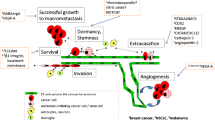
Abstract
The incidence of brain metastasis is increasing, however, little is known about the molecular mechanisms responsible for metastasis of peripheral tumor cells and their colonization of the brain. After tumor cells metastasize to the brain, they encounter a completely different microenvironment from that in the periphery. The interactions between tumor cells and glial cells, mainly astrocytes and microglia, including soluble factors released from these cells, are still under investigation. However this knowledge will contribute to understanding the mechanisms of cell-cell interactions in the brain and identify possible therapeutic targets on resident brain cells that could effect brain metastasis formation and treatment. In addition to the complex interactions between metastatic tumor cells and the brain’s resident cells, factors from endothelial cells and endogenous plasma factors also affect the blood-brain barrier and may change tumor cell characteristics. Therefore the totality of the brain microenvironment must be considered. The cell types and soluble factors that contribute to the brain microenvironment surrounding metastatic tumor cells are discussed herein.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In the metastatic process, the microenvironment of the metastatic site plays an important role in tumor cells invasion and proliferation in the target tissues [1]. Such a microenvironment contains many resident cell types in addition to tumor cells as well as migratory hematopoietic cells. In the brain or central nervous system (CNS) the microenvironment is composed of neurons and glial cells (microglia, astrocytes, and oligodendrocytes). Endothelial cells and pericytes that compose the blood-brain barrier (BBB) are also present.
In the CNS, activated glial cells contribute to the innate immune response and produce a large variety of different inflammatory mediators as a chronic inflammatory reaction [2]. A similar mechanism could function in mediating tumor cell survival, proliferation and colonization, and invasion and motility in the microenvironment of brain metastases [3, 4]. The involvement of brain-resident and infiltrating cells in the pathology of primary and metastatic brain tumors is poorly understood. Therefore, a better understanding of the tumor microenvironment in the brain and interactions between each cell type is necessary. Accordingly, some of the known interactions between metastatic tumor cells in the brain and different stromal cells were already described [5]. These understandings and additional information would be expected to contribute to the development of improved therapies for brain metastasis that are urgently needed due to poor treatment options for these malignancies.
Experimental models of brain metastasis will aid in our study of the brain as a microenvironment to support metastatic growth (see Chap. 2 for a discussion of experimental models of brain metastasis). In brain metastasis mouse models using human lung cancer cell lines, tumor cells metastasized to whole regions of the brain. At 3 weeks after the inoculation of tumor cells into the cardiac ventricle, metastatic foci were found in midbrain-lateral cortex (Noda, unpublished observation). At 4–6 weeks, metastatic foci of various sizes were found throughout the brain. It is important to understand the interactions between invaded tumor cells and resident brain cells to understand how tumor cells grow and rapidly colonize the brain. Similar results were seen with breast cancer brain metastasis models [4, 6]. Further, this understanding could help prevent the growth of metastatic tumors in the brain. In this chapter, cell types in the brain are defined, pathology of invaded tumor cells and surrounding cells are described and the interactions between tumor cells and individual resident brain cells are discussed.
2 Cell Types in the Brain
2.1 Neurons
Neurons are the fundamental cells in the brain and all other cells are mainly devoted to the support of neurons. This traditional concept is, however, only partially correct under certain physiological condition, especially from a synaptic point of view [7]. Under many pathological conditions, including brain metastases, neurons are damaged and destined to die, leading to neuronal loss. The fate of damaged neurons is controlled by interactions between the metastatic tumor cells and neuron-supporting cells, mainly glial cells.
2.2 Microglia
In the brain, microglial cells are considered as the pathologic response element [8–10]. They are sometimes referred to as the macrophage of the brain, however, when the brain is damaged, blood monocyte-derived macrophages are also present [11, 12]. Microglial cells are dispersed throughout the entire CNS, exhibiting a ramified morphology under normal conditions, and their physiological role is gradually becoming unveiled [13]. Recently it was shown that microglial processes are highly dynamic in the intact brain, suggesting that microglial cells scan the brain parenchyma with their processes and potentially shield it from injury [14, 15]. Under pathologic conditions such as a lesion (traumatic brain injury), stroke, neurodegenerative disorder or tumor cell invasion, activated microglia migrate rapidly to the affected site of the CNS. At the same time, microglial activation is accompanied by the release of immunocompetent molecules such as cytokines or chemokines, and other molecules such as growth factors [16].
In brain metastasis mouse models, bigger metastatic foci attracted increased numbers of microglia (Fig. 3.1a). Though the incidence of brain metastasis was different in each mouse, the highest incidence was generally observed in the cerebral cortex and the hippocampus [17]. In the cerebral cortex, less activated microglia were often observed around large metastatic foci (Fig. 3.1b), the reason for this is being still interrogated.
In a mouse xenograft model of brain metastasis, microglia accumulate around lung cancer cells in the brain parenchyma. (a) Typical example of microgliosis (green) around the tumor (red), and microglia accumulate in relation to the size of the metastatic foci. Red; cytokeratin. Green; Iba1. (b) Typical example showing that more microglia accumulated around metastatic foci in the hippocampus than in the cerebral cortex. Staining antibodies the same as in (a) (Reproduced from Noda et al. 2009)
2.3 Astrocytes
Among the glial cells, astrocytes are the characteristically star-shaped cells in the brain and are the most abundant glial cell population. Astrocytes play an important role in maintaining homeostasis of the brain [18], including biochemical support of endothelial cells that form the BBB, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, and they also play a role in the repair and scarring process of the brain and spinal cord following traumatic injuries. Recently, the function of astrocytes has been reconsidered, and they are now thought to play a number of active roles in the brain as well, including the secretion or absorption of neural transmitters and maintenance of the BBB [13]. Astrocytes, therefore, may be considered one of the most influential cell types in the brain, and they interact with metastatic tumor cells. In brain metastasis mouse models using lung cancer cells, glial fibrillary acidic protein (GFAP)-positive astrocytes, so-called “activated astrocytes”, accumulated according to the size of the metastatic tumor [19]. Similarly, accumulation of astrocytes around brain metastases was also observed in surgical specimens from breast cancer patient craniotomies [4] and autopsy cases from a number of primary tumor types [20]. These observations suggest that astrocytes may be essential to metastatic tumor cells in the microenvironment of brain metastases.
2.4 Oligodendrocytes
The main function of oligodendrocytes in the brain is the insulation of axons (the long projection of nerve cells) [21]. The same function is performed by Schwann cells in the peripheral nervous system. This functional importance is obvious in myelination. Although the role of oligodendrocytes in pathology is unclear, it is suggested that they may participate at an early stage in amyotrophic lateral sclerosis [22]. The role of oligodendrocytes in brain metastasis is unknown.
2.5 Pericytes
Pericytes are specifically located surrounding the endothelial cell layer of the capillary network in the brain. Pericytes play an integral role in the maintenance of the BBB as well as several other homeostatic and hemostatic functions of the brain [23]. These cells regulate capillary blood flow and BBB permeability, and are responsible for clearance and phagocytosis of cellular debris. Pericytes are also a key component of the neurovascular unit, which includes astrocytes and neurons as well as endothelial cells [24]. Recent studies suggest that pericytes in the CNS are bone marrow derived, although a respective precursor still remains enigmatic [25]. It has also been revealed recently that a lack of pericytes in the CNS can cause a breakdown of the BBB and lead to other degenerative changes in the brain [23, 26, 27]. As well as leakage of neurotoxins due to pericyte dysfunction and BBB breakdown, a role for pericytes in limiting tumor cell metastasis was demonstrated [28].
3 Cancer Stem Cells
Neoplastic clones are maintained exclusively by a rare fraction of cells with stem-like properties, known as cancer stem cells. It is believed that tumors grow from a type of “cancer stem cell” that gives rise to other cancerous cells and the identification of brain tumor initiating cells provided insight into human brain tumor pathogenesis, giving strong support for the cancer stem cell hypothesis as the basis for many solid tumors [29]. The role of cancer stem cells in the organ tropism of breast cancer metastasis as well as brain tumors was recently reported [30]. Additionally, Calabrese et al., reported the existence of a perivascular niche for brain tumor stem cells [31] and it is tempting to speculate that this niche in the microenvironment may also exist for metastatic tumor cells in the brain. Therefore, a better understanding of the interactions between cancer stem cells in invading tumor cell populations in the brain and other organs is essential for the development of novel therapeutic targets for metastatic disease. However, an in depth discussion of this is beyond the scope of this chapter.
4 Interactions Between Cell Types
4.1 Metastatic Tumor Cell-Neuron Interactions
The neuronal network in the brain has a highly compact framework devoid of large amounts of extracellular space thus the presence of a growing metastatic lesion is thought to cause neuronal damage and death as it displaces this network. Direct interactions between metastatic cells and neurons have not been fully elucidated, and whether the damage to neurons is the result of direct cell-to-cell contact or the result of toxic soluble factors secreted by tumor cells is not known. In a model on lung cancer brain metastases, a correlation was observed between the size of the metastatic lesion that was present and a vacant area of brain surrounding the lesion, suggesting neuronal loss in the brain (Noda, unpublished data). Using an intracranial implantation model with breast cancer cells, neuronal death was quantified by the identification of Fluorojade-B positive neurons [32]. The injection of the breast cancer cells induced a 15-fold increase in neuronal damage compare to a saline control injection [32]. Similarly, in vitro co-culture of primary neurons with lung tumor cells or addition of conditioned medium of lung tumor cells showed that tumor cells released factors that were toxic for neurons. The addition of the lung tumor cells inhibited the survival of primary cultured neurons depending on the number of tumor cells (Fig. 3.2). When the number of lung tumor cells was five times of that of neurons (T5 in Fig. 3.2a), the number of neuronal cells decreased to almost 50%. Importantly, tumor cell-induced neuronal damage may be responsible for cognitive symptoms experienced by some brain metastasis patients.
The influence of microglia on the interactions between tumor cells and neurons. (a) Lung tumor cells inhibited neuronal survival in a dose-dependent manner in vitro. C; no tumor cells. T1; equal amounts of tumor cells and neurons. T5; Fivefold the number of tumor cells compared to neurons. T10; Tenfold the number of tumor cells compared to neurons. (b) Primary cultured neurons from mouse cerebral cortex (top left) were co-culture with tumor cells without microglia (bottom left) or with microglia (top right) and microglia and tumor cells (bottom right). Neurons are seen as MAP2 -positive red staining cells. (c) Number of neurons present in each culture from (b). Neurons were counted in 4–7 images per condition. *p < 0.05, **p < 0.01. N neurons, T tumor cells, M microglia (Modified from Noda et al. 2009)
4.2 Metastatic Tumor Cell-Microglia Interactions
The importance of microglia in the brain necessitates that these cells would interact with invading metastatic tumor cells. In 3 experimental models of brain metastasis, activated microglia were seen surrounding single or small clusters of tumor cells just 7 days after the tumor cells were injected into the circulation [33]. Exactly what the microglia is doing at the metastatic site is still under investigation. In in vitro co-culture experiments with lung cancer cells and microglia or just by the addition of microglial conditioned medium to the lung cancer cells, tumor cell proliferation was significantly inhibited by unknown microglial factors [17]. Importantly, no significant TUNEL staining was observed in the tumor cells exposed to microglial conditioned medium, while BrdU-positive cells decreased over time, this suggested that inhibition of tumor cell proliferation by microglial factors may due to cell-cycle arrest but not apoptosis [17]. In contrast, when breast cancer cells were grown in soft agar in the presence of microglia, colony formation was increased almost fivefold compared to breast cancer cells in soft agar alone [4]. These differing effects could be primary tumor cell type specific or result from experimental differences, such as purity of the microglial cultures, and should be further investigated.
Since microglia inhibited the proliferation of lung tumor cells, it could be hypothesized that microglia may rescue damaged neurons and promote their survival. The addition of microglia to neurons in the absence of tumor cells did not show any significant effect on the neurons (Fig. 3.2b, Neuron/microglia; N/M in Fig. 3.2c), assessed by counting MAP2 (microtubule-associated protein 2, a neuronal cell marker)-positive cells. However, when microglia were added to the co-culture of neurons with lung tumor cells (Fig. 3.2b, Neuron/tumor/microglia; N/T/M in Fig. 3.2c), the number of MAP2 positive cells present in the culture was significantly increased compared to the neurons cultured with the tumor cells in the absence of microglia (Fig. 3.2c, N/T/M vs N/T).
On the contrary, microglia exposed to tumor cell-conditioned medium appeared to have a stimulated morphology and increased proliferation (Fig. 3.3). This is not unexpected as the metastatic lesion is an insult in the brain and should trigger a host cell response. In line with this, as noted above, when neurons were cultured with tumor cells and microglia, the microglia appeared to support neuronal cell viability (Fig. 3.2). This suggests that microglia, at least in vitro, serve in their immune cell function rescuing neurons from harm. These results indicate that there are not only cell-cell interactions but also complex multi-cell interactions at play in the presence of a metastatic lesion in the brain.
4.3 Metastatic Tumor Cell-Astrocyte Interactions
Since astrocytes, in conjunction with microglia, play a critical role in neuronal cell survival, it has been postulated that they can also support tumor cell survival in the brain. Activated astrocytes produce a number of inflammatory cytokines. IL-1, one such inflammatory cytokine, has been shown to stimulate the growth of tumor cells in hepatic and/or lung metastases of melanoma cells in vivo [34–36]. In vitro, astrocytes through secretion of IL-6, TGF-β and/or IGF stimulated the growth of a breast cancer cell line, which was derived from a brain metastasis [37]. In brain metastasis of melanoma, it was reported that astrocytes produce neurotrophin-regulated heparanase which was shown to increase tumor cell invasion [38, 39].
Using lung tumor cells, astrocytes were activated by tumor cell-oriented factors; MIF, IL-8 and PAI-1. These activated astrocytes then produced IL-6, TNF-α and IL-1β, which in turn promoted tumor cell proliferation. The addition of mouse recombinant IL-6, TNF-α and IL-1β to human lung cancer cells mimicked the effects of activated astrocytes [19]. Semi-quantitative immunocytochemistry showed that expression of receptors for IL-6 and its subunits gp130 on human lung cancer cells were up-regulated with time, while receptors for TNF-α and IL-1β were down-regulated after co-culture with astrocytes. These results suggest that astrocyte-derived inflammatory cytokines and their receptors, especially IL-6 receptors, may have an important role on the development of metastatic lesions in the brain and therefore might be therapeutic targets in brain metastases of lung cancer and other cancers.
Aside from those mentioned above astrocytes have been also been shown to produce IL-3, TNF-α, TGF-β, IGF-1 and PDGF [40–43]. Among them, it was suggested that IL-6, TGF-β and IGF-1 may contribute to the development of brain metastasis by breast cancer cells [37].
In the human brain, an immunohistochemical study was conducted on the peritumoral gliosis which is produced around hematogenous metastases. Eighty-five percent of the cases with metastases showed expression of endothelin-like immunoreactivity in the peritumoral astrocytes. Activation of microglial cells was another frequent and widespread glial cell alteration around the metastases [20]. Recently, it was also reported that co-culture of human breast cancer cells or lung cancer cells with murine astrocytes (but not murine fibroblasts) led to the up-regulation of survival genes, including GSTA5, BCL2L1, and TWIST1, in the tumor cells [44].
Astrocytes not only interact with and affect tumor cells at the site of metastatic growth, they also interact with microglia and neurons at the site. Activated astrocytes express glial cell line-derived neurotrophic factor (GDNF) followed by releasing of TNF-α and IL-1β by astrocytes then promotes the survival and growth of dopaminergic neurons [45]. Cultured astrocytes activated by lung tumor cells also express GDNF (Noda et al., unpublished data). Though which factors released from activated astrocytes are responsible is unknown, these factors attenuated microglial-tumor cell interactions and tumor cell proliferation showed less inhibition with microglia-astrocyte co-culture medium than that with microglia-conditioned medium alone (Noda et al., unpublished data). There results suggest that there are complicated cell-cell interaction between tumor cells and glial cells that remain to be fully understood.
5 Soluble Factors in the Brain Microenvironment
In addition to cell-cell interactions and the soluble factors mentioned above, numerous other soluble factors have been shown to play a role in influencing metastatic tumor growth in the brain microenvironment. Bradykinin, a plasma protein, has been shown to be involved in tumor metastasis by increasing BBB permeability mediated by adenosine 5’-triphosphate-sensitive potassium channel [46] and TNF-α [47], or blood-tumor barrier permeability [48]. It was suggested that bradykinin, acting via bradykinin-2 receptors (B2R), acts as an important signal for directing the invasion of glioma cells toward blood vessels [49]. These results suggest that not only brain tumor cells but also invaded peripheral tumor cells may show increased chemotaxis in the brain. Therefore, clinically approved B2R antagonists could be used as anti-invasive drugs in the future.
Recently, the cytokine pigment epithelium-derived factor (PEDF) was shown to affect both metastatic tumor cells in the brain and neurons [32]. PEDF is a secreted factor that was down regulated in a cohort of human breast cancer brain metastasis specimens compared to unlinked primary tumors [50]. When breast cancer cells were forced to overexpress PEDF and implanted into the brains of nude mice, PEDF expressing tumor cells showed increased apoptosis compared to control cells. Additionally, when the amount of neuronal damage surrounding the implanted cells was quantified by fluorojade B staining, there was a 3.5-fold decrease in damaged neurons surrounding the PEDF expressing cells compared to the control cells [32]. These data suggest that restoring the expression of PEDF in metastatic tumor cells might limit their spread. Additionally, PEDF peptides, if delivered to the brain, might help alleviate neuronal damage and thus cognitive symptoms in brain metastasis patients.
6 Summary
Figure 3.4 illustrates the cell-cell interactions and their effects discussed herein. Much of what was discussed has only been elucidated in the last decade and we still have much to learn about the brain microenvironment during metastatic tumor cell colonization and growth. Interestingly, competitive cross-species hybridization of microarray experiments showed that the brain microenvironment induces complete reprogramming of metastasized cancer cells residing there, resulting in a gain of neuronal cell characteristics and mimicking neurogenesis during development [51]. This suggests that identifying target molecules on tumor cells that could restrict these characteristic changes would be a useful strategy to prevent brain metastasis in the future.
Proposed schema for neuron-glia-tumor cell interactions. Microglial cells release soluble factors that suppress the proliferation of tumor cells by inducing cell-cycle arrest. Tumor cells, in turn, release soluble factors that activate astrocytes and suppress neuronal survival. Astrocytes are also involved in the regulation of tumor cell proliferation by the release of various soluble factors. Astrocytes may interact with microglia and neurons surround the metastatic lesion. In addition, plasma kinin and kallikrein increase blood-brain and blood-tumor permeability and may influence resident brain cells, too. Arrows indicate activation or stimulation and dotted lines are speculated signaling interactions
References
Fidler IJ, Yano S, Zhang RD et al (2002) The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol 3(1):53–57
Aloisi F, Ria F, Adorini L (2000) Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today 21(3):141–147
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545
Fitzgerald DP, Palmieri D, Hua E et al (2008) Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis 25(7):799–810
Lorger M (2012) Tumor microenvironment in the brain. Cancer 4(1):218–243
Heyn C, Ronald JA, Ramadan SS et al (2006) In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med 56(5):1001–1010
Farber K, Kettenmann H (2005) Physiology of microglial cells. Brain Res Brain Res Rev 48(2):133–143
Kim SU, de Vellis J (2005) Microglia in health and disease. J Neurosci Res 81(3):302–313
Kreutzberg GW. (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8):312–318
Perry VH, Matyszak MK, Fearn S (1993) Altered antigen expression of microglia in the aged rodent CNS. Glia 7(1):60–67
Santambrogio L, Belyanskaya SL, Fischer FR et al (2001) Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA 98(11):6295–6300
Morantz RA, Wood GW, Foster M et al (1979) Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg 50(3):305–311
Kettenmann H, Hanisch UK, Noda M et al (2011) Physiology of microglia. Physiol Rev 91(2):461–553
Davalos D, Grutzendler J, Yang G et al (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8(6):752–758
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40(2):140–155
Noda M, Seike T, Fujita K et al (2009) The role of immune cells in brain metastasis of lung cancer cells and neuron-tumor cell interaction. Ross Fiziol Zh Im I M Sechenova 95(12):1386–1396
Miller RH, Ffrench-Constant C, Raff MC (1989) The macroglial cells of the rat optic nerve. Annu Rev Neurosci 12:517–534
Seike T, Fujita K, Yamakawa Y et al (2011) Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis 28(1):13–25
Zhang M, Olsson Y (1995) Reactions of astrocytes and microglial cells around hematogenous metastases of the human brain. Expression of endothelin-like immunoreactivity in reactive astrocytes and activation of microglial cells. J Neurol Sci 134(1–2):26–32
Kettenmann H, Ransom BR (2005) Neuroglia, Oxford University Press, Oxford
Kang SH, Fukaya M, Yang JK et al (2010) NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68(4):668–681
Winkler EA, Bell RD, Zlokovic BV (2010) Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener 5:32
Dore-Duffy P, Cleary K (2011) Morphology and properties of pericytes. Methods Mol Biol 686:49–68
Krueger M, Bechmann I (2010) CNS pericytes: concepts, misconceptions, and a way out. Glia 58(1):1–10
Winkler EA, Bell RD, Zlokovic BV (2011) Central nervous system pericytes in health and disease. Nat Neurosci 14(11):1398–1405
Quaegebeur A, Segura I, Carmeliet P (2010) Pericytes: blood-brain barrier safeguards against neurodegeneration? Neuron 68(3):321–323
Xian X, Hakansson J, Stahlberg A et al (2006) Pericytes limit tumor cell metastasis. J Clin Invest 116(3):642–651
Singh SK, Hawkins C, Clarke ID et al (2004) Identification of human brain tumour initiating cells. Nature 432(7015):396–401
Chu JE, Allan AL (2012) The role of cancer stem cells in the organ tropism of breast cancer metastasis: a mechanistic balance between the “seed” and the “soil”? Int J Breast Cancer 2012:209748
Calabrese C, Poppleton H, Kocak M et al (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11(1):69–82
Fitzgerald DP, Subramanian P, Deshpande M et al (2012) Opposing effects of pigment epithelium-derived factor on breast cancer cell versus neuronal survival: implication for brain metastasis and metastasis-induced brain damage. Cancer Res 72(1):144–153
Lorger M, Felding-Habermann B (2010) Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol 176(6):2958–2971
Giavazzi R, Garofalo A, Bani MR et al (1990) Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res 50(15):4771–4775
Vidal-Vanaclocha F, Alvarez A, Asumendi A et al (1996) Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro. J Natl Cancer Inst 88(3–4):198–205
Vidal-Vanaclocha F, Amezaga C, Asumendi A et al (1994) Interleukin-1 receptor blockade reduces the number and size of murine B16 melanoma hepatic metastases. Cancer Res 54(10):2667–2672
Sierra A, Price JE, Garcia-Ramirez M et al (1997) Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Lab Invest 77(4):357–368
Marchetti D, Denkins Y, Reiland J et al (2003) Brain-metastatic melanoma: a neurotrophic perspective. Pathol Oncol Res 9(3):147–158
Denkins Y, Reiland J, Roy M et al (2004) Brain metastases in melanoma: roles of neurotrophins. Neuro Oncol 6(2):154–165
Aloisi F, Care A, Borsellino G et al (1992) Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol 149(7):2358–2366
Hertz L, McFarlin DE, Waksman BH (1990) Astrocytes: auxiliary cells for immune responses in the central nervous system? Immunol Today 11(8):265–268
Lee SC, Liu W, Dickson DW et al (1993) Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150(7):2659–2667
Wang FW, Jia DY, Du ZH et al (2009) Roles of activated astrocytes in bone marrow stromal cell proliferation and differentiation. Neuroscience 160(2):319–329
Kim SJ, Kim JS, Park ES et al (2011) Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia 13(3):286–298
Iravani MM, Sadeghian M, Leung CC et al (2012) Lipopolysaccharide-induced nigral inflammation leads to increased IL-1beta tissue content and expression of astrocytic glial cell line-derived neurotrophic factor. Neurosci Lett 510(2):138–142
Zhang H, Gu YT, Xue YX (2007) Bradykinin-induced blood-brain tumor barrier permeability increase is mediated by adenosine 5’-triphosphate-sensitive potassium channel. Brain Res 1144:33–41
Qin LJ, Gu YT, Zhang H et al (2009) Bradykinin-induced blood-tumor barrier opening is mediated by tumor necrosis factor-alpha. Neurosci Lett 450(2):172–175
Ma T, Liu L, Wang P et al (2012) Evidence for involvement of ROCK signaling in bradykinin-induced increase in murine blood-tumor barrier permeability. J Neurooncol 106(2):291–301
Montana V, Sontheimer H (2011) Bradykinin promotes the chemotactic invasion of primary brain tumors. J Neurosci 31(13):4858–4867
Palmieri D, Fitzgerald D, Shreeve SM et al (2009) Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol Cancer Res 7(9):1438–1445
Park ES, Kim SJ, Kim SW et al (2011) Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc Natl Acad Sci USA 108(42):17456–17461
Acknowledgment
This work was supported by Grants-in Aid for Scientific Research from the Japanese Society for Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Noda, M. (2012). The Brain Microenvironment. In: Palmieri, D. (eds) Central Nervous System Metastasis, the Biological Basis and Clinical Considerations. Cancer Metastasis - Biology and Treatment, vol 18. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5291-7_3
Download citation
DOI: https://doi.org/10.1007/978-94-007-5291-7_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5290-0
Online ISBN: 978-94-007-5291-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)