Abstract
Monocyte chemoattractant protein-1 (MCP-1) has a pathogenic role in murine lupus nephritis (LN). We recruited 25 pediatric and adolescent systemic lupus erythematosus (SLE) patients from our lupus clinic [13 (52%) patients with LN and 12 (48%) lupus non-nephritis patients] and evaluated their urinary and plasma MCP-1 levels compared to adult and childhood controls. The median age and SLE disease duration of patients were 14.4 and 5.5 years, respectively. LN patients had a higher median renal (p = 0.01) British Isles Lupus Assessment Group (BILAG) index, with a tendency for higher total BILAG scores (p = 0.2). There were significantly increased urinary MCP-1 levels in the LN patients compared to healthy controls (p < 0.001) whose values were significantly higher than lupus non-nephritis children (p< 0.004). Urinary MCP-1 levels correlated well with total BILAG scores (r = 0.82, p = 0.04). There were no differences in plasma MCP-1 levels between SLE patient groups and pediatric controls, although the levels in the childhood controls were elevated compared to those of the adult controls (p < 0.04). These results provide evidence of increased urinary—but not plasma—MCP-1 levels in children with LN, which correlates well with SLE disease activity as measured by the BILAG index.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a lifelong, life-limiting, multi-systemic autoimmune disease with elusive etiopathogenetic factors. Children and adolescents present with a myriad of clinical features typically associated with flares of disease activity and varying immunological manifestations. There is a significant morbidity and mortality associated with SLE, which has not improved dramatically over the last decade, despite an increasing armamentarium of immunosuppressive agents [1–4]. The prognosis of SLE can be predicted from renal involvement, with younger children having an increased incidence, severity and morbidity of lupus nephritis (LN) compared to adult-onset disease [5–10]. However, neither clinical nor serological data can accurately predict the histopathological lesion of LN as classified by the International Society of Nephrology/Renal Pathology Society (ISN/RPS) Working Group in 2003 [11, 12]. This classification has been used in children, adolescents and adults with SLE and LN [13–15].
There is increased enthusiasm for investigating and validating biomarkers for SLE and LN disease activity and damage, although there is a lack of reliable, specific biomarkers from which to make clinical management decisions [16]. The ideal biomarker would predict flares of disease activity, impending renal relapse, relapse severity and the potential for recovery. Urinary proteomics studies in adult SLE patients have demonstrated an increase in the hepcidin-20 amino acid peptide 4 months before renal flare, which returned to baseline at renal flare, with a decrease in the hepcidin-25 amino acid peptide at renal flare, which returned to baseline 4 months after the flare [17].
The role of the chemokine network is important in regulating renal leucocyte recruitment in LN, with chemotactic factors induced by immune complexes responsible for mobilizing monocytes, T lymphocytes and neutrophils, which infiltrate the kidney and mediate tissue injury and renal dysfunction [18]. Monocyte chemoattractant protein-1 (MCP-1) or chemokine ligand 2 (CCL2), a monomeric polypeptide produced as a protein precursor, is a small cytokine belonging to the CC chemokine family. It plays a significant role in the recruitment of monocytes and lymphocytes and can enhance endothelial and leucocyte adhesiveness and endothelial permeability in the kidneys of murine and human LN models where there is evidence of intra-renal inflammation and lymphocyte activation [19].
Due to genetically mandated differences in MCP-1 and intrarenal cytokine gene expression in SLE [19, 20], we previously investigated and demonstrated a correlation between the glomerular expression of MCP-1 and macrophages in kidney biopsy specimens of children with SLE and LN [21]. There is a variety of organ involvement in SLE, but urine is an excellent non-invasive resource to utilize in investigating local immunopathogenesis of LN. Urinary MCP-1 was shown to be a sensitive and specific biomarker of renal SLE flare and its severity in adults with SLE who were on immunosuppressive agents [22]. However, there is little data on biomarkers in childhood SLE [23]. The aim of the study reported here was to evaluate urinary and plasma MCP-1 as biomarkers in children with SLE with and without LN.
Methods
Patients
The SLE patients enrolled in this study were identified through searches in the Pediatric Nephrology and Histopathology databases at Great Ormond Street Hospital for Children NHS Trust and were currently attending our pediatric nephrology and rheumatology lupus clinic. All the patients fulfilled at least four of the 11 revised American College of Rheumatology (ACR) classification criteria for SLE [24, 25]. Patients were deemed to have renal involvement with LN based on clinical and histopathological findings and confirmation of LN. SLE patients without LN (non-nephritis) fulfilled the ACR classification criteria for SLE in the absence of renal involvement at the time of analysis (none of these patients had hypertension, urinary sediment, renal dysfunction or percutaneous renal biopsy performed).
Healthy childhood controls were children who were undergoing routine elective surgical procedures. The controls were age and gender matched with the patient cohort and confirmed to have no known medical illnesses, active infections or drug ingestion at the time of the study. We also included adult controls to look at the differences in pediatric and adult healthy control populations. Plasma and early morning urine samples were obtained from our control populations.
Full assessment with history and examination was undertaken for all patients with SLE disease activity scored by the British Isles Lupus Assessment Group (BILAG) index. Blood tests included investigative markers of disease activity (with erythrocyte sedimentation rate, C-reactive protein, antinuclear antibody, anti-double stranded DNA antibody, C3, and C4). Other investigations included serum electrolytes, creatinine, albumin and full blood count. Urine investigations included quantification of proteinuria with urine albumin:creatinine ratios.
MCP-1 laboratory techniques
Both plasma and urinary MCP-1 levels were measured by a sandwich enzyme-linked immunosorbent assay (ELISA) using murine monoclonal antibody (R & D Systems, Abingdon, UK). All EDTA samples were separated and aliquoted immediately and stored at −80°C until analyzed. For urinary MCP-1 analysis, early morning urine samples were collected aseptically into sterile containers, centrifuged immediately and stored at −80°C until analyzed. The commercial kit was used according to the protocol provided by the manufacturer. In brief, micro-titer wells coated with anti-human MCP-1 antibody were incubated with diluted plasma and urine from each of the study groups, standards and controls. After a 2-h incubation at room temperature, peroxidase-conjugated polyclonal anti-human MCP-1 antibody and then substrate were added. The reaction was stopped by adding acid, and the plate was read at 450 nm using Multiskan EX Ascent software ver. 2.6 (Thermo Lab Systems, Thermo Electron Corp, Franklin MA). A standard curve was generated by plotting logarithmic absorbance versus logarithmic human MCP-1 concentration, and the best fit line was determined by regression analysis. The intra-assay and inter-assay coefficient of variation were <7.8 and <6.8%, respectively, and the minimum sensitivity of the assay was 5.0 ng/ml.
Statistical methods
The data were analyzed on SPSS ver. 14.0 software for Windows (SPSS, Chicago, IL). Statistical significance was derived using non-parametric tests, including the Mann–Whitney, Wilcoxon and Kruskal–Wallis tests. To quantify the relationships between the continuous variables, we first obtained a column scatterplot and then calculated the correlation either by the Spearman’s rank or Pearson correlation method. P values <0.05 were considered to be statistically significant.
Ethics
Ethical approval was obtained for this study from the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust Research Ethics Committee.
Results
Clinical data
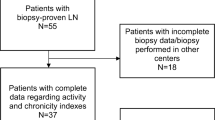
Twenty-five patients aged 4.6–17.8 (median 14.4) years of whom 84% (21/25) were female were recruited from outpatient clinics. The patients had been diagnosed with SLE for 0.1–13.2 (median 5.5) years, and 52% (13/25) had biopsy-proven LN of whom 46% (6 patients) had ISN/RPS Class III (A/C) LN, 23% (3) had Class IV [2 G(A/C) and 1 S(A)] LN, 23% (3) had Class II LN and 8% (1) had Class I LN.
There were similar baseline characteristics between SLE patients in the nephritis and non-nephritis groups. Both groups had the same median age of 14.4 years [age range 4.6–17.8 years (LN patients) vs. 7.7–17.6 (non-nephritis patients)]. LN patients had a higher median renal BILAG score [3 vs. 1 (non-nephritis patients); p = 0.01] with a tendency to have higher total BILAG scores [15 vs. 7 (non-nephritis); p = 0.2]. The only differences in patients’ blood and urine results at the time of plasma and urinary MCP-1 analysis was that LN patients had higher urine albumin:creatinine ratios (10.5 vs. 1.3 (non-nephritis patients) mg/mmol; p = 0.03; Table 1).
All patients, apart from one lupus non-nephritis child who had just been diagnosed, were on immunosuppressive therapies at the time of the plasma and urinary MCP-1 analyses. The median corticosteroid dosages were similar between the LN [0.15 (range 0.05–0.5) mg/kg/day] and lupus non-nephritis [0.12 (range 0.04–0.4) mg/kg/day] groups. The LN group had a tendency to have received more immunosuppressive agents, although this was not statistically significant.
Biomarker data
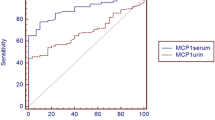
Children with LN had significantly increased urinary MCP-1 levels than the children and adult controls (p < 0.001) and lupus non-nephritis children (p < 0.001). Surprisingly, the healthy controls had significantly higher urinary MCP-1 levels than lupus non-nephritis children (p < 0.004), possibly due to the immunosuppressive therapies in lupus non-nephritis children (Fig. 1). The urinary MCP-1 levels correlated well with the total BILAG scores (r = 0.82, p = 0.04; Fig. 2).
There were no differences in plasma MCP-1 levels between the lupus patient groups and childhood controls (Fig. 3), although plasma MCP-1 levels were elevated in children compared to the adult controls (Fig. 4; p < 0.04).
Due to the increased numbers of subgroups within the ISN/RPS classification of LN and there only being 13 biopsy-proven LN patients in our cohort of 25 SLE patients, no statistically significant differences were observed, although there was a tendency for increased urinary MCP-1 levels in children with Class IV LN. However, the degree of disease activity could also be modified by immunosuppressive therapies due to the delay between LN patients having percutaneous renal biopsies and this study measuring the urinary and plasma MCP-1 levels.
Discussion
Our study demonstrated increased urinary but not plasma MCP-1 levels in children with SLE and LN compared to lupus non-nephritis patients. Urinary MCP-1 levels correlated well with SLE disease activity as measured by the BILAG index. There were no differences in plasma MCP-1 levels between those patients with and without LN, although levels were significantly higher in childhood compared to adulthood controls. These results emphasize the need for novel biomarkers in clinical pediatric studies in order to obtain normative data for children, which cannot be extrapolated from healthy adult controls.
Certain MCP-1 genetic polymorphisms have been implicated in the development of SLE and LN [26, 27], although there is still an ongoing debate in the literature on this association [28]. The data from these studies indicate that SLE patients with specific genotypes may be at higher risk of developing LN through the modulation of MCP-1 expression, with the measurement of urinary levels of MCP-1 correlating with proteinuria and thus being a useful tool for the detection and management of LN. Therefore, MCP-1 may have a role in the etiopathogenesis of SLE and LN and could be utilized as a potential biomarker of disease activity.
Children with SLE develop LN earlier and in a more severe form. This occurs in up to 82% of patients, with half of these developing a diffuse LN associated with the poorest renal prognosis (proteinuria and reduced glomerular filtration rate) [2]. Murine LN models have shown upregulation of MCP-1 with the recruitment and activation of inflammatory cells [29], with MCP-1 antagonists [30] and anti-MCP-1 gene therapy [31] halting the initiation and progression of disease.
Our study is an extension of our previous study of percutaneous renal biopsies from children with SLE and LN, which showed a correlation between glomerular expression of MCP-1, PGM1 (anti-CD68) and renal prognosis in childhood LN [21]. There is upregulation of MCP-1 with increasing intra-renal inflammation and severity of LN, highlighting the possible pathogenic role of MCP-1. This correlation has previously been demonstrated in both murine [32] and adult [33] LN models and suggests the use of MCP-1 as a prognostic marker. There is intra-glomerular expression of target genes, with MCP-1 expression related to the total and renal severity of the SLE of LN [19].
Increased glomerular and interstitial expression of MCP-1 has been demonstrated in adults with anti-neutrophilic cytoplasmic antibodies-associated vasculitis and crescentic glomerulonephritis, with urinary MCP-1 acting as a useful non-invasive biomarker of renal involvement and disease monitoring [34]. Another study showed that urinary MCP-1 levels correlates well with the degree of renal involvement in adults with crescentic glomerulonephritis [35].
MCP-1 lures monocytes and memory T lymphocytes to sites of cellular immune reactions and has potential pathogenic effects in diseases characterized by a monocyte inflammatory component. MCP-1 is produced by renal tubular epithelial cells in proteinuric disorders, which can be a factor in the development of renal dysfunction and chronic kidney disease [36].
MCP-1 may be a useful biomarker of SLE and LN disease activity. However, more recently, other potential biomarkers have been implicated [37], although each biomarker needs to be evaluated in both pediatric and adult SLE disease, with and without LN, in terms of sensitivity and specificity. There is evidence of the importance of urinary neutrophil gelatinase-associated lipocalin as a biomarker of LN in childhood-onset systemic lupus erythematosus [38, 39].
Based on the results from this study together with our previous evidence of increased glomerular expression of MCP-1 in children with LN [21], we propose multi-center, prospective, longitudinal studies evaluating urinary, plasma and intra-renal biomarkers. When we finally have a good understanding of the involvement of these biomarkers in the etiopathogenesis, activity, chronicity and progression of SLE and LN, we may be able to use them to guide patient treatment.
References
Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, Urowitz M, Fortin PR, Petri M, Barr S, Gordon C, Bae SC, Isenberg D, Zoma A, Aranow C, Dooley MA, Nived O, Sturfelt G, Steinsson K, Alarcon G, Senecal JL, Zummer M, Hanly J, Ensworth S, Pope J, Edworthy S, Rahman A, Sibley J, El-Gabalawy H, McCarthy T, St Pierre Y, Clarke A, Ramsey-Goldman R (2006) Mortality in systemic lupus erythematosus. Arthritis Rheum 54:2550–2557
Cameron JS (1994) Lupus nephritis in childhood and adolescence. Pediatr Nephrol 8:230–249
Chambers SA, Allen E, Rahman A, Isenberg D (2009) Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology (Oxford) 48:673–675
Walravens PA, Chase HP (1976) The prognosis of childhood systemic lupus erythematosus. Am J Dis Child 130:929–933
Baqi N, Moazami S, Singh A, Ahmad H, Balachandra S, Tejani A (1006) Lupus nephritis in children: a longitudinal study of prognostic factors and therapy. J Am Soc Nephrol 7:924–929
Brunner HI, Gladman DD, Ibanez D, Urowitz MD, Silverman ED (2008) Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum 58:556–562
Emre S, Bilge I, Sirin A, Kilicaslan I, Nayir A, Oktem F, Uysal V (2001) Lupus nephritis in children: prognostic significance of clinicopathological findings. Nephron 87:118–126
Glidden RS, Mantzouranis EC, Borel Y (1983) Systemic lupus erythematosus in childhood: clinical manifestations and improved survival in fifty-five patients. Clin Immunol Immunopathol 29:196–210
Marini R, Costallat LT (1999) Young age at onset, renal involvement, and arterial hypertension are of adverse prognostic significance in juvenile systemic lupus erythematosus. Rev Rhum Engl Ed 66:303–309
Stichweh D, Arce E, Pascual V (2004) Update on pediatric systemic lupus erythematosus. Curr Opin Rheumatol 16:577–587
Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15:241–250
Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65:521–530
Markowitz GS, D’Agati VD (2007) The ISN/RPS 2003 classification of lupus nephritis: an assessment at 3 years. Kidney Int 71:491–495
Marks SD, Sebire NJ, Pilkington C, Tullus K (2007) Clinicopathological correlations of paediatric lupus nephritis. Pediatr Nephrol 22:77–83
Yokoyama H, Wada T, Hara A, Yamahana J, Nakaya I, Kobayashi M, Kitagawa K, Kokubo S, Iwata Y, Yoshimoto K, Shimizu K, Sakai N, Furuichi K (2004) The outcome and a new ISN/RPS 2003 classification of lupus nephritis in Japanese. Kidney Int 66:2382–2388
Liu CC, Ahearn JM (2009) The search for lupus biomarkers. Best Pract Res Clin Rheumatol 23:507–523
Zhang X, Jin M, Wu H, Nadasdy T, Nadasdy G, Harris N, Green-Church K, Nagaraja H, Birmingham DJ, Yu CY, Hebert LA, Rovin BH (2008) Biomarkers of lupus nephritis determined by serial urine proteomics. Kidney Int 74:799–807
Rovin BH (2008) The chemokine network in systemic lupus erythematous nephritis. Front Biosci 13:904–922
Chan RW, Lai FM, Li EK, Tam LS, Chow KM, Lai KB, Li PK, Szeto CC (2007) Intrarenal cytokine gene expression in lupus nephritis. Ann Rheum Dis 66:886–892
Brown KS, Nackos E, Morthala S, Jensen LE, Whitehead AS, Von Feldt JM (2007) Monocyte chemoattractant protein-1: plasma concentrations and A(-2518)G promoter polymorphism of its gene in systemic lupus erythematosus. J Rheumatol 34:740–746
Marks SD, Williams SJ, Tullus K, Sebire NJ (2008) Glomerular expression of monocyte chemoattractant protein-1 is predictive of poor renal prognosis in pediatric lupus nephritis. Nephrol Dial Transplant 23:3521–3526
Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN (2005) Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol 16:467–473
Das L, Brunner HI (2009) Biomarkers for renal disease in childhood. Curr Rheumatol Rep 11:218–225
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Kim HL, Lee DS, Yang SH, Lim CS, Chung JH, Kim S, Lee JS, Kim YS (2002) The polymorphism of monocyte chemoattractant protein-1 is associated with the renal disease of SLE. Am J Kidney Dis 40:1146–1152
Tucci M, Barnes EV, Sobel ES, Croker BP, Segal MS, Reeves WH, Richards HB (2004) Strong association of a functional polymorphism in the monocyte chemoattractant protein 1 promoter gene with lupus nephritis. Arthritis Rheum 50:1842–1849
Nakashima H, Akahoshi M, Shimizu S, Inoue Y, Miyake K, Ninomiya I, Igawa T, Sadanaga A, Otsuka T, Harada M (2004) Absence of association between the MCP-1 gene polymorphism and histological phenotype of lupus nephritis. Lupus 13:165–167
Wagrowska-Danilewicz M, Stasikowska O, Danilewicz M (2005) Correlative insights into immunoexpression of monocyte chemoattractant protein-1, transforming growth factor beta-1 and CD68+ cells in lupus nephritis. Pol J Pathol 56:115–120
Hasegawa H, Kohno M, Sasaki M, Inoue A, Ito MR, Terada M, Hieshima K, Maruyama H, Miyazaki J, Yoshie O, Nose M, Fujita S (2003) Antagonist of monocyte chemoattractant protein 1 ameliorates the initiation and progression of lupus nephritis and renal vasculitis in MRL/lpr mice. Arthritis Rheum 48:2555–2566
Shimizu S, Nakashima H, Masutani K, Inoue Y, Miyake K, Akahoshi M, Tanaka Y, Egashira K, Hirakata H, Otsuka T, Harada M (2004) Anti-monocyte chemoattractant protein-1 gene therapy attenuates nephritis in MRL/lpr mice. Rheumatology (Oxford) 43:1121–1128
Zoja C, Liu XH, Donadelli R, Abbate M, Testa D, Corna D, Taraboletti G, Vecchi A, Dong QG, Rollins BJ, Bertani T, Remuzzi G (1997) Renal expression of monocyte chemoattractant protein-1 in lupus autoimmune mice. J Am Soc Nephrol 8:720–729
Dai C, Liu Z, Zhou H, Li L (2001) Monocyte chemoattractant protein-1 expression in renal tissue is associated with monocyte recruitment and tubulo-interstitial lesions in patients with lupus nephritis. Chin Med J (Engl) 114:864–868
Tam FW, Sanders JS, George A, Hammad T, Miller C, Dougan T, Cook HT, Kallenberg CG, Gaskin G, Levy JB, Pusey CD (2004) Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant 19:2761–2768
Wada T, Furuichi K, Segawa-Takaeda C, Shimizu M, Sakai N, Takeda SI, Takasawa K, Kida H, Kobayashi KI, Mukaida N, Ohmoto Y, Matsushima K, Yokoyama H (1999) MIP-1alpha and MCP-1 contribute to crescents and interstitial lesions in human crescentic glomerulonephritis. Kidney Int 56:995–1003
Murali NS, Ackerman AW, Croatt AJ, Cheng J, Grande JP, Sutor SL, Bram RJ, Bren GD, Badley AD, Alam J, Nath KA (2007) Renal upregulation of HO-1 reduces albumin-driven MCP-1 production: implications for chronic kidney disease. Am J Physiol Renal Physiol 292:F837–F844
Rovin BH, Zhang X (2009) Biomarkers for lupus nephritis: the quest continues. Clin J Am Soc Nephrol 4:1858–1865
Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, Mishra J, Devarajan P (2006) Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum 54:2577–2584
Hinze CH, Suzuki M, Klein-Gitelman M, Passo MH, Olson J, Singer NG, Haines KA, Onel K, O’Neil K, Silverman ED, Tucker L, Ying J, Devarajan P, Brunner HI (2009) Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum 60:2772–2781
Acknowledgments
SM received a grant from the Peel Medical Research Trust to carry out this research and would like to express his gratitude to them.
Conflicts of interest
None of the authors have any relationships with companies that may have a financial interest in the information contained in the manuscript. There are no financial interests or arrangements with a company whose product was used in a study or is referred to in a manuscript. There are no financial interests of arrangement with a competing company. There are no direct payments to an author(s) from any source for the purpose of writing the manuscript. There are no other financial connections, direct or indirect, or other situations that might raise the question of bias in the work reported or the conclusions, implications, or opinions stated including pertinent commercial or other sources of funding for the individual author(s) or for the associated department(s) or organization(s), personal relationships, or direct academic competition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marks, S.D., Shah, V., Pilkington, C. et al. Urinary monocyte chemoattractant protein-1 correlates with disease activity in lupus nephritis. Pediatr Nephrol 25, 2283–2288 (2010). https://doi.org/10.1007/s00467-010-1605-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1605-z