Abstract
Ankylosing spondylitis (AS) is a chronic inflammatory disease characterized by pathological osteogenesis and inflammation. However, the pathogenesis of AS and the pathological relationship between osteogenesis and inflammation in this disease remain largely unknown. Mesenchymal stem cells (MSCs) are multipotent progenitor cells capable of osteogenic differentiation and immunoregulation. Recently, we demonstrated that MSCs from AS patients (ASMSCs) have a greater potential for osteogenic differentiation than MSCs from healthy donors (HDMSCs), which therefore seems to be a component of pathological osteogenesis in AS. Previous studies have indicated that the immunoregulatory abilities of MSCs change following differentiation. However, the subsequent effects of ASMSCs during abnormal osteogenic differentiation are unclear. Here, we further demonstrated that ASMSCs secreted more monocyte chemoattractant protein 1 (MCP1) than HDMSCs during osteogenic differentiation. This enhanced MCP1 secretion augmented monocyte migration, increased classical macrophage polarization, and enhanced TNF-α secretion. Inhibiting MCP1 secretion from osteogenic differentiated ASMSCs using lentiviruses encoding short hairpin RNAs ameliorated these dysfunctions. Blocking the ERK1/2 pathway in ASMSCs with U0126 corrected the abnormal osteogenic differentiation, inhibited MCP1 overexpression, and prevented subsequent monocyte dysfunction. Finally, MCP1 expression was up-regulated during osteogenic differentiation in ASMSCs in vivo and was locally augmented in osteoblasts at ossification sites in AS patients. In summary, our study determined that MCP1 overexpression during abnormal osteogenic differentiation of ASMSCs triggers monocyte dysfunctions. We propose the novel hypothesis that pathological osteogenesis can lead to inflammation in AS. This hypothesis may contribute to reveal the precise pathological relationship between osteogenesis and inflammation in the field of osteoimmunology.
Key message
-
ASMSCs secreted more MCP1 during abnormal osteogenic differentiation.
-
MCP1 overexpression leads to monocyte dysfunctions.
-
Pathological osteogenesis can lead to inflammation in AS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease characterized by chronic inflammation and pathological osteogenesis [1]. Increasing research efforts have focused on the pathogenesis of AS. However, the precise mechanisms underlying inflammation and pathological osteogenesis remain poorly understood, and controversies exist regarding the relationship between these two processes in AS [2, 3]. Some studies have demonstrated that inflammation predominates and results in pathological osteogenesis [4, 5]. Other studies have shown that these two processes are uncoupled [6, 7]. However, whether pathological osteogenesis leads to inflammation in AS has never been investigated.
Mesenchymal stem cells (MSCs), multipotent stromal cells capable of immunoregulation and tri-lineage differentiation, participate in both inflammation and osteogenesis [8, 9]. It is acknowledged that MSC dysfunctions contribute to various inflammatory diseases [10–12]. We recently demonstrated that because they secrete more bone morphogenetic protein 2 (BMP2) but less Noggin, MSCs from AS patients (ASMSCs) have greater capacities for osteogenic differentiation than MSCs from healthy donors (HDMSCs), which is a mechanism underlying pathological osteogenesis in AS [13]. It has been reported that immunoregulatory abilities of MSCs change following differentiation [14]. Whether ASMSCs lead to subsequent dysfunctions, especially in immunoregulation, during abnormal osteogenic differentiation remains unclear.
Chemokines, immunomodulatory factors secreted by MSCs, are small heparin-binding proteins that participate in inflammatory reactions [15]. Monocyte chemoattractant protein 1 (MCP1), known as C-C motif chemokine ligand 2 (CCL2), is one of the most important chemokines [15, 16]. Produced by various types of cells including MSCs, MCP1 affects monocyte migration and subsequent macrophage polarization [16, 17]. Previous studies have demonstrated that MCP1 parasecretion contributes to the development of inflammatory diseases, including AS [18–22]. Particularly, MCP1 oversecretion during MSC osteogenic differentiation induces immune disorder [23]. However, MCP1 expression patterns and their effects on monocyte during abnormal osteogenic differentiation of ASMSCs remain unknown.
Here, we demonstrated that because of extracellular signal-regulated kinase 1/2 (ERK1/2) pathway overactivation, ASMSCs secreted more MCP1 during abnormal osteogenic differentiation than HDMSCs, resulting in augmented monocyte migration, enhanced M1 macrophage polarization, and increased tumor necrosis factor-α (TNF-α) secretion. We speculate that MCP1 triggers monocyte dysfunction during abnormal osteogenic differentiation of ASMSCs and propose the novel hypothesis that pathological osteogenesis leads to inflammation in AS.
Materials and methods
Ethics statement
This study conforms to the Declaration of Helsinki and was approved by the Ethics Committee and the Animal Ethical and Welfare Committee of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China. Before the study, all healthy donors and AS patients were informed of the clinical requirements and possible risks of all operations, and signed informed consents were obtained.
Cell isolation and culture
Twenty-one healthy donors and 19 AS patients were recruited. AS patients were diagnosed according to the modified New York criteria [24]. The characteristics of study subjects are presented in Supplemental Table 1. After bone marrow punctures, MSCs were immediately isolated and cultured as described [13]. MSCs were used at passage 3.
Peripheral blood mononuclear cells (PBMCs) were isolated via density gradient centrifugation. CD14+ monocytes were isolated from PBMCs using CD14 MicroBeads (Miltenyi Biotec).
Flow cytometry
To assess the purity, CD14+ monocytes were incubated with CD14 antibody (Miltenyi Biotec). For macrophage polarization assays, cells were incubated with antibodies against HLA-DR-PE or CD206-PE (BD Pharmingen) and then incubated with fixation medium (Invitrogen) for 15 min. After three washes, cells were incubated with a permeabilization medium (Invitrogen) plus CD68 antibody (BD Pharmingen). All labeled cells were detected using BD Influx cell sorter (BD Biosciences).
Osteogenic differentiation
MSCs were seeded in 12-well plates at a density of 1.5 × 104 cells/cm2 and cultured in osteogenic differentiation medium composed of Dulbecco’s modified Eagle’s medium (DMEM, GIBCO) supplemented with 10% fetal bovine serum (FBS, GIBCO), 0.1 μM dexamethasone, 10 mM β-glycerol phosphate, 50 μM ascorbic acid, 100 IU/ml penicillin, and 100 IU/ml streptomycin (Sigma-Aldrich). The culture medium was replaced every 3 days.
Quantitative real-time PCR
Total RNA was extracted using RNAiso Plus and reserve transcribed into cDNA using PrimeScript™ reagent kits (TaKaRa). High-throughput microfluidics fluorescence qRT-PCR was performed as described [25]. Briefly, sample pre-mix and assay pre-mix were added into a 48.48 Dynamic Array Integrated Fluidic Circuit (IFC; Fluidigm), which was primed and loaded in IFC Controller (Fluidigm). Reactions were performed in BioMark™ HD System (Fluidigm). Data were first normalized to GAPDH expression and second to 0 day values of HDMSCs. Data were analyzed with PCR analysis software (Fluidigm). For ordinary qRT-PCR, reactions were performed in the LightCycler®480 PCR system (Roche) using SYBR® Premix Taq™ kits (TaKaRa). The relative expression levels were analyzed using the 2−ΔΔCt method. Primers for each gene are shown in Supplemental Table 2.
Migration assay
Migration assays were performed using Polycarbonate Membrane Transwell® Inserts (5.0-μm pores; Corning). MSCs (2 × 104) in 600 μl culture supernatant were placed in the lower chambers. In some assays, 600 μl cell-free MSC culture supernatant was placed in the lower chambers. After 8 h, the upper chambers, containing 1 × 106 CD14+ monocytes suspended in 100 μl DMEM with 10% FBS, were placed into the wells. After 4 h incubation, the numbers of migrated CD14+ monocytes in the medium in the lower chamber were counted via flow cytometry. The numbers of migrated cells are expressed as cells per minute.
Macrophage polarization assay
Macrophage polarization assays were performed using Polycarbonate Membrane Transwell® Inserts (0.4-μm pores; Corning). CD14+ monocytes (2 × 105) suspended in 600 μl RPMI-1640 (GIBCO) with 10% FBS were placed in the lower chambers. MSCs (2 × 104) suspended in 100 μl RPMI-1640 with 10% FBS were placed in the upper chambers. After 5 days incubation, flow cytometry was performed to determine the ratios of pro-inflammatory classically activated macrophages (M1 macrophages) or anti-inflammatory alternatively activated macrophages (M2 macrophages) in the lower chambers.
Enzyme-linked immunosorbent assay
MCP1 and TNF-α in cell culture supernatants or serum were measured using human MCP1 or TNF-α enzyme-linked immunosorbent assay (ELISA) kits according to the instruction manuals (BD OptEIA™).
Alizarin red S (ARS) assay
MSCs were fixed and stained with 1% ARS for 15 min. Stained cells were observed via microscopy (40×). For ARS quantification, stained cells were incubated with 10% cetylpyridinium chloride monohydrate, and absorbance was measured at 562 nm.
Alkaline phosphatase assay
MSCs were fixed and treated with alkaline dye solution for 15 min in the dark. Stained cells were observed via microscopy (40×). To detect alkaline phosphatase (ALP) activity using ALP activity kits (Nanjing Jiancheng Biotech), MSCs were lysed using RIPA buffer containing protease and phosphatase inhibitors (Roche). Lysates were centrifuged and supernatants were incubated with reaction buffer at 37 °C for 15 min. After adding stop solution, absorbance was measured at 405 nm. Protein concentrations were measured using the BCA assay kit (Sigma-Aldrich). ALP activity is shown as units per gram protein per 15 min (U/gpro/15 min).
Lentiviruses assay
Four lentiviruses encoding short hairpin RNA (shRNA) for MCP1 (Lv-MCP1) and a negative control shRNA (Lv-NC) were constructed. Lentiviruses were generated via co-transfecting pGLVH1/GFP/Puro (GenePharma) and packing plasmids (pGag/Pol, pRev, and pVSV-G) into 293T cells. Cell culture supernatants containing lentiviruses were filtered and concentrated 72 h after transfection. Lentiviruses (109 TU/ml) with 5 μg/ml polybrene were incubated with MSCs for 24 h at a MOI of 50. Transduction efficiency was confirmed by green fluorescent protein (GFP)-positive MSCs and MCP1 ELISA. The most effective Lv-MCP1 with a target sequence of 5′-GGCTCGCGAGCTATAGAAGAA-3′ was chosen for experiments. The sequence for Lv-NC was 5′-TTCTCCGAACGTGTCACGTTTC-3′. Related experiments were performed on day 10 of induction.
ERK1/2 pathway blocking
U0126 (Sigma) was added (10 μM) as MSCs underwent osteogenic differentiation. Related experiments were performed on day 10 of induction.
Western blotting
Equal concentrations of proteins were separated via sodium dodecylsulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore), followed by blotting with primary antibodies against GAPDH, MCP1, C-C chemokine receptor 2 (CCR2), ERK1/2, and pERK1/2 (1:1000, Cell Signaling Technology). The membrane was washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:3000, Cell Signaling Technology). Specific antibody-antigen complexes were detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore).
Bone formation assay
In vivo bone formation assays were performed as described [26]. Briefly, MSCs (5 × 105) were loaded onto 40 mg hydroxyapatite/tricalcium phosphate (HA/TCP; Zimmer) and implanted subcutaneously into the dorsal sides of 8-week-old BALB/c-nu/nu female mice (Laboratory Animal Center of Sun Yat-Sen University). Operations were performed under anesthesia achieved via intraperitoneal injection of ketamine and xylazine. At 2, 4, and 8 weeks, mice were killed and implants were obtained.
Enthesial biopsies assay
Six AS patients and six non-AS patients (two patients with lumbar intervertebral disc herniation and four patients with scoliosis) were recruited. The sites of ossifying enthesis were confirmed via pre-surgery image analyses and visual observations during surgery. Ossifying tissues were obtained during lumbar spine surgeries. The characteristics of study subjects are presented in Supplemental Table 3.
Immunofluorescence assay
Tissues obtained above were successively fixed, decalcified, and embedded in paraffin. Sections were deparaffinized, hydrated, and incubated in 1% Triton X-100/PBS. After antigen retrieval in citrate buffer and blocking in goat serum, sections were incubated with anti-MCP1 and anti-osteocalcin (OCN) antibodies (Abcam) overnight at 4 °C. Sections were washed and incubated with fluorescein secondary antibody and 4′,6-diamidino-2-phenylindole (DAPI; Thermo). Sections were examined with the Axio Observer fluorescence microscope (Carl Zeiss).
Statistical analysis
All the results were determined based on at least three separate experiments containing at least triplicate samples. All data are expressed as means ± standard deviations. T tests and one-way analyses of variance followed by Bonferroni tests and Pearson correlation tests were performed in SPSS (SPSS Inc). The n values indicated numbers of individuals in each experiment. P values less than 0.05 were considered statistically significant.
Results
ASMSCs secreted more MCP1 than HDMSCs during osteogenic differentiation
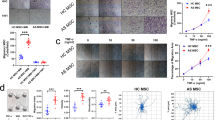
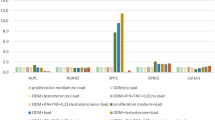
High-throughput microfluidics fluorescence qRT-PCR results show that MSCs expressed 27 main chemokines, with MCP1 expression increasing gradually, reaching a peak on day 10 of induction, and then decreasing. ASMSCs expressed more MCP1 than HDMSCs on days 10 and 14 of induction. No differences were observed among the remaining 26 chemokines (Fig. 1a). Ordinary qRT-PCR produced consistent results (Fig. 1b). MCP1 protein expression in ASMSCs was higher than that in HDMSCs on days 10 and 14 of induction (Fig. 1c, d). Besides, MCP1 secretion was positively correlated with ALP activity, a marker of early osteogenesis in MSCs, on day 10 of induction (Supplemental Fig. 1).
ASMSCs secreted more MCP1 than HDMSCs during osteogenic differentiation. a Gene expression of chemokines was detected via high-throughput qRT-PCR. The heat maps show ΔΔCT values. Each column represents one sample, and each row represents one gene. MCP1/CCL2 expression was higher in ASMSCs (A) than HDMSCs (H) during osteogenic differentiation. b MCP1 expression was confirmed via ordinary qRT-PCR. Results show that ASMSCs had higher MCP1 expression levels than HDSMCs on days 10 and 14 of induction. c ELISA results show that ASMSCs secreted more MCP1 than HDMSCs on days 10 and 14 of induction. d MCP1 expression at protein level was detected via Western blotting, confirming that ASMSCs expressed more MCP1 than HDMSCs on days 10 and 14 of induction. Data are presented as means ± SD of 12 samples per group. * indicates P < 0.05 between HDMSCs and ASMSCs
ASMSCs outperformed HDMSCs in recruiting monocytes during osteogenic differentiation
Monocytes migrate and differentiate into macrophages, which subsequently polarize into either M1 or M2 macrophages [27]. MCP1 functions during monocyte migration and polarization [16]. CD14+ monocytes were isolated, and their purity was confirmed (Supplemental Fig. 2). Corresponding to MCP1 expression results, monocyte migration induced by MSCs increased during MSC osteogenic differentiation, peaked on day 10 of induction and then decreased. ASMSCs had greater capacities to induce monocyte migration than HDMSCs on days 10 and 14 of induction (Fig. 2a). Monocyte migration induced by MSC culture supernatant showed similar results (Fig. 2b). We respectively isolated monocytes from healthy donors (HDMs) and AS patients (ASMs). However, regardless of whether monocyte migration was induced by MSCs directly or by MSC culture supernatant, only the source of MSCs, rather than the source of monocytes, had effects on migration (Fig. 2c). Moreover, no differences were observed in MCP1 and CCR2 expressions between HDMs and ASMs (Supplemental Fig. 3).
ASMSCs outperformed HDMSCs in recruiting monocytes during osteogenic differentiation. Numbers of migrated CD14+ monocytes were counted using flow cytometry. a MSCs promoted monocyte migration. ASMSCs (n = 12) showed a greater ability to promote monocyte migration than HDMSCs (n = 12) on days 10 and 14 of induction. b MSC culture supernatant promoted monocyte migration. ASMSCs (n = 12) culture supernatant exhibited a greater ability to promote monocyte migration than HDMSCs (n = 12) culture supernatant on days 10 and 14 of induction. c Monocytes from healthy donors (HDMs; n = 12) and monocytes from AS patients (ASMs; n = 12) showed equal migration capacities when induced by the same types of MSCs or culture supernatants on day 10 of induction. The experiments were done in triplicate and data are presented as means ± SD. * indicates P < 0.05 between HDMSCs and ASMSCs
Osteogenically differentiated ASMSCs exhibit reduced capacities to inhibit M1 macrophage polarization
Regardless of osteogenic differentiation status, MSCs inhibit monocytes polarization into M1 macrophages, which were positive for CD68 and HLA-DR. This capacity reduced on day 10 of induction. ASMSCs exhibited lower capacities than HDMSCs to inhibit M1 macrophage polarization on day 10 of induction. Surprisingly, no significant differences were observed in the effects of MSCs on M2 macrophage polarization, which were positive for CD68 and CD206 (Fig. 3a). Similar to migration assay results, HDMs and ASMs showed no differences in M1 macrophage polarization when cultured with the same MSCs on day 10 of induction (Fig. 3b). TNF-α is a pro-inflammatory cytokine primarily secreted by M1 macrophages [28]. Similarly, MSCs inhibited TNF-α secretion from M1 macrophages, and this inhibition was reduced following osteogenic differentiation. On day 10 of induction, ASMSCs exhibited lower capacities than HDSMCs to inhibit TNF-α secretion (Fig. 3c).
Osteogenically differentiated ASMSCs exhibit reduced capacities to inhibit M1 macrophage polarization. a M1 macrophage polarization ratios decreased after culturing with MSCs. M1 macrophage polarization ratios in cultures with ASMSCs (n = 12) were equal to those with HDMSCs (n = 12) on day 0 of induction. M1 macrophage polarization ratio with ASMSCs (n = 12) was higher than those with HDMSCs (n = 12) on day 10 of induction. M2 macrophage polarization ratios were almost not affected by HDMSCs (n = 12) or ASMSCs (n = 12). b When cultured with the same MSCs on day 10 of induction, M1 macrophage polarization ratios of HDMs (n = 12) showed no statistical differences with those of ASMs (n = 12). c TNF-α secretion by macrophage decreased after culturing with MSCs. TNF-α secretion of macrophage cultured with ASMSCs (n = 12) was equal to that of those cultured with HDMSCs (n = 12) on day 0 of induction. TNF-α secretion of macrophage cultured with ASMSCs (n = 12) was higher than those cultured with HDMSCs (n = 12) on day 10 of induction. The experiments were done in triplicate and data are presented as means ± SD. * indicates P < 0.05 between HDMSCs and ASMSCs
Lv-MCP1 ameliorated dysfunctions in monocyte migration and polarization caused by osteogenically differentiated ASMSCs
Transfection and inhibition efficiencies were assessed in HDMSCs and ASMSCs (Fig. 4a). ARS and ALP assay results confirmed that ASMSCs had greater osteogenic differentiation capacities than HDMSCs. Lv-MCP1 did not affect osteogenic differentiation in either HDMSCs or ASMSCs (Fig. 4b). Moreover, Lv-MCP1 significantly decreased monocyte migration induced by HDMSCs and ASMSCs to equal levels on day 10 of induction (Fig. 4c). Similarly, inhibiting MCP1 in HDMSCs and ASMSCs significantly decreased M1 macrophages ratios to equal levels in the co-culture system (Fig. 4d). TNF-α secretion from M1 macrophages showed consistent results (Fig. 4e).
Lv-MCP1 ameliorated dysfunctions in monocyte migration and polarization caused by osteogenically differentiated ASMSCs. a Transduction efficiency in MSCs was confirmed based on GFP-positive cells and ELISA results. Scale bar = 200 μm. b ARS and ALP assay results show that ASMSCs (n = 9) had greater osteogenic differentiation capacity than HDSMCs (n = 9) on day 10 of induction. Lv-MCP1 did not alter osteogenic differentiation capacity of MSCs. Scale bar = 50 μm. c The capacities of HDMSCs (n = 9) and ASMSCs (n = 9) to induce monocyte migration on day 10 of induction were decreased to equal levels by Lv-MCP1. d The capacities of HDMSCs (n = 9) and ASMSCs (n = 9) to inhibit M1 macrophage polarization on day 10 of induction were increased to equal levels by Lv-MCP1. e The capacities of HDMSCs (n = 9) and ASMSCs (n = 9) to inhibit TNF-α secretion on day 10 of induction were increased to equal levels by Lv-MCP1. The experiments were done in triplicate and data are presented as means ± SD. * indicates P < 0.05 between HDMSCs and ASMSCs. & indicates P < 0.05 for Lv-MCP1 group compared with induction group. Control indicates MSCs cultured in growth medium
ERK1/2 pathway overactivation during abnormal osteogenic differentiation of ASMSCs leads to MCP1 overexpression and subsequent monocyte dysfunction
We previously demonstrated that BMP2 and Noggin imbalance led to ERK1/2 pathway overactivation, resulting in abnormal ASMSC osteogenic differentiation [13]. Furthermore, ERK1/2 pathway modulates MCP1 expression [29]. We confirmed that ERK1/2 pathway was more active in ASMSCs than HDMSCs on day 10 of induction. Adding U0126, a specific ERK1/2 inhibitor, reduced ERK1/2 pathway activation in HDMSCs and ASMSCs to equal levels (Fig. 5a). Moreover, U0126 completely inhibited osteogenic differentiation in HDMSCs and ASMSCs (Fig. 5b). On day 10 of induction, MCP1 secretion from HDMSCs and ASMSCs was significantly inhibited by U0126 and was consistent with that from undifferentiated MSCs (Fig. 5c). Blocking ERK1/2 pathway in HDMSCs and ASMSCs on day 10 of induction inhibited monocyte migration and decreased M1 macrophage ratios in the co-culture systems to equal levels (Fig. 5d, e). TNF-α secretion showed results consistent with macrophage polarization assays (Fig. 5f).
ERK1/2 pathway overactivation during abnormal osteogenic differentiation of ASMSCs leads to MCP1 overexpression and subsequent monocyte dysfunction. a Western blotting showed that ASMSCs (n = 9) had higher ERK1/2 levels than HDMSCs (n = 9) on day 10 of induction. U0126 decreased ERK1/2 pathway activation in HDMSCs (n = 9) and ASMSCs (n = 9) to equal levels. b ARS and ALP assays confirmed the greater osteogenic differentiation capacity of ASMSCs (n = 9) relative to HDMSCs (n = 9) on day 10 of induction, which was decreased to equal levels by U0126. Scale bar = 50 μm. c MCP1 secretion levels were higher in ASMSCs (n = 9) than in HDMSCs (n = 9) on day 10 of induction and were decreased to equal levels following treatment with U0126. d ASMSCs (n = 9) had a greater capacity to induce monocyte migration than HDMSCs (n = 9) on day 10 of induction, but these capacities were equalized by U0126. e ASMSCs (n = 9) had a lower capacity to inhibit M1 macrophage polarization than HDMSCs (n = 9) on day 10 of induction. This was restored by treatment with U0126. f The capacity to inhibit TNF-α secretion was lower in ASMSCs (n = 9) than in HDMSCs (n = 9) on day 10 of induction but was equalized to equal levels by U0126. The experiments were done in triplicate and data are presented as means ± SD. * indicates P < 0.05 between HDMSCs and ASMSCs. & indicates P < 0.05 for OM group compared with OM with U0126 group. OM indicates osteogenic differentiation medium
MCP1 expression in MSCs during osteogenic differentiation in vivo
To detect MCP1 expression in MSCs during osteogenic differentiation in vivo, we constructed a mouse model bone formation assay [26]. OCN expression in MSCs, a marker of osteogenesis, increased gradually from 2 to 8 weeks, indicating osteogenic differentiation. MCP1 expression in MSCs peaked at 4 weeks and decreased by week 8. Both the expression of MCP1 in ASMSCs were markedly higher than were observed for HDMSCs at 4 weeks (Fig. 6a, white arrows).
MCP1 expression in MSCs during osteogenic differentiation in vivo and MCP1 expression at local sites of ossifying enthesis in AS patients. a OCN expression increased gradually from 2 to 8 weeks, indicating the osteogenic differentiation of MSCs. MCP1 expression of MSCs in the scaffolds peaked at 4 weeks and then decreased at 8 weeks. The expressions of MCP1 in ASMSCs were markedly higher than were observed for HDMSCs at 4 weeks (white arrows). b MCP1 expression was higher in OCN+ osteoblasts at sites of ossifying enthesis in AS patients compared with non-AS patients (white arrow heads). S indicates scaffold; M indicates MSCs. Scale bar = 50 μm
MCP1 expression at local sites of ossifying enthesis in AS patients
MCP1 expression at local sites of ossifying enthesis in vivo in AS has never been investigated. Therefore, we evaluated MCP1 expression in osteoblasts, which come from MSCs and are major cells responsible for new bone formation [30], at local sites of ossifying enthesis in AS. The expression of MCP1 in OCN+ osteoblasts was significantly higher in AS patients than in non-AS patients (Fig. 6b, white arrow heads). Serum MCP1 concentrations in AS patients were essentially the same as in healthy donors (Supplemental Fig. 4).
Discussion
MCP1 is a crucial chemokine for maintaining immunity homeostasis [16]. Previous studies demonstrated that serum MCP1 concentrations are elevated in patients with inflammatory diseases [31, 32]. However, whether MCP1 levels are elevated in AS is controversial [18, 33]. Here, we demonstrated that serum MCP1 levels are equal between healthy donors and AS patients. However, MCP1 expression in osteoblasts at sites of ossifying enthesis in vivo was higher in AS patients than in non-AS patients, indicating that MCP1 is locally, rather than systemically, elevated in AS. MSCs are primary sources of osteoblasts and one of the main producers of MCP1 [30, 34]. Besides, MSCs continuously secrete MCP1 during osteogenic differentiation [23]. Therefore, we hypothesized that MCP1 overexpression in osteoblasts at local osteogenesis sites in AS patients derives from ASMSCs undergoing abnormal osteogenic differentiation. Supporting this hypothesis, we showed that ASMSCs secreted more MCP1 than HDMSCs during osteogenic differentiation both in vitro (10 days) and in vivo (4 weeks) at time points corresponding to early stages of differentiation. It is acknowledged that MCP1 parasecretion from MSCs results in immune dysfunction [22]. Whether MCP1 overexpression during abnormal osteogenic differentiation of ASMSCs leads to subsequent immunocyte dysfunction remains unknown.
MCP1 has various effects on monocytes, one of which is inducing monocyte migration [16]. Under physiological conditions, MSCs recruit monocytes for tissue repair by secreting MCP1 [8]. However, MCP1 overexpression in MSCs accelerates monocyte migration and results in pathological conditions [35]. We observed that the capacity of ASMSCs to recruit monocytes increased during osteogenic differentiation and corresponded to MCP1 overexpression. Moreover, Lv-MCP1 could ameliorate this dysfunction. These results suggest that the enhanced ability of ASMSCs to induce monocyte migration results from MCP1 overexpression during abnormal osteogenic differentiation.
MCP1 affects monocyte polarization and cytokine secretion after migration [16, 36]. Although it has been demonstrated that MSCs inhibit M1 macrophage polarization [37], we found that this capacity was reduced after osteogenic differentiation. Moreover, osteogenically differentiated ASMSCs had lower capacities to inhibit M1 macrophage polarization than HDMSCs. This dysfunction, along with aberrant induction of monocyte migration, was not only consistent with MCP1 overexpression patterns but also ameliorated by Lv-MCP1. These results suggest that the above changes resulted from MCP1 overexpression. Actually, the effects of MCP1 on monocyte polarization remain controversial. Some studies concluded that MCP1 promotes monocyte polarization into M2 macrophages rather than M1 macrophages [36, 38]. Conversely, other studies, consistent with our results, indicated that MCP1 is positively related to M1 macrophage ratio and promotes pro-inflammatory cytokine secretion [39–41]. These discrepancies may result from differences in the microenvironments, culture systems, and cell origins used in each study. TNF-α, mainly secreted by M1 macrophages, is the most important inflammatory cytokine in AS [42]. In this study, TNF-α secretion patterns were fully consistent with M1 macrophage polarization, further supporting to our conclusions and providing insights into why high levels of TNF-α exist at specific locations in AS.
MCP1 can be secreted by monocytes and exerts effects by binding to CCR2, a specific receptor for MCP1 [16, 36]. However, no differences in MCP1 and CCR2 expression were observed between HDMs and ASMs. Besides, HDMs and ASMs exhibited similar migration and polarization capabilities when cultured with the same MSCs. Moreover, inhibiting MCP1 expression in HDMSCs and ASMSCs removed any differences in monocyte migration and polarization. These results suggest that MCP1 overexpression during abnormal osteogenic differentiation of ASMSCs triggers monocyte dysfunctions.
ERK1/2 pathway contributes to osteogenesis [43]. Particularly, its activation promotes MSC osteogenic differentiation [44]. We previously demonstrated that an imbalance between BMP2 and Noggin resulted in excessive ERK1/2 pathway activation, leading to abnormal osteogenic differentiation of ASMSCs. Decreasing BMP2 or increasing Noggin rectified ERK1/2 pathway overactivation and abnormal osteogenic differentiation of ASMSCs [13]. Additionally, ERK1/2 pathway activation leads to MCP1 secretion [29, 45]. We found that blocking ERK1/2 pathway inhibited abnormal osteogenic differentiation of ASMSCs and reduced the subsequent MCP1 overexpression, indicating that MCP1 overexpression results from ERK1/2 pathway overactivation during abnormal osteogenic differentiation. These conclusions not only emphasize the key role of ERK1/2 pathway in both abnormal osteogenic differentiation and MCP1 overexpression but also illustrate an inherent relationship between these two abnormalities in ASMSCs. Evidentially, a positive correlation was found between ALP activity and MCP1 expression in MSCs. However, Lv-MCP1 did not alter osteogenic differentiation capacity in HDMSCs or ASMSCs, indicating that MCP1 overexpression does not cause abnormal osteogenic differentiation of ASMSCs. We speculate that MCP1 overexpression results from abnormally osteogenic differentiation of ASMSCs. Therefore, we further conclude that the effects of MCP1 overexpression on monocyte migration and polarization are in order the result of abnormal ASMSC osteogenic differentiation.
The relationship between pathological osteogenesis and inflammation in AS remain controversial [2]. Previous studies found that CD14+ monocytes and CD68+ macrophages infiltrate close to the area of new bone formation in AS [42, 46]. This in vivo phenomenon is similar to the in vitro results of our study. Do inflammatory cells infiltrate before or after osteogenesis? To the best of our knowledge, whether pathological osteogenesis leads to inflammation remains unknown in AS. We previously demonstrated that ASMSCs have enhanced capacities for osteogenic differentiation [13]. We further demonstrated that ASMSCs secreted more MCP1 during abnormal osteogenic differentiation, resulting in monocyte dysfuncions that are the typical of inflammation in AS [42]. Therefore, we propose a novel mechanism that pathological osteogenesis can lead to inflammation in AS. During abnormal osteogenic differentiation, MCP1 secretion by ASMSCs greatly increases, which recruits additional monocytes from circulating blood to osteogenesis sites. These recruited monocytes polarize into M1 macrophages and secrete TNF-α under the effects of MCP1, leading to local inflammation that is observed in AS (Supplemental Fig. 5). This mechanism may suggests a vicious cycle between pathological osteogenesis and inflammation, and pathological osteogenesis may be one of the dynamic factors of inflammation in AS. Furthermore, this mechanism explains why inhibiting inflammation alone does not delay pathological osteogenesis [7], and it indicates that simultaneous treatments targeting pathological osteogenesis and inflammation may have surprising benefits for AS patients.
Here, we demonstrated that MCP1 triggers inflammation during abnormal osteogenic differentiation of MSCs in AS, providing insights into AS pathogenesis and the relationship between pathological osteogenesis and inflammation. However, several limitations exist in this study. How does the complex in vivo environment affect MSC osteogenic differentiation and monocyte function? Continuous imaging studies and histological examinations are required to support this hypothesis, which should be addressed in future studies.
References
Braun J, Sieper J (2007) Ankylosing spondylitis. Lancet 369:1379–1390
Maksymowych WP, Elewaut D, Schett G (2012) Motion for debate: the development of ankylosis in ankylosing spondylitis is largely dependent on inflammation. Arthritis Rheum 64:1713–1719
Sieper J, Poddubnyy D (2014) Inflammation, new bone formation and treatment options in axial spondyloarthritis. Ann Rheum Dis 73:1439–1441
Maksymowych WP, Morency N, Conner-Spady B, Lambert RG (2013) Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis 72:23–28
Chiowchanwisawakit P, Lambert RG, Conner-Spady B, Maksymowych WP (2011) Focal fat lesions at vertebral corners on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis. Arthritis Rheum 63:2215–2225
Lories RJ, Derese I, de Bari C, Luyten FP (2007) Evidence for uncoupling of inflammation and joint remodeling in a mouse model of spondylarthritis. Arthritis Rheum 56:489–497
van der Heijde D, Landewe R, Einstein S, Ory P, Vosse D, Ni L, Lin SL, Tsuji W, Davis JJ (2008) Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 58:1324–1331
Bernardo ME, Fibbe WE (2013) Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13:392–402
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8:726–736
Guiducci S, Manetti M, Romano E, Mazzanti B, Ceccarelli C, Dal Pozzo S, Milia AF, Bellando-Randone S, Fiori G, Conforti ML et al (2011) Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Ann Rheum Dis 70:2011–2021
Chen H, Shi B, Feng X, Kong W, Chen W, Geng L, Chen J, Liu R, Li X, Chen W et al (2015) Leptin and neutrophil-activating peptide 2 promote mesenchymal stem cell senescence through activation of the phosphatidylinositol 3-kinase/Akt pathway in patients with systemic lupus erythematosus. Arthritis Rheumatol 67:2383–2393
Sun Y, Deng W, Geng L, Zhang L, Liu R, Chen W, Yao G, Zhang H, Feng X, Gao X et al. (2015) Mesenchymal stem cells from patients with rheumatoid arthritis display impaired function in inhibiting Th17 cells. J Immunol Res :284215
Xie Z, Wang P, Li Y, Deng W, Zhang X, Su H, Li D, Wu Y, Shen H (2016) Imbalance between BMP2 and Noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol 68:430–440
Chen X, McClurg A, Zhou GQ, McCaigue M, Armstrong MA, Li G (2007) Chondrogenic differentiation alters the immunosuppressive property of bone marrow-derived mesenchymal stem cells, and the effect is partially due to the upregulated expression of B7 molecules. Stem Cells 25:364–370
Charo IF, Ransohoff RM (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354:610–621
Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interf Cytokine Res 29:313–326
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Romero-Sanchez C, Tsou HK, Jan MS, Wong RH, Chang IC, Londono J, Valle-Onate R, Howe HS, Yu D, Leung BP (2011) Serum monocyte chemotactic protein-1 concentrations distinguish patients with ankylosing spondylitis from patients with mechanical low back pain. J Spinal Disord Tech 24:202–207
Harigai M, Hara M, Yoshimura T, Leonard EJ, Inoue K, Kashiwazaki S (1993) Monocyte chemoattractant protein-1 (MCP-1) in inflammatory joint diseases and its involvement in the cytokine network of rheumatoid synovium. Clin Immunol Immunopathol 69:83–91
Nakamachi Y, Kawano S, Takenokuchi M, Nishimura K, Sakai Y, Chin T, Saura R, Kurosaka M, Kumagai S (2009) MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum 60:1294–1304
Marks SD, Shah V, Pilkington C, Tullus K (2010) Urinary monocyte chemoattractant protein-1 correlates with disease activity in lupus nephritis. Pediatr Nephrol 25:2283–2288
Che N, Li X, Zhang L, Liu R, Chen H, Gao X, Shi S, Chen W, Sun L (2014) Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J Immunol 193:5306–5314
Molloy AP, Martin FT, Dwyer RM, Griffin TP, Murphy M, Barry FP, O'Brien T, Kerin MJ (2009) Mesenchymal stem cell secretion of chemokines during differentiation into osteoblasts, and their potential role in mediating interactions with breast cancer cells. Int J Cancer 124:326–332
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Citri A, Pang ZP, Sudhof TC, Wernig M, Malenka RC (2012) Comprehensive qPCR profiling of gene expression in single neuronal cells. Nat Protoc 7:118–127
Chen L, Holmstrom K, Qiu W, Ditzel N, Shi K, Hokland L, Kassem M (2014) MicroRNA-34a inhibits osteoblast differentiation and in vivo bone formation of human stromal stem cells. Stem Cells 32:902–912
Ginhoux F, Jung S (2014) Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14:392–404
Bradley JR (2008) TNF-mediated inflammatory disease. J Pathol 214:149–160
Kojima R, Taniguchi H, Tsuzuki A, Nakamura K, Sakakura Y, Ito M (2010) Hypertonicity-induced expression of monocyte chemoattractant protein-1 through a novel cis-acting element and MAPK signaling pathways. J Immunol 184:5253–5262
Bianco P (2015) Stem cells and bone: a historical perspective. Bone 70:2–9
Ellingsen T, Buus A, Stengaard-Pedersen K (2001) Plasma monocyte chemoattractant protein 1 is a marker for joint inflammation in rheumatoid arthritis. J Rheumatol 28:41–46
Dominguez-Gutierrez PR, Ceribelli A, Satoh M, Sobel ES, Reeves WH, Chan EK (2014) Elevated signal transducers and activators of transcription 1 correlates with increased C-C motif chemokine ligand 2 and C-X-C motif chemokine 10 levels in peripheral blood of patients with systemic lupus erythematosus. Arthritis Res Ther 16:R20
Choe JY, Lee MY, Rheem I, Rhee MY, Park SH, Kim SK (2008) No differences of carotid intima-media thickness between young patients with ankylosing spondylitis and healthy controls. Joint Bone Spine 75:548–553
Rafei M, Hsieh J, Fortier S, Li M, Yuan S, Birman E, Forner K, Boivin MN, Doody K, Tremblay M et al (2008) Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood 112:4991–4998
Guilloton F, Caron G, Menard C, Pangault C, Ame-Thomas P, Dulong J, De Vos J, Rossille D, Henry C, Lamy T et al (2012) Mesenchymal stromal cells orchestrate follicular lymphoma cell niche through the CCL2-dependent recruitment and polarization of monocytes. Blood 119:2556–2567
Sierra-Filardi E, Nieto C, Dominguez-Soto A, Barroso R, Sanchez-Mateos P, Puig-Kroger A, Lopez-Bravo M, Joven J, Ardavin C, Rodriguez-Fernandez JL et al (2014) CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol 192:3858–3867
Coulson-Thomas VJ, Gesteira TF, Hascall V, Kao W (2014) Umbilical cord mesenchymal stem cells suppress host rejection: the role of the glycocalyx. J Biol Chem 289:23465–23481
Francois M, Romieu-Mourez R, Li M, Galipeau J (2012) Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther 20:187–195
Moreno M, Bannerman P, Ma J, Guo F, Miers L, Soulika AM, Pleasure D (2014) Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. J Neurosci 34:8175–8185
Biswas SK, Sodhi A (2002) In vitro activation of murine peritoneal macrophages by monocyte chemoattractant protein-1: upregulation of CD11b, production of proinflammatory cytokines, and the signal transduction pathway. J Interf Cytokine Res 22:527–538
Abe T, Su CA, Iida S, Baldwin WR, Nonomura N, Takahara S, Fairchild RL (2014) Graft-derived CCL2 increases graft injury during antibody-mediated rejection of cardiac allografts. Am J Transplant 14:1753–1764
Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, Eggens U, Distler A, Sieper J (1995) Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum 38:499–505
Greenblatt MB, Shim JH, Glimcher LH (2013) Mitogen-activated protein kinase pathways in osteoblasts. Annu Rev Cell Dev Biol 29:63–79
Mei Y, Bian C, Li J, Du Z, Zhou H, Yang Z, Zhao RC (2013) miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J Cell Biochem 114:1374–1384
Lin SK, Kok SH, Yeh FT, Kuo MY, Lin CC, Wang CC, Goldring SR, Hong CY (2004) MEK/ERK and signal transducer and activator of transcription signaling pathways modulate oncostatin M-stimulated CCL2 expression in human osteoblasts through a common transcription factor. Arthritis Rheum 50:785–793
Francois RJ, Neure L, Sieper J, Braun J (2006) Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor alpha in two patients with early disease and transforming growth factor beta in three more advanced cases. Ann Rheum Dis 65:713–720
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (81672128, 81672097), the Engineering Technology Research Center for Comprehensive Diagnosis and Treatment of Ankylosing Spondylitis of Guangdong Higher Education Institutes (GCZX-A1301), and the Science and Technology Project of Guangdong Province (2015B020228001, 2015B090903059).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study conforms to the Declaration of Helsinki and was approved by the Ethics Committee and the Animal Ethical and Welfare Committee of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China.
Conflict of interest
The authors declare that they have no conflicts of interests.
Additional information
Zhongyu Xie, Peng Wang, and Jinteng Li have contributed equally to this work.
Electronic supplementary material
ESM 1
(PDF 446 kb)
Rights and permissions
About this article
Cite this article
Xie, Z., Wang, P., Li, J. et al. MCP1 triggers monocyte dysfunctions during abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. J Mol Med 95, 143–154 (2017). https://doi.org/10.1007/s00109-016-1489-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1489-x