Abstract
Metastasizing cancer cells that arrest in brain microvessels have to face an organ microenvironment that is alien, and exclusive. In order to survive and thrive in this foreign soil, the malignant cells need to successfully master a sequence of steps that includes close interactions with pre-existing brain microvessels, and other nonmalignant cell types. Unfortunately, a relevant number of circulating cancer cells is capable of doing so: brain metastasis is a frequent and devastating complication of solid tumors, becoming ever more important in times where the systemic tumor disease is better controlled and life of cancer patients is prolonged. Thus, it is very important to understand which environmental cues are necessary for effective brain colonization. This review gives an overview of the niches we know, including those who govern cancer cell dormancy, survival, and proliferation in the brain. Colonization of pre-existing niches related to stemness and resistance is a hallmark of successful brain metastasis. A deeper understanding of those host factors can help to identify the most vulnerable steps of the metastatic cascade, which might be most amenable to therapeutic interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases are challenging targets for therapy and prevention [1, 2]. The organ that is colonized is unlike any other in the body. Its anatomical, physiological, and molecular microenvironment is special, and its importance for physical well-being and personality is obvious. Today, metastasis to the brain is an increasing problem for patients suffering from solid tumors who are otherwise responding well to local and systemic therapies [1]. Many therapeutics that work well on systemic metastases have much less, if any, activity in the brain, making it to a “sanctuary site” [2]. The reason for that is much debated: historically, it is widely assumed that the blood-brain barrier (BBB) is the most important factor, protecting tumor cells from systemic therapeutics. However, emerging data suggests that this is only one explanation, and organ-specific metastatic niche(s) contribute to the protective nature of the brain microenvironment.
Lung cancer is the leading primary site, causing more than 50 % of all brain metastases; breast cancer and melanoma follow [3]. Brain metastases are associated with a dismal survival, a high morbidity reducing the quality of life, and limited treatment options. Thus, strategies to prevent this complication hold the promise to translate into a significant benefit for cancer patients [2, 4]. With more and more targeted therapies emerging that show promising activity on established brain metastases [5], there is hope that tumor cells dissemination to the brain is a process that can in principle be controlled by systemic treatments. To optimize those treatments, and to explore new avenues of better disease control, we need to improve our understanding of the brain metastatic process, particularly of the niche where successful tumor cell survival and brain colonization takes place. This review aims to present the most relevant available data in this respect, provides a discussion of its implications, and highlights fields of research where progress is urgently needed. Thus, a clear focus of this review will lie on the brain microenvironment as the soil of metastases growth, and not on the seed, i.e., special properties of the brain metastasis-initiating cancer cell (BMIC).
Hints for a pre-metastatic niche in the brain
The pre-metastatic niche is an interesting concept with high implications for the biology of the metastatic process, and therapeutic interactions with it. However, not all findings in this respect are unequivocal and/or reproducible [6, 7]. This is mainly caused by the fact that it has been extremely difficult, if not impossible, to longitudinally track metastasizing cancer cells in high resolution over time, including the microenvironment at a specific site before successful metastasis takes place here. However, there is hope that this might change with novel animal models of intravital imaging which allow to track the brain metastatic process of an individual cancer cell over prolonged periods of time [8].
Fong et al. found that cancer cell-secreted circulating miR-122 can suppress glucose uptake by astrocytes and neurons, two important brain resident cell types by downregulating the glycolytic enzyme pyruvate kinase. Inhibition of miR-122 restored glucose uptake in the brain and other organs, and decreased the incidence of metastasis [9]. The authors hypothesize that, by decreasing glucose consumption of other cell types, cancer cells favor their own access to glucose, thus creating a “pre-metastatic metabolic niche” in the brain. Moreover, heterogeneous expression of E-selectin at brain endothelial cells might govern the site where successful brain metastasis takes place [10]. Other than that, data about the pre-metastatic niche in the brain is scarce yet, and the question whether such a niche exists at all needs further supportive data.
The blood-brain barrier: a special gatekeeper
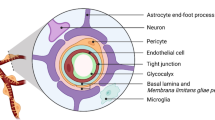
The specific anatomical and molecular constitution of the BBB prevents sufficient access of many molecules to the (healthy) brain: specialized endothelial cells connected by tight junctions, the vascular basement membrane, pericytes, astrocytic foot processes, and specialized transporter systems strictly regulate extravasation, while active exclusion mechanisms like glycoprotein P (P-gp), breast cancer resistance protein (BCRP), and the family of multidrug-resistance proteins exclude xenobiotics effectively [11]. Thus, in the normal brain, microvessels have an ultra-low permeabilty, and do not allow significant extravasation of circulating cell types into the brain parenchyma. However, it is also clear that the majority of large, macroscopic brain metastases experience breakdown of the BBB, although to a various extent [12]. In accordance with that, highly variable tumor levels have been reported for different chemotherapeutic agents in clinical specimens [13]. Together with clinical exceptions such as reported activity of primary chemotherapy in lung cancer brain metastasis [14], the picture becomes obscure, and it is increasingly doubtful that sole barrier functions are responsible for the reduced activity of systemic therapies on brain metastases.
The BBB is, however, the structure that every circulating cancer cell has to cross for access to the brain (Fig. 1). Since the brain has no classical lymphatics (and the lymphatic vessels that have been recently described around dural sinuses cannot explain the patterns of brain metastases formation [15]), hematogeneous metastasis is the exclusive way for tumor cells to access the brain. Bos et al. identified the prostaglandin-synthesizing enzyme cyclooxygenase 2 (COX2), the heparin-binding epidermal growth factor (HBEGF), and the alpha-2,6-sialyltranserase ST6GALNAC5 as mediators of breast cancer cell passage through the BBB [16]. Sialyltransferases catalyze the addition of sialic acid to cell-surface gangliosides and glycoproteins (sialylation) and promote cell-cell interactions. Normally restricted to the brain, the expression of ST6GALNAC5 in breast cancer cells enhances their adhesion to brain endothelial cells and their BBB passage [16]. Furthermore, the authors showed that treatment of mice with an antagonist to the receptor of HBEGF, cetuximab (which is routinely used for treatment of non-small cell lung cancer (NSCLC), colorectal cancer, and head and neck cancer), decreased the brain metastatic activity of breast cancer cells. Similarly, expression levels of membrane-bound melanotransferrin correlated with the ability of melanoma cells to cross the BBB [17]. Rho kinase signaling, which is involved in intracellular junction disruption, has also been found to be active in transendothelial migration of SCLC cells [18]. Likewise, the chemokine receptor CXCR4 and its ligand CXCL12 (also referred to as stromal cell-derived factor 1-alpha (SDF-1 alpha)) have been shown to participate in migration of breast cancer cells through brain endothelial cells via increase of vascular permeability and activation of the PI-3K/AKT pathway [19]. Furthermore, cathepsin S was found to specifically mediate BBB transmigration through proteolytic processing of the junctional adhesion molecule JAM-B [20]. One recent report found that tumor-derived miRNA-181c containing extracellular vesicles dysregulate the dynamics of intracellular actin in brain endothelial cells, which could be another mechanism of BBB breakdown [21]. Finally, even physical exercise has been associated with higher BBB integrity and decreased brain metastases formation [22].
Successful brain metastasis depends on colonization of a distinct perivascular niche, where multiple interactions of cancer cells with the brain microenvironment take place. After vascular arrest, a circulating cancer cell has to extravasate by overcoming the blood-brain barrier. After that, most of the cancer cells die. A small fraction, however, is able to survive in the alien microenvironment and can remain dormant in a perivascular niche, or successfully grow to a macrometastasis. Both brain invasion via perivascular pathways and induction of sprouting angiogenesis can be employed to successfully grow in the brain. Furthermore, an adaptation to brain-specific survival pathways seems to play a role. Examples of known molecular players that guide these interactions are given. Further details are given in the text. NSCLC non-small cell lung cancer
In addition, integrin-mediated interaction of tumor cells with host cells may play a role for the early steps of the brain metastatic cascade. Activation of integrin alpha-v-beta-3 supported tumor cell arrest in the blood flow through interaction with platelets and incorporation into microthrombi in a mouse model of breast cancer [23]. AlphaB-crystallin promoted adhesion of cancer cells to brain endothelial cells through an alpha3beta1 integrin-dependent mechanism [24]. Integrin beta-4 signaling indirectly supported the adherence to the brain vasculature by inducing ErbB2-dependent upregulation of VEGF in tumor cells [25]. Indeed, activated alpha-v-beta 3 was associated with brain metastatic growth through upregulation of VEGF under normoxic conditions [26]. VEGF can decrease the integrity of the endothelial cell layer and thus enhance the transendothelial migration of tumor cells [27]. In contrast to these findings, by making use of a novel animal model that allows to visualize and quantify all steps of the brain metastatic cascade, it was found that inhibition of VEGF-A did indeed slow down, but did not change the overall extravasation rate of cancer cells in another model of lung cancer brain metastases [8]. Another pro-angiogenic and permeability-increasing molecule, angiopoietin-2, has also been associated with early BBB breakdown and metastatic brain colonization in breast cancer [28].
Collectively, these data provide ample evidence that the multi-layered BBB, usually tight and impenetrable, must somehow become more permissive at the site where blood-borne cancer cells are able to extravasate into the brain. This appears highly plausible, even if other extravasation strategies (e.g., luminal growth of endothelial cells over cancer cells, pushing them out of the blood lumen) might exist, at least under certain conditions. There is limited information, however, about the relative importance of the single molecular players of extravasation, and even less about tumor type-specific mechanisms—which would be crucial informations about the most promising strategy to prevent the earliest steps of brain metastases formation by a systemic drug in patients.
A perivascular niche for cancer cell survival, dormancy, and stemness
After overcoming the BBB, tumor cells are confronted with a highly alien microenvoriment: the extracellular matrix differs when compared to other sites in the body, as do resident parenchymal cells (primarily astrocytes, microglia, and neurons, and brain-specific endothelial cells and pericytes), and also paracrine signaling molecules such as cytokines and growth factors. Early and prolonged cancer cell survival, and subsequent tumor growth requires adaption to and successful interaction with these factors.
There is ample data that the perivascular niche is of crucial importance for the brain metastatic process (Fig. 1). We and others have shown that strict perpetuation of a direct contact to the perfused brain microvessel is obligatory for cancer cells to survive after extravasation [8, 29, 30]. Whenever this contact is lost, death was inevitable for brain-metastasizing lung cancer and melanoma cells—no matter whether this happened initially, or after days and weeks [8]. Next to the prime access to oxygen and nutrients that comes with this microanatomical position, other factors likely play a role. The vascular basement membrane with its high content in collagen, laminin, and other extracellular matrix proteins can provide important cues for growth and survival via interactions with the beta1 integrin subunit on the surface of metastasizing cancer cells [29]. Thus, the basement membrane could make the brain microenvironment—which normally is devoid of these extracellular matrix proteins—to a less foreign and less hostile “soil” for colonizing malignant cells of alien origin.
Importantly, a few percent of both melanoma and lung cancer cells enter a dormant state after extravasation, and rest as single cells without proliferation or regression over many weeks, which again is strictly limited to a position in the perivascular niche [8]. Since dormant cancer cells may be responsible for late manifestations or relapses of brain metastases, it is crucial to better understand their biology, including which factors release them from dormancy. Unfortunately, very little is published about this point today. Conceptually, extravasated cancer cells that remain dormant over prolonged periods of time in the perivascular niche might share features with primary brain tumor cells that harbor stem-like features, which could speak for a role of Ephrin B2, nitric oxide, osteopontin, CXCL12, laminins, and/or Notch ligands [31]. For brain metastasis, Ghajar and colleagues confirmed that dormancy in the brain and also in the lung and bone marrow indeed was restricted to a position in the perivascular niche. Thrombospondin-1, released from resting endothelium and located to the vascular basement membrane, including that of brain vessels, mediated cancer cell quiescence [32]. In contrast, TGF-beta1 and periostin were identified as factors released from activated endothelial cells that sparked micrometastatic outgrowth. While the functional role of these mechanisms for gain and loss of dormancy in the brain microenvironment have to be further confirmed, this data provides interesting hints how microvessels can in principle control tumor cell dormancy.
The perivascular niche also has a crucial role for normal neurogenesis, which depends on tight and reciprocal interactions of blood vessel components, particularly endothelial cells, with neural stem and progenitor cells [33]. One crucial player for mitotic expansion of neural progenitor cells in the perivascular niche is the VEGF pathway [34], which has also been implicated in successful brain metastases formation for different cancer types [8, 35–38]. Furthermore, perivascular nitric oxide (NO) plays a complex role in neurogenesis. While it was long regarded as its tonical suppressor under normal conditions, it seems to potentiate stem cell proliferation under pathological conditions including hypoxia [33]. Likewise, in breast cancer, inhibition of inducible nitric oxide synthase (iNOS, NOS2) inhibited the formation of brain metastases [39]. More information comes from primary brain tumors, where a distinct perivascular niche has been described for glioma stem cells [40], and implicated in radiation resistance of medulloblastoma stem-like cells [41]. Perivascular nitric oxide gradients generated by the endothelial NOS (eNOS) appear to be one crucial mediator of promoting stemness of glioma cells [42]. Others have found that glioma stem-like cells depend on NO generated by their own iNOS, while non-stem-like cells do not [43]. Moreover, activation of NOTCH signaling is involved in the modulation of stemness of astroglia-like neural stem cells [44], and upregulated by NO in glioma stem-like cells [42]. Interestingly, proficient NOTCH signaling has also been involved in successful brain metastasis [45, 46]. Other important players of neurogenesis in the perivascular niche, such as CD24, purinergic receptors, and brain-derived neurotrophic factor (BDNF) [33] have not been investigated in the context of brain metastases so far, but are interesting avenues for future studies.
All in all, the perivascular niche plays a central role for brain metastases survival and dormancy, but also for promotion of stemness, including that of brain tumor cells. It is not clear, however, whether microregional differences in niche composition, or differences resulting from the heterogeneity of brain metastasis-initiating cancer cells are responsible for the dichotomous behavior in it, i.e., quiescence in a dormant state, or rapid growth and invasion [8].
The brain microenvironment: friend or foe for metastatic tumor cells?
Another potential resistance mechanism for metastatic tumor cells in the brain is the interaction with brain resident cells. The high inefficiency of the brain metastatic process (with 95–99 % of brain-arrested cancer cells failing to successfully grow to a macrometastasis [8, 47]) raises the question whether some of these components are more foe than friend for extravasating cancer cells in the brain. While interactions with brain endothelial cells have been extensively discussed above, and the role of brain microvascular pericytes remains elusive, a growing body of evidence suggests that cellular interactions with astrocytes, microglia cells, and also neurons can play a role for brain colonization of cancer cells. Importantly, all three cell types are also in direct contact to the perivascular niche in the brain [48].
Astrocytes
Astrocytes, specifically their end feet that wrap cerebral blood vessels, are among the first cellular structures encountered by extravasating cancer cells. Data from several groups indicate that astrocytes can support brain invasion, e.g., by expressing the ECM-degrading enzyme heparanase [49]. Moreover, cytokine production by astrocytes may contribute to brain metastases growth by paracrine signaling [50]. Furthermore, astrocyte-secreted factors contributed to MAPK activation and subsequent overexpression of MMP2 in breast cancer cells [51]. Finally, Xing et al. demonstrated that brain metastatic breast cancer cells express high levels of IL-1beta, which in turn activates surrounding astrocytes, augmenting their expression of JAG1. This stimulated NOTCH signaling in stem-like cancer cells [45]. In contrast, a recent report suggested that plasmin can convert membrane-bound FasL of astrocytes into a paracrine death signal for brain-metastasizing lung and breast cancer cells, which have to express high levels of anti-plasminogen activator—serpins to withstand this brain protective mechanism [30]. Taken together, it appears that astrocytes can play both brain metastases-promoting and brain metastases-suppressing roles. Taken that reactive astrogliosis, i.e., a wall of activated astrocytes, surrounds most brain metastases in mice and men, it is important to better understand whether, and if so under which conditions, these opposing effects occur in patients.
Isaiah Fidler and co-workers added another role for astrocytes, demonstrating how they can help cancer cells to withstand the deleterious effects of chemotherapy. First describing a connexin 43 gap-junction mediated, helpful direct “buffering” of increased calcium concentrations in melanoma cells [52], they later found a gap-junction-dependent upregulation of survival genes (GSTA5, BCL2L1, and TWIST1) that correlated with chemoresistance in lung- and breast cancer cell lines [53]. In further cell culture experiments, they found that astrocyte-derived endothelin isoforms activate their receptors on cancer cells, leading to increased survival via the AKT and MAPK pathways [54]. In turn, cancer cells secrete IL-6 and IL-8 after gap-junction coupling with astrocytes to increase endothelin expression in those astrocytes. This data speaks for a complex, recriprocal cross-talk between the two cell types. Both endothelin receptors (ETAR and ETBR) are required to mediate chemoprotection of cancer cells by astrocytes. Finally, another question is whether the potentially protective effects of brain resident cells are limited to chemotherapy. Gap-junction intercellular communication between astrocytes makes them resistant to the deleterious effects of reactive oxygen species [55], thus providing a hint that astrocytes might be capable of protecting cancer cells from the adverse events of radiotherapy, too.
Microglia
Microglial cells are the main immune effectors of the CNS, and thus prime candidates to defend the CNS from entry of metastasizing cancer cells. Activated microglia is frequently found at and around human brain metastases [56], and can be tumoricidal and lyse melanoma and carcinoma cells by release of nitric oxide [57], similar to findings in gliomas [58]. In accordance with this, expression of the neurotrophin NT-3 was associated with increased brain metastases formation, and reduced activation of cytotoxic microglia [59]. To the contrary, Pukrop et al. reported that carcinoma cells may use microglial processes as “guiding tracks” of invasion into the brain parenchyma, which was WNT-dependent [60]. Again, the same nonmalignant cell type appears to play divergent roles under different conditions with respect to the brain metastatic process.
Neurons
Neurotrophins including the prototypic nerve growth factor (NGF) are a family of proteins expressed primarily in the CNS, but might be important for brain metastases biology, too [61]. Neurotrophins exert anti-apoptotic, pro-mitotic, and chemotactic properties. The similarities between neuronal development in the perivascular niche, and brain tumor cell biology have been highlighted in the last chapter. Human melanoma cells express the neurotrophin receptors p75(NTR) and TrkC, possibly in relation to the common embryologic origin of melanocytes and neuronal cell populations [62]. Interestingly, brain metastatic breast cancer cells displayed GABAergic properties, similar to that of neuronal cells, which could speak for a metastasis-promoting role of co-inhabitation of the neuronal niche in the brain [63]. Recently, it has been demonstrated that neuronal activity lead to secretion of neuroligin-3, stimulating glioma growth in a paracrine manner [64]. It has to be seen whether activity of mature neurons also has brain metastases-promoting effects.
Successful growth in the brain by blood vessel interactions
The brain with its high requirement for oxygen and nutrients is among the places with the highest vascular density in the body. This makes pre-existing brain microvessels to a niche that is abundantly available for brain metastases progression. Indeed, melanoma cells [1, 8], but also breast cancer cells (Liao et al., unpublished) exploit pre-existing microvessels as the leading tracks for invasion and proliferation in the brain, a growth pattern called “vascular cooption” (Fig. 2). Molecular determinants seem to include growth cues from the vascular basement membrane [29], but also the axon pathfinding molecule L1CAM [30], which is an interesting example how pathways from (neuronal) development are hijacked by metastasizing cancer cells. While vascular cooption is so successful that “real” sprouting angiogenesis is a very late (if at all present) event in melanoma and breast cancer brain metastases formation, angiogenesis was detected very early in brain metastases of non-small cell lung cancer (NSCLC) lines [8]. When VEGF-A was inhibited by a therapeutic antibody, small micrometastases consisting of up to 10 single cancer cells where arrested in a dormant state, unable to grow further. Quantification revealed that this was indeed the only relevant effect of VEGF-A inhibition, however very effective, since macrometastases did not form any more in these mice; in contrast, VEGF-A inhibition in melanoma cells that grew by vascular cooption did not significantly change the brain metastatic process [8]. Interestingly, the micrometastases in the therapy-induced dormant state again strictly inhabited the perivascular niche, causing extensive vascular remodeling at the very place of cancer cell-blood contact. This phenomenon which results in a significant increase of the surface area available for cancer cell-blood vessel interactions is also observed during the natural progression of melanoma [8] and breast cancer cells (Liao et al., unpublished) in the brain, and also in primary brain tumors [65]. It seems that cancer cells can create their own niche in the brain, by increasing the access to the crucial perivascular area, resulting in optimal cues for survival.
Metastasis of a melanoma cell (red) in the mouse brain by exploitation of the pre-existing perivascular niche. After vascular arrest in a brain capillary (green, day 1), the cancer cell extravasates into the perivascular space (day 3), and starts to invade the brain parenchyma perivascularly (day 6). Strict perpetuation of this perivascular niche position is also evident during further proliferation of this metastatic nodule (days 9–28), resulting in a tumor that grows finger-like into the healthy brain by vascular cooption. Images are acquired by in vivo two-photon microscopy, deep in the live brain. From: Kienast et al. [8]
Summary and outlook
With an increasing number of research groups getting involved in brain metastasis research in the last decade, we have already learned important lesions about how cancer cells hijack existing niches and organ-specific pathways to survive and thrive in the foreign soil. It is expected that further studies about the interaction of metastasizing cancer cells with the brain parenchyma will greatly increase our knowledge on how we can better target cancer cells in the brain, how we can break their treatment resistance, and to what extent findings from primary brain tumors can be transferred to metastatic ones. That underscores the absolute necessity to further characterize the helpful vs. detrimental effects of brain resident cells, and dynamic niche compositions. This must include the use of good preclinical animal models, which—even if opposing effects from different cell types and/or cellular activation states exist—will finally show us the net effects regarding modulation of the metastatic process in the live brain, for different disease stages, and tumor types. One important but unresolved question is what awakes dormant cancer cells in their perivascular niche. It might include angiogenic stimuli [32], which are likely to occur in the context of brain microinfarctions—a very frequent, clinically silent event in elderly humans. All in all, a better understanding of the mandatory niches for brain metastases formation, the complex interactions within them, and tumor (sub-) type-specific mechanisms of brain colonization will lead to a clearer rationale how to better treat this feared complication of tumor diseases—ideally how to prevent it altogether.
References
Preusser M, Capper D, Ilhan-Mutlu A, Berghoff AS, Birner P, Bartsch R, Marosi C, Zielinski C, Mehta MP, Winkler F et al (2012) Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathol 123:205–22
Steeg PS, Camphausen KA, Smith QR (2011) Brain metastases as preventive and therapeutic targets. Nat Rev Cancer 11:352–63
Davis FG, Dolecek TA, McCarthy BJ, Villano JL (2012) Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro-Oncology 14:1171–7
Kienast Y, Winkler F (2010) Therapy and prophylaxis of brain metastases. Expert Rev Anticancer Ther 10:1763–77
Ahluwalia MS, Winkler F (2015) Targeted and immunotherapeutic approaches in brain metastases. Am Soc Clin Oncol Edu Book 35:67–74
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA et al (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827
Duda DG, Jain RK (2010) Premetastatic lung “niche”: is vascular endothelial growth factor receptor 1 activation required? Cancer Res 70:5670–3
Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16:116–122
Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, Chow A, O’Connor ST, Li S, Chin AR et al (2015) Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol 17:183–94
Kang SA, Hasan N, Mann AP, Zheng W, Zhao L, Morris L, Zhu W, Zhao YD, Suh KS, Dooley WC et al (2015) Blocking the adhesion cascade at the premetastatic niche for prevention of breast cancer metastasis. Mol Ther 23:1044–54
Daneman R (2012) The blood-brain barrier in health and disease. Ann Neurol 72:648–72
Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA et al (2010) Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 16:5664–78
Pitz MW, Desai A, Grossman SA, Blakeley JO (2011) Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neuro-Oncol 104:629–38
Barlesi F, Gervais R, Lena H, Hureaux J, Berard H, Paillotin D, Bota S, Monnet I, Chajara A, Robinet G (2011) Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol 22:2466–70
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–41
Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA et al (2009) Genes that mediate breast cancer metastasis to the brain. Nature 459:1005–9
Rolland Y, Demeule M, Fenart L, Beliveau R (2009) Inhibition of melanoma brain metastasis by targeting melanotransferrin at the cell surface. Pigment Cell Melanoma Res 22:86–98
Li B, Zhao WD, Tan ZM, Fang WG, Zhu L, Chen YH (2006) Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett 580:4252–60
Lee BC, Lee TH, Avraham S, Avraham HK (2004) Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res 2:327–38
Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ et al (2014) Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol 16:876–88
Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lotvall J, Nakagama H, Ochiya T (2015) Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun 6:6716
Wolff G, Davidson SJ, Wrobel JK, Toborek M (2015) Exercise maintains blood-brain barrier integrity during early stages of brain metastasis formation. Biochem Biophys Res Commun 463:811–7
Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A et al (2001) Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A 98:1853–8
Malin D, Strekalova E, Petrovic V, Deal AM, Al Ahmad A, Adamo B, Miller CR, Ugolkov A, Livasy C, Fritchie K et al (2014) alphaB-crystallin: a novel regulator of breast cancer metastasis to the brain. Clin Cancer Res 20:56–67
Fan J, Cai B, Zeng M, Hao Y, Giancotti FG, Fu BM (2011) Integrin beta4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-mediated secretion of VEGF. Ann Biomed Eng 39:2223–41
Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B (2009) Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci U S A 106:10666–71
Lee TH, Avraham HK, Jiang S, Avraham S (2003) Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 278:5277–84
Avraham HK, Jiang S, Fu Y, Nakshatri H, Ovadia H, Avraham S (2014) Angiopoietin-2 mediates blood-brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J Pathol 232:369–81
Carbonell WS, Ansorge O, Sibson N, Muschel R (2009) The vascular basement membrane as “soil” in brain metastasis. PLoS One 4:e5857
Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E et al (2014) Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156:1002–16
Ghajar CM (2015) Metastasis prevention by targeting the dormant niche. Nat Rev Cancer 15:238–47
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY et al (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15:807–17
Goldman SA, Chen Z (2011) Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci 14:1382–9
Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36:827–35
Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S (2006) Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res 66:3188–3196
Kim LS, Huang S, Lu W, Lev DC, Price JE (2004) Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin Exp Metastasis 21:107–118
Kusters B, Leenders WP, Wesseling P, Smits D, Verrijp K, Ruiter DJ, Peters JP, van Der Kogel AJ, de Waal RM (2002) Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res 62:341–345
Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, Davis DW, McConkey DJ, Fidler IJ (2000) Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res 60:4959–4967
Heinecke JL, Ridnour LA, Cheng RY, Switzer CH, Lizardo MM, Khanna C, Glynn SA, Hussain SP, Young HA, Ambs S et al (2014) Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. Proc Natl Acad Sci U S A 111:6323–8
Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M et al (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11:69–82
Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC (2008) PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev 22:436–48
Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC (2010) Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 6:141–152
Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, Misuraca KL, Lathia JD, Forrester MT, Lee J, Stamler JS et al (2011) Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell 146:53–66
Andreu-Agullo C, Morante-Redolat JM, Delgado AC, Farinas I (2009) Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci 12:1514–23
Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Wilber A, Mo YY, Moore BE et al (2013) Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol Med 5:384–96
Nam DH, Jeon HM, Kim S, Kim MH, Lee YJ, Lee MS, Kim H, Joo KM, Lee DS, Price JE et al (2008) Activation of notch signaling in a xenograft model of brain metastasis. Clin Cancer Res 14:4059–4066
Heyn C, Ronald JA, Ramadan SS, Snir JA, Barry AM, MacKenzie LT, Mikulis DJ, Palmieri D, Bronder JL, Steeg PS et al (2006) In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med 56:1001–1010
Osswald M, Winkler F (2013) Insights into cell-to-cell and cell-to-blood-vessel communications in the brain: in vivo multiphoton microscopy. Cell Tissue Res 352:149–59
Marchetti D, Li J, Shen R (2000) Astrocytes contribute to the brain-metastatic specificity of melanoma cells by producing heparanase. Cancer Res 60:4767–70
Sierra A, Price JE, Garcia-Ramirez M, Mendez O, Lopez L, Fabra A (1997) Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Lab Investig 77:357–68
Mendes O, Kim HT, Lungu G, Stoica G (2007) MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clin Exp Metastasis 24:341–51
Lin Q, Balasubramanian K, Fan D, Kim SJ, Guo L, Wang H, Bar-Eli M, Aldape KD, Fidler IJ (2010) Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia 12:748–54
Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, Maya M, He J, Kim SW, Weihua Z et al (2011) Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia 13:286–98
Kim SW, Choi HJ, Lee HJ, He J, Wu Q, Langley RR, Fidler IJ, Kim SJ (2014) Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro-Oncology 16:1585–98
Le HT, Sin WC, Lozinsky S, Bechberger J, Vega JL, Guo XQ, Saez JC, Naus CC (2014) Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J Biol Chem 289:1345–54
Berghoff AS, Lassmann H, Preusser M, Hoftberger R (2013) Characterization of the inflammatory response to solid cancer metastases in the human brain. Clin Exp Metastasis 30:69–81
Brantley EC, Guo L, Zhang C, Lin Q, Yokoi K, Langley RR, Kruzel E, Maya M, Kim SW, Kim SJ et al (2010) Nitric oxide-mediated tumoricidal activity of murine microglial cells. Transl Oncol 3:380–8
Hwang SY, Yoo BC, Jung JW, Oh ES, Hwang JS, Shin JA, Kim SY, Cha SH, Han IO (2009) Induction of glioma apoptosis by microglia-secreted molecules: the role of nitric oxide and cathepsin B. Biochim Biophys Acta 1793:1656–68
Louie E, Chen XF, Coomes A, Ji K, Tsirka S, Chen EI (2013) Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene 32:4064–77
Pukrop T, Dehghani F, Chuang HN, Lohaus R, Bayanga K, Heermann S, Regen T, Van Rossum D, Klemm F, Schulz M et al (2010) Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 58:1477–89
Termini J, Neman J, Jandial R (2014) Role of the neural niche in brain metastatic cancer. Cancer Res 74:4011–5
Denkins Y, Reiland J, Roy M, Sinnappah-Kang ND, Galjour J, Murry BP, Blust J, Aucoin R, Marchetti D (2004) Brain metastases in melanoma: roles of neurotrophins. Neuro-Oncology 6:154–65
Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, Kowolik CM, Li H, Hambrecht AC, Roberts E, Jandial R (2014) Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci U S A 111:984–9
Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS et al (2015) Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161:803–16
Winkler F, Kienast Y, Fuhrmann M, von Baumgarten L, Burgold S, Mitteregger G, Kretzschmar H, Herms J (2009) Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia 57:1306–1315
Acknowledgments
This work was supported by a grant from the German Research Foundation (DFG), WI 1930/5-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winkler, F. The brain metastatic niche. J Mol Med 93, 1213–1220 (2015). https://doi.org/10.1007/s00109-015-1357-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1357-0