.org/10.1007/s00261-014-0311-x.
Zhao M, Li C, Zheng J, Yan M, Sun K, Wang Z. Cystic lymphangioma-like adenomatoid tumor of the adrenal gland: report of a rare case and review of the literature. Int J Clin Exp Pathol. 2013;6(5):943–50 (PMID: 23638228; PMCID: PMC3638107).
Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH Jr, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics. 2011;31(4):949–76. https://doi.org/10.1148/rg.314095132.
Yang DM, Jung DH, Kim H, Kang JH, Kim SH, Kim JH, Hwang HY. Retroperitoneal cystic masses: CT, clinical, and pathologic findings and literature review. Radiographics. 2004;24(5):1353–65. https://doi.org/10.1148/rg.245045017.
Hoang VT, Trinh CT, Le TB, Le TK. Recurrence of retroperitoneal mature cystic teratoma in an adult: a case report. Radiol Case Rep. 2019;14(6):692–6. https://doi.org/10.1016/j.radcr.2019.03.008.
Xiao J, Shao Y, Zhu S, He X. Characteristics of adult abdominal cystic Lymphangioma: a single-center Chinese cohort of 12 cases. BMC Gastroenterol. 2020;20(1):244. https://doi.org/10.1186/s12876-020-01388-8.
Surabhi VR, Menias C, Prasad SR, Patel AH, Nagar A, Dalrymple NC. Neoplastic and non-neoplastic proliferative disorders of the perirenal space: cross-sectional imaging findings. Radiographics. 2008;28(4):1005–17. https://doi.org/10.1148/rg.284075157.
Funaki K, Fukunishi H, Tsuji Y, Maeda T, Takahashi T. Giant cystic leiomyoma of the uterus occupying the retroperitoneal space. J Radiol Case Rep. 2013;7(12):35–40. https://doi.org/10.3941/jrcr.v7i12.1447.
Gemici K, Buldu İ, Acar T, Alptekin H, Kaynar M, Tekinarslan E, Karatağ T, Efe D, Çolak H, Küçükkartallar T, İstanbulluoğlu MO. Management of patients with retroperitoneal tumors and a review of the literature. World J Surg Oncol. 2015;13:143. https://doi.org/10.1186/s12957-015-0548-z.
Kumar N, Yadav P, Ansari MS, Lal H. Surgical management of giant retroperitoneal lymphangioma in a child. BMJ Case Reports CP. 2020;13: e234447. https://doi.org/10.1136/bcr-2020-234447.
Borman P. Lymphedema diagnosis, treatment, and follow-up from the view point of physical medicine and rehabilitation specialists. Turk J Phys Med Rehabil. 2018;64(3):179–97. https://doi.org/10.5606/tftrd.2018.3539.
Rodriguez-Laguna L, Agra N, Ibañez K, Oliva-Molina G, Gordo G, Khurana N, et al. Somatic activating mutations in PIK3CA cause generalized lymphatic anomaly. J Exp Med. 2019;216(2):407–18. https://doi.org/10.1084/jem.20181353.
Geissler EK. The influence of mTOR inhibitors on immunity and the relationship to post-transplant malignancy. Transplant Res. 2013;2(Suppl 1):S2. https://doi.org/10.1186/2047-1440-2-S1-S2.
Ricci KW. Advances in the medical management of vascular anomalies. Semin Intervent Radiol. 2017;34(3):239–49. https://doi.org/10.1055/s-0037-1604297.
Teachey DT, Greiner R, Seif A, Attiyeh E, Bleesing J, Choi J, et al. Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. Br J Haematol. 2009;145(1):101–6. https://doi.org/10.1111/j.1365-2141.2009.07595.x.
Mofenson LM, Brady MT, Danner SP, Dominguez KL, Hazra R, Handelsman E, et al. Guidelines for the prevention and treatment of opportunistic infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58(RR-11):1–166.
Margolin JF, Soni HM, Pimpalwar S. Medical therapy for pediatric vascular anomalies. Semin Plast Surg. 2014;28(2):79–86. https://doi.org/10.1055/s-0034-1376264.
Padia R, Bly R, Bull C, Geddis AE, Perkins J. Medical management of vascular anomalies. Curr Treat Options Pediatr. 2018;4(2):221–36. https://doi.org/10.1007/s40746-018-0130-3.
Institute of Medicine (US) Committee on Accelerating Rare Diseases Research and Orphan Product Development. Rare Diseases and Orphan Products: Accelerating Research and Development. In: Field MJ, Boat TF, (eds). National Academies Press, Washington (DC), USA, 2010. PMID: 21796826
Funding
No financial support.
Author information
Authors and Affiliations
Contributions
VTH contributed to the conception and design of the manuscript and data acquisition and wrote the initial manuscript. HATV critically revised the manuscript. MDN and DTH supported the revision and critically reviewed the paper. All authors participated in the approval of the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The manuscript does not contain patient information.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hoang, V.T., Nguyen, M.D., Van, H.A.T. et al. Review of diagnosis, differential diagnosis, and management of retroperitoneal lymphangioma. Jpn J Radiol 41, 283–301 (2023). https://doi.org/10.1007/s11604-022-01356-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-022-01356-0