Abstract
Ginsenoside-Rg1 (Rg1) has been used in the traditional Chinese medicine for over 2,000 years. The present study was performed to test our hypothesis that Rg1 provides pro-angiogenic and anti-fibrotic benefits in the ischemic myocardium in a rat model of myocardial infarction. The expression of vascular endothelial growth factor (VEGF) and phosphorylation/activation of PI3K, Akt, and p38 MAPK signaling pathways were examined in human umbilical vein endothelial cells and in the myocardial samples of rats. In addition, the expression levels of TNF-α and collagen I level, the number of newly formed blood vessels, the extent of myocardial fibrosis, and left ventricular function were measured in vivo. Our results demonstrated that administration of Rg1 increased VEGF expression levels, activated PI3K/Akt, and inhibited p38 MAPK in vitro and in vivo. Furthermore, Rg1 increased the density of newly formed vessels, decreased TNF-α and collagen I expression levels and area of myocardial fibrosis, and improved left ventricle function in vivo. PI3K inhibitor LY294002 significantly attenuated Rg1-enhanced VEGF expression and capillary density. As well, inhibition of p38 MAPK slightly increased VEGF expression in vitro and in vivo, increased capillary density, and decreased TNF-α and collagen I expression levels and area of myocardial fibrosis in vivo. Rg1-induced activation of PI3K/Akt also contributed to the downregulation of p38 MAPK. Thus, Rg1 is effective in promoting angiogenesis and attenuating myocardial fibrosis, resulting in ameliorated left ventricular function. The possible mechanisms may involve activation of PI3K/Akt, inhibition of p38 MAPK, and cross talk between the two signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease is one of the leading causes of morbidity and affects more than 10 million people in the USA and millions worldwide [1]. Tissue injury from myocardial infarction (MI) activates local and systemic neurohormonal systems, which triggers left ventricular (LV) remodeling. Persistent LV remodeling leads to functional decompensation and, eventually, heart failure and even death [2]. Previous studies have confirmed that myocardial fibrosis is an essential factor in the process of LV remodeling after MI [3]. Thus, it is of paramount importance to enhance regional myocardial blood flow and attenuate myocardial fibrosis in the ischemic zone after myocardial infarction.
Recent studies revealed that ginsenoside-Rg1 (Rg1), the most prevalent and active constituent of ginseng, is a potent pro-angiogenic factor and may potentially be useful in therapeutic angiogenesis [4–6]. At present, ginseng is one of the most extensively used alternative medications throughout the world and has appeared in the pharmacopoeias of several western countries, including the USA and European countries with indications for cardiovascular diseases and other conditions [4, 7, 8].
Angiogenesis is fundamental in reproduction, development, and repair of vasculature [9]. Vascular endothelial growth factor (VEGF) is considered the chief mediator of angiogenesis because of its ability to stimulate endothelial cell proliferation, migration, and capillary formation [10]. Leung et al. found that Rg1 enhanced production of VEGF by the PI3K/Akt pathway via the glucocorticoid receptor [4]. Additionally, there is accumulating evidence that mitogen-activated protein kinase (MAPK) phosphorylation is involved in the biosynthesis of VEGF in hypoxia [11]. Issbrucker et al. showed that inhibition of p38 MAPK activity enhanced VEGF-induced angiogenesis in vitro and in vivo [12]. However, whether Rg1 stimulates VEGF-induced angiogenesis through the p38 MAPK signaling pathway is unknown.
In addition to the efficacy in angiogenesis, Rg1 has been found to inhibit the production of the inflammatory cytokine tumor necrosis factor alpha (TNF-α) [13]. In clinical and animal studies, elevated plasma and myocardial levels of TNF-α were linked to interstitial fibrosis and ventricular remodeling [14]. Inflammatory stimuli activate many intracellular signaling pathways, including the nuclear factor-κB pathway and three MAPK pathways mediated through extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK [15–17] with p38 MAPK considered as a central regulator of inflammation. Treatment with the selective p38 inhibitor SB203580 reduced p38 MAPK activity, blocked TNF-α secretion, and attenuated extracellular matrix (ECM) accumulation in the myocardium of MKK6bE transgenic mice [17]. Wu et al. suggested that ginsenosides potently suppress TNF-α secretion through inhibiting the phosphorylation of JNK and ERK [13]. However, whether Rg1 suppresses TNF-α-induced myocardial fibrosis through the p38 MAPK pathway is unclear. Thus, the present study was carried out to test our hypothesis that Rg1 is effective in promoting angiogenesis and ameliorating left ventricular remodeling due to myocardial infarction via PI3K/Akt and p38 MAPK pathways.
Materials and methods
Drugs and reagents
Ginsenoside-Rg1 (purity > 98%) was obtained from the Shanghai Medical Research Institute, Chinese Academy of Science (Shanghai, China). A stock solution of Rg1 (150 nM) was prepared in sterile double-distilled H2O for cell culture. The p38 MAPK inhibitor SB and PI3K inhibitor LY were purchased from Calbiochem (San Diego, CA). Medium 199 was from Invitrogen (Carlsbad, CA). Fetal bovine serum was from Hyclone Laboratories (Logan, UT). Anti-VEGF (sc-7269), anti-p-p38 MAPK (sc-7973), anti-p-Akt (sc-7985-R), anti-TNF-α (sc-80383), anti-TNFR1 (sc-8436), anti-TNFR2 (sc-7862), and anti-collagen I (sc8784) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-vWF antibody was purchased from Abcam (Cat #ab6994, Abcam, Cambridge, MA).
Cell culture
The study followed guidelines of the Helsinki Declaration, and ethical approval was obtained from the Shandong University Research and Ethics Committee for the procurement of human umbilical veins from healthy term pregnant women. The human umbilical vein endothelial cells (HUVECs) were cultured in medium 199 (Invitrogen, Carlsbad, CA) supplemented with 20% fetal bovine serum and endothelial cell growth supplement (120 mg/l; Sigma, St. Louis, MO) in a 25-ml gelatin-coated culture flask at 37°C in 5% CO2 and 95% air. HUVECs from passage 3 to 6 were used for all experiments. Cells were randomly divided into the following five groups: normal group under normoxic conditions (21% oxygen), Rg1 + LY-treated group (preincubation with 10 μM LY for 30 min and then with 150 nM Rg1), Rg1-treated group (150 nM Rg1), SB-treated (10 μM SB), and control group under hypoxic condition (1.5% oxygen) [18]. After treatment for 12, 24, and 48 h, the medium was collected for analysis.
Animal model
All animal care and experimental protocols complied with the Animal Management Rules of the Ministry of Health of the People’s Republic of China (document no. 55, 2001) and the Guidelines for the Care and Use of Laboratory Animals of Shandong University. Male Wistar rats (n = 106) weighing 230–290 g were purchased from the Animal Center of Shandong University (Shandong, Jinan). Rats were housed in temperature (22 ± 2°C) and humidity (55 ± 5%) controlled rooms with a 12/12-h light/dark cycle. Solid rodent chow and tap water were given freely. Animals were allowed to acclimatize for 1 week before entry into the experimental protocol. Then they were intraperitoneally anesthetized by the use of 10% chloral hydrate (300 mg/kg) and ventilated with a VIP Bird ventilator (Bird Products Corp., Palm Springs, CA) with a tidal volume of 3.0 ml and a respiratory rate of 60 cycles/min. Six rats died during the anesthetic induction. Twenty rats were randomly chosen as the sham group which underwent the same open-chest surgery without coronary artery ligation. The myocardial infarction model was created by ligation of the left anterior descending coronary artery approximately 3 mm distal from its origin with the use of a 6–0 polypropylene suture in the remaining 80 rats. After surgery, rats with myocardial infarction were randomly divided into the following four groups (n = 20) for intraperitoneal treatment: Rg1 (10 mg/kg/day) +LY (0.3 mg/kg every 3 days)-treated group [19], Rg1-treated group (10 mg/kg/day) [20], SB-treated group (2 mg/kg every 3 days) [1], and MI model group without any treatment as control group. Five rats died of acute heart failure or arrhythmias, leading to an overall mortality of 10.4%. No deaths involved cardiac rupture in all groups. Rg1, LY, and SB were dissolved in sterile double H2O. The sham group received sterile double H2O. These five groups of rats were further divided into two subgroups each for 2- and 4-week treatment, respectively.
Quantitative real-time RT-PCR
Tissues were homogenized and total RNA was extracted with Trizol reagent (Invitrogen) according to the manufacturer’s instructions. RNA was treated with phenol and underwent chloroform extraction and ethanol precipitation. The quality of RNA was determined by spectrophotometry (DU®800, Beckman, Palo Alto, CA). cDNA was prepared from 500 ng total RNA in a final volume of 20 μl in a thermal cycler (Tgradient 96, Whatman Biometra, Niedersachsen, Germany). Samples were incubated at 37°C for 60 min and 95°C for 5 min. cDNA was stored at −20°C. The rat-specific primers for VEGF, collagen I, and β-actin were designed with the use of Primer Premier 5 (Premier Biosoft, Palo Alto, CA). The rat-specific primers for VEGF were 5′-TGT GCG GGC TGC TGC AAT GAT-3′ (forward) and 5′-TGT GCT GGC TTT GGT GAG ATT TGA-3′ (reverse), primers for collagen I were 5′-TTG GGA TGG AGG GAG TTT AC-3′ (forward) and 5′-TTC ACC TAC AGC ACG CTT GT-3′ (reverse), and primers for β-actin were 5′-TGT TGC CCT AGA CTT CGA GCA-3′ (forward) and 5′-GGA CCC AGG AAG GAA GGC T-3′ (reverse). Primers were synthesized by BioAsia (Shanghai, China). The genes of interest and a housekeeping gene (β-actin) were amplified with the use of LightCycler (Roche Diagnostics, Mannheim, Germany) with SYBR Green I in the SYBR RT-PCR Kit (Perfect Real Time, TaKaRa, Kyoto, Japan). The reaction involved an initial denaturing at 95°C for 10 s, then 45 cycles of 68°C (for VEGF) or 60°C (for collagen I) for 10 s and 72°C for 15 s, terminated by a cooling step, 30 s at 40°C. Data were analyzed by use of LightCycler Software 4.0 with the 2−ΔΔCt method [21]. PCR products (5 μl) were separated by electrophoresis on 2% agarose gels. Gel images were captured by the use of a FAS-III free-system DS-30 (Toyobo Inc., Tokyo, Japan).
Western blot analysis
Equivalent amounts of endothelial cellular protein or myocardial extracts were lysed in RIPA Lysis Buffer (Beyotime, Shanghai, China). Protein concentration was measured by the Micro BCA™ Protein Assay Kit (Pierce, Rockford, IL, USA). Western blot analysis was performed using previously described method [22]. Data analysis involved use of an automated image analysis system (QuantityOne, Bio-Rad).
Histopathological and immunohistochemical staining
Fibrotic areas in the ischemic myocardium were determined with picrosirius red and Masson’s trichrome staining. Three sections of each rat were randomly selected for histomorphometrical analysis to calculate the relative area of fibrosis (the percentage of the total area of interstitial fibrosis to the total connective tissue and cardiomyocytes area). Immunohistochemical staining with antibodies for multiclonal mouse anti-VEGF (dilution, 1:200), goat anti-collagen I (dilution, 1:200), and goat anti-von Willebrand factor (vWF, dilution, 1:800) was used to determine the relative content of VEGF, the relative content of collagen I protein, and capillary density, respectively. Transverse sections of the myocardia (4 μm thick) were obtained and incubated with primary antibodies overnight at 4°C, followed by secondary antibodies (1:200) for 1 h at room temperature and used for staining with diaminobenzidine substrate (Beijing Zhongshan Golden Bridge Biotechnology, Beijing). Three sections from each rat underwent histomorphometrical analysis by use of an Olympus BX51 microscope (Olympus, Japan). The digital images were analyzed to calculate the relative area of fibrosis and capillary density (mean number of capillaries per high-power field) by the use of Image-Pro Plus 5.0 (Media Cybernetics, Bethesda, MD). Myocardial capillaries or area of fibrosis was quantified from ten randomly selected fields.
PI3K activity assay
PI3K activity was determined by use of the PhosphoSensor kit (Antibody-free kinase measurement; PerkinElmer life sciences, Waltham, MA) to quantify the phosphorylation of phosphatidyl inositol diphosphate (PIP2) according to the manufacturer’s instructions. Myocardial extracts and cellular extracts of HUVECs were incubated at room temperature for 1 h with biotinylated PIP2 (500 nM) and adenosine triphosphate (3 μM). Detection of phosphorylated PIP2 involved use of fluorescent streptavidin conjugates and measuring the emission of fluorescence at 520 nm (iEMS analyzer, Labsystems, Finland).
Echocardiographic imaging
At weeks 2 and 4 after treatment, MI rats underwent two-dimensional echocardiography by use of an ultrasound scanner (SONOS 7500, Philips Medical Systems, Andover, MA) and the left ventricular end-diastolic diameter (LVEDd) and left ventricular end-systolic diameter (LVESd) were measured. Left ventricular ejection fraction (LVEF) was determined from the apical two- and four-chamber views by use of a modified Simpson’s algorithm. Left ventricular fractional shortening (FS) was calculated as FS% = (LVEDd − LVESd)/LVEDd × 100%. LV wall thickness was measured at the border of the infarction segment at both end-diastole (DWT) and end-systole (SWT), and LV systolic wall thickening (WT) was calculated as WT% = (SWT − DWT)/DWT × 100% using our previously reported method [9].
Statistical analysis
All values are expressed as means ± SD. Data analysis involved use of SPSS 16.0 (SPSS, Chicago, IL). Before test, the normal distribution test was conducted in the variables. Intergroup differences were analyzed by one-way ANOVA followed by the LSD analysis for multiple comparisons. A P < 0.05 was considered significant.
Results
PI3K/Akt and p38 MAPK signaling pathways is involved in Rg1-enhanced VEGF expression in vitro
As VEGF plays a key role in angiogenesis, we examined whether VEGF is upregulated by Rg1 in HUVECs. The results showed that VEGF protein expression level at 12 and 24 h in hypoxia-injured HUVECs of the control group was slightly higher than that in HUVECs of the normal group but significantly downregulated at 48 h compared with that at 24 h. After stimulation with 150-nM Rg1, VEGF protein expression level was increased at 12 h, peaked at 24 h, and was slightly downregulated at 48 h. VEGF expression level in Rg1 group was significantly higher than that in the control (P < 0.001) and normal group (P < 0.001; Fig. 1a and b).
Assay for PI3K activity, expression of VEGF, p-Akt, and p-p38 MAPK protein in HUVECs. a Representative expression of VEGF and b normalization curves relative to the total β-actin at 12, 24, and 48 h in normal, Rg1 + LY-treated, Rg1-treated, SB-treated, and control groups of HUVECs. c Relative PI3K activity in HUVECs in five groups of HUVECs at 12, 24, and 48 h. d Representative expression of p-Akt and e normalization curves relative to the total Akt in the five groups of HUVECs at 12, 24, and 48 h. f Representative expression of p-p38 MAPK and g normalization curves relative to the total p38 MAPK levels in the five groups of HUVECs at 12, 24, and 48 h. Data were means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group; †††P < 0.001 vs. normal group; ‡P < 0.05, ‡‡P < 0.01 vs. Rg1-treated group
To further study the roles of p38 MAPK and PI3K/Akt in Rg1-induced VEGF expression, we detected the expression of p38 and PI3K/Akt in HUVECs before and after the use of p38 MAPK inhibitor SB203580 (SB) and PI3K inhibitor LY294002 (LY). Treatment with Rg1 (150 nM) at 12, 24, and 48 h significantly increased PI3K activity (Fig.1c) by 53.4%, 57.9%, and 55.1%, respectively, and enhanced phosphorylation of Akt (Fig.1d and e) by 1.43-, 1.29-, and 1.63-fold, respectively, but inhibited phosphorylation of p38 MAPK (Fig. 1f and g) by 46.5%, 33.3%, and 18.6%, respectively, as compared with control group (all P < 0.001). In contrast, treatment with LY downregulated the Rg1-enhanced expression of VEGF by 50.08%, 53.5%, and 49.18% at 12, 24, and 48 h, respectively, as compared with Rg1-treated group (P < 0.01, Fig. 1b), whereas LY administration completely blocked the Rg1-induced activation of PI3K and phosphorylation of Akt (Fig. 1d and e). This suggests that Rg1-induced VEGF expression is mediated only in part via activation of the PI3K/Akt pathway. Meanwhile, compared with Rg1-treated group, Rg1 + LY-treated group showed significantly higher phosphorylation of p38 MAPK (P < 0.05, Fig. 1f and g), which demonstrates that phosphorylation of PI3K/Akt plays a negative role in the phosphorylation of p38 MAPK. Therefore, in addition to directly inhibiting p38 MAPK by Rg1, Rg1-induced activation of PI3K/Akt also contributed to the downregulation of p38 MAPK. As well, inhibition of p38 MAPK activity with SB slightly increased the VEGF expression level in a time-dependent manner as compared with the control group (P < 0.01, Fig. 1b). However, SB exhibited no effect on activation and expression of PI3K and Akt (P > 0.05, Fig. 1c–e).
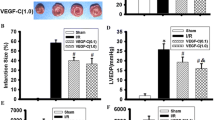
Effect of Rg1 on angiogenesis in vivo
To study the effect of Rg1 on angiogenesis in vivo, rats with myocardial infarction received treatment with Rg1 + LY, Rg1 or SB, or no treatment (control group). At the border of infarct, the density of newly formed capillaries was stained with anti-vWF antibody (Fig. 2a); the density of capillaries was determined. VEGF protein and mRNA expression was evaluated with immunohistochemical staining (Fig. 2b) and quantitative real-time PCR (Fig. 2c), respectively. At week 2, Rg1-treated group showed a significantly higher capillary number than the other groups (Fig. 2d). To assess the stability of these newly established capillaries, immunohistochemical staining was performed again at week 4 which showed that treatment with Rg1 resulted in a higher amount of new capillaries than the control group (Fig. 2e), a result similar to that at week 2. Similarly, we found that Rg1-treated group exhibited greater VEGF mRNA expression level than the control group at weeks 2 (Fig. 2f) and 4 (Fig. 2g). VEGF protein expression level was also higher in the Rg1-treated group than in the control group (Fig. 2b) at week 4.
Newly formed capillaries and VEGF mRNA and protein expression levels in the ischemic myocardium. a Capillaries in the myocardium at the infarct border stained with anti-vWF antibody in the sham, Rg1 + LY-treated, Rg1-treated, SB-treated, and control groups of rats 4 weeks after treatment (magnification ×20). Arrows indicated capillaries. b Protein expression of VEGF in the myocardium of the five groups of rats 4 weeks after treatment (magnification ×40, scale bar = 100 μm. c Representative gel electrophoresis of the PCR products of β-actin and VEGF in the five groups of rats 4 weeks after treatment. Product size—VEGF, 103 bp and β-actin, 155 bp. d, e Capillary density expressed as the mean per high-power visual field in the five groups of rats at weeks 2 and 4, respectively. f, g Quantification of VEGF mRNA expression in the five groups of rats at weeks 2 and 4, respectively. Data were means ± SD. *P < 0.05, **P < 0.01 vs. the control group; ‡P < 0.05, ‡‡P < 0.01 vs. the Rg1-treated group
Rg1 induced angiogenesis via activation of PI3K/Akt and inhibition of p38 MAPK signaling pathways
In vivo studies showed that Rg1 treatment increased the activity of PI3K by 49.4% and 55.8% (Fig. 3a) at weeks 2 and 4, respectively, and phosphorylation of Akt by 57.1% and 83.8% (Fig. 3b and c) at weeks 2 and 4, respectively, and decreased that of p38 MAPK by 55.2% and 46.2% (Fig. 3d and e) at weeks 2 and 4, respectively, as compared with the control group (all P < 0.001). Treatment with Rg1 ± LY completely blocked the Rg1-induced activation of PI3K (Fig. 3 a) and phosphorylation of Akt (Fig. 3b and c) and downregulated the Rg1-enhanced expression of VEGF by 2.46- and 3.16-fold at weeks 2 and 4, respectively (both P < 0.01, Fig. 2f and g), as compared with Rg1-treated group. Therefore, the PI3K inhibitor failed to completely inhibit the Rg1-enhanced VEGF expression and new capillary formation, which suggests that Rg1-stimulated angiogenesis is mediated only in part via activation of the PI3K/Akt pathway. As well, inhibition of p38 MAPK activity with SB slightly increased the VEGF expression level in vivo (Fig. 2c, f, and g), compared with the control group (P < 0.05). The SB group showed a trend, although not significant, for higher capillary index than the control group at weeks 2 and 4 (Fig. 2a, d, and e).
Assay for PI3K activity, p-Akt, and p-p38 MAPK protein expression in vivo. a Relative PI3K activity in the myocardium of the sham, Rg1 + LY-treated, Rg1-treated, SB-treated, and control groups of rats 2 and 4 weeks after treatment. b, c Representative immunoblots in the five groups of rats and quantification of the expression of p-Akt relative to the total Akt level at weeks 2 and 4, respectively. d, e Representative immunoblots in the five groups of rats and quantification of the expression of p-p38 MAPK relative to the total p38 MAPK level at weeks 2 and 4, respectively. Data were means ± SD. ***P < 0.001 vs. the control group; ‡P < 0.05 vs. the Rg1-treated group
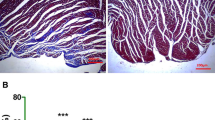
Rg1 attenuated LV myocardial fibrosis
Previous studies demonstrated that Rg1 was able to attenuate myocardial fibrosis [23]. In the present study, the extent of myocardial interstitial fibrosis was analyzed by picrosirius red staining and Masson’s trichrome staining. The results of picrosirius red staining showed narrow myocytes surrounded by a faint collagen network in the sham group but extensive fibrosis (red color) in both interstitial spaces and between bundles of fibers in the control group (Fig. 4a and b). The percentage of the myocardial fibrosis area was reduced by 51.35% and 39.73%, respectively, in the Rg1-treated group (Fig. 4c and d) at weeks 2 and 4 as compared with the control group (both P < 0.001). However, the percentage of the myocardial fibrosis area in the Rg1 + LY-treated group was higher than that in the Rg1-treated group (P < 0.05), albeit still less than in the control group (P < 0.01), suggesting that administration of PI3K inhibitor LY largely reversed the therapeutic effects of Rg1 on myocardial fibrosis. On the other hand, treatment with p38 MAPK inhibitor SB decreased the percentage of the myocardial fibrosis area to similar extent with the Rg1 + LY group, compared with the control group (P < 0.05). These results were further confirmed by Masson’s trichrome staining (Fig. 4e).
Assessment of myocardial fibrosis and collagen I expression in vivo. a, b Representative photomicrographs of picrosirius red staining of the myocardium in the sham, Rg1 + LY-treated, Rg1-treated, SB-treated, and control groups of rats 4 weeks after treatment. Collagens were stained as red (magnification ×40, scale bar = 200 μm). c, d Quantitative assessment of the relative area of fibrosis of the myocardium in the five groups of rats at weeks 2 and 4, respectively. e Representative Masson’s trichrome staining in the five groups of rats at week 4 (magnification ×40, scale bar = 200 μm). f Representative histopathological staining of collagen I protein in the five groups of rats at week 4 (magnification ×40, scale bar = 100 μm). g Representative gel electrophoresis of the PCR products of β-actin and collagen I in the five groups of rats at week 4. Product size—collagen I, 196 bp and β-actin, 155 bp. h, i Quantification of collagen I mRNA expression in the five groups of rats at weeks 2 and 4, respectively. Data were means ± SD. *P < 0.05, ***P < 0.001 vs. the control group; ‡P < 0.05 vs. the Rg1-treated group
As collagen I is the major component of cardiac extracellular matrix proteins [17], we detected the expression levels of collagen I in the ischemic myocardium which were found to be significantly higher in the control group than in all other groups. Treatment with Rg1 significantly reduced collagen I protein and mRNA expression levels (Fig. 4f and g) with a 51.9% and 64.1% decrease of collagen I mRNA expression level (Fig. 4h and i) at weeks 2 and 4, respectively, as compared with the control group (all P < 0.001). These findings provide evidence that Rg1 plays an important role in attenuating LV myocardial fibrosis.
Effects of TNF-α on LV myocardial fibrosis
Since TNF-α has been implicated in the development of cardiac remodeling after MI [24], we detected the expression of TNF-α in the ischemic myocardium and found that the expression of TNF-α was markedly higher I in the control group than in all other groups. In contrast, treatment with Rg1 significantly reduced the expression levels of TNF-α protein by 49.6% and 48.2% at weeks 2 and 4, respectively, as compared with the control group (Fig. 5a and b).
TNF-α and TNF-α receptors expression. a, b Representative immunoblots in the sham, Rg1 + LY-treated, Rg1-treated, SB-treated, and control groups of rats 2 and 4 weeks after treatment and quantification of TNF-α expression relative to the β-actin level at weeks 2 and 4, respectively. c, d Representative immunoblots in the five groups of rats 2 and 4 weeks after treatment quantification of TNFR1 expression relative to the β-actin level at weeks 2 and 4, respectively. e, f Representative immunoblots in the five groups of rats 2 and 4 weeks after treatment and quantification of TNFR2 expression relative to the β-actin level at weeks 2 and 4, respectively. Data were means ± SD. *P < 0.05, ***P < 0.001 vs. the control group; ‡P < 0.05 vs. the Rg1-treated group
It has been shown that TNF-α exerted its effects by binding two different receptors, TNF-α receptor 1 and 2 (TNFR1 and TNFR2) [25]. To further explore the role of these receptors in TNF-α-mediated myocardial fibrosis, we examined the TNFR1 and TNFR2 expression by western blot and found that TNFR1 expression was higher in the control group than in all other groups (Fig. 5c and d). Although TNFR1 expression was significantly lower in the Rg1-treated and Rg1+LY-treated groups than in the control group (both P < 0.05), it showed no significant difference between Rg1-treated and Rg1+LY-treated groups (P > 0.05). Similarly, treatment with p38 MAPK inhibitor SB decreased TNFR1 expression compared with the control group (P < 0.05). These results show that p38 MAPK, but not PI3K/Akt, involves in Rg1-induced TNFR1 downregulation and myocardial fibrosis attenuation. On the contrary, TNFR2 expression level was higher in the Rg1-treated group than in all other groups (Fig. 5e and f). Inhibition of PI3K with LY significantly reduced the Rg1-enhanced TNFR2 expression (P < 0.05). No significant difference in TNFR2 expression was found between the SB and the control group (P > 0.05). These results indicate that Rg1-enhanced TNFR2 expression is mediated through PI3K/Akt pathway but unaffected by p38 MAPK. In summary, Rg1 attenuates TNF-α-mediated myocardial fibrosis by suppressing TNFR1 expression while promoting TNFR2 expression.
Rg1 attenuated LV myocardial fibrosis via inhibiting p38 MAPK pathway and cross talk between PI3K/Akt and p38 MAPK pathways
To further investigate the anti-fibrosis mechanism of Rg1 in the ischemic myocardium, we examined whether PI3K/Akt and p38 MAPK signaling pathways were involved in this process. Our results showed that TNF-α and collagen I expression levels were significantly higher in the Rg1 + LY-treated group than in the Rg1-treated group (both P < 0.05), but still lower than the control group (both P < 0.05, Figs. 4f–i and 5a and b), suggesting that Rg1-mediated reduction of TNF-α expression was partly through PI3K signaling pathway. The SB-treated group showed that TNF-α and collagen I expression levels were lower than the control group (both P < 0.05) but still higher than that in the Rg1-treated group (both P < 0.05). These findings suggest that the effects of Rg1 on TNF-α and collagen I expression are achieved through activation of PI3K/Akt as well as inhibition of p38 MAPK signaling pathways.
In addition to downregulated expression of TNF-α and collagen I, the Rg1-treated group also showed significantly inhibited phosphorylation of p38 MAPK as compared with the control group (P < 0.001, Fig. 3d and e), a result similar to that in the Rg1 + LY-treated group albeit to a less extent (P < 0.05), which suggests that phosphorylation of PI3K/Akt plays a negative role in the phosphorylation of p38 MAPK. Meanwhile, Rg1-induced activation of PI3K/Akt also contributed to downregulation of p38 MAPK. Thus, a cross talk may exist between PI3K/Akt pathway and p38 MAPK pathway which plays an important role in mediating the effects of Rg1 on pro-angiogenesis and anti-fibrosis in ischemic myocardium.
Improvement of global and regional myocardial function
Two and four weeks after treatment, the Rg1-treated group showed a significant decrease in LVESd and LVEDd and an increase in WT, FS, and LVEF, compared with the control group (Table 1). LVESd and LVEDd were lower in the Rg1-treated group than those in the Rg1+LY-treated and SB-treated groups (both P < 0.05). WT and FS were significantly higher in the Rg1+LY-treated and SB-treated groups than in the control group (both P < 0.05), but still lower than in the Rg1-treated group (both P < 0.05). Compared with Rg1-treated group, there was a trend towards decreased LVEF in the Rg1+LY-treated group but no significant difference was found. These results demonstrate that Rg1 could inhibit the progression of LV remodeling (LVESd and LVEDd expansion) and improve LV regional and global function (WT, FS, and LVEF). Moreover, WT and FS were lower in the Rg1+LY-treated group than in the Rg1-treated group (P < 0.05), which indicate that the PI3K inhibitor attenuates the beneficial effects of Rg1 on left ventricle function.
Discussion
The major finding of the present study was that treatment with Rg1 significantly enhanced angiogenesis and attenuated LV remodeling, which leads to functional improvement of the left ventricle in a rat model of myocardial infarction. Rg1 enhanced VEGF-induced angiogenesis and attenuated TNF-α-induced myocardial fibrosis via activating PI3K/Akt, inhibiting p38 MAPK and cross talk between the two signaling pathways.
As one of the most popular Chinese herbal medicine, ginseng has been used for cancer, diabetes, and cardiovascular diseases for thousands of years in China and many other countries [26, 27]. However, the pharmacological mechanisms of ginseng are still poorly understood. Rg1, as an important ginsenoside in the extract of ginseng, has been identified to be an angiogenic factor, which can induce neovascularization in vivo and promote proliferation and tubulogenesis of endothelial cells in vitro [28].
Angiogenesis plays an important role in many physiology and pathological conditions and VEGF has been recognized as the most important angiogenic cytokine [29]. Many other angiogenic cytokines not only directly induce new vessel formation but also upregulate VEGF expression. In the present study, we found a marked increase in VEGF expression in vitro and in vivo and increased amount of new capillaries in Rg1-treated rats. The results confirmed that Rg1-induced angiogenesis was associated with higher VEGF expression.
Previous studies revealed that the PI3K/Akt signaling pathway is required for the angiogenic effects of Rg1 [4, 28]. In this study, we observed a direct stimulatory effect of Rg1 on VEGF-induced angiogenesis, in part, via activation of the PI3K/Akt signaling pathway. However, different mechanisms may be involved in Rg1 upregulated VEGF expression and angiogenesis. Apart from being a potent signaling pathway for angiogenesis, PI3K/Akt is capable of directly downregulating the phosphorylation of p38 MAPK as proved by current and other studies [30]. Our results also demonstrated that activation of PI3K/Akt pathway by Rg1 treatment reduced myocardial fibrosis area and improved LV regional function in the Rg1-treated group compared with the Rg1+LY-treated group. However, LVEF tended to be higher in the Rg1-treated group than in the Rg1+LY-treated group with no significant difference. These results suggest that activation of PI3K/Akt pathway by Rg1 improves wall motion at the border of the infarcted zone with only minor changes in the LV global function.
In the present study, we provided evidence that Rg1 promoted angiogenesis via two mechanisms. First, Rg1-induced inhibition of p38 MAPK leads to slightly increased expression level of VEGF and new capillaries. Second, Rg1-induced inhibition of p38 MAPK and high expression of VEGF jointly enhance angiogenesis, which was confirmed by previous studies in which a combination of a p38 MAPK inhibitor and a growth factor (such as VEGF or FGF1) produced better results for angiogenesis than with a p38 MAPK inhibitor or a growth factor alone [1, 11].
In the setting of myocardial infarction, injury from ischemic and necrotic myocardium disturbs loading conditions within the heart and triggers LV remodeling characterized by fibrosis and thinning of the infarcted zone and LV dilation and dysfunction [1]. During the pathological process of LV remodeling, TNF-α stimulates the production or expression of interleukin-1β (IL-1β), collagenase, and type I and III collagens [13, 31], and p38 MAPK plays a key role in inducing inflammatory cytokines [16]. Thus, inhibition of p38 MAPK has been found to be effective in attenuating TNF-α expression and ameliorating ECM remodeling in the heart [16]. TNF-α acts by binding two different cell surface receptors, TNFR1 and TNFR2 [25]. In the present study, Rg1 attenuated TNF-α-mediated myocardial fibrosis by suppressing TNFR1 while promoting TNFR2 expression. These findings are consistent with a previous report by Monden et al. who demonstrated that TNFR1 activation exacerbated LV remodeling whereas TNFR2 activation exerted cardioprotective effects in a mouse model of myocardial infarction [32]. The major components of cardiac ECM proteins are collagens, of which collagen type I comprises approximately 85% [33]. We found that Rg1 treatment significantly decreased the phosphorylation of p38 MAPK and expression level of TNF-α, collagen I, and fibrosis area as compared with the control group, which implies that the protective effect of Rg1 in attenuating inflammation-induced myocardial fibrosis was mediated in part via inhibition of the p38 MAPK pathway.
Our in vitro and in vivo evidence showed that the cross talk between PI3K/Akt and p38 MAPK signaling pathways in the myocardium plays an important role in regulating not only the processes of angiogenesis but also LV myocardial fibrosis. Inhibition of PI3K activity by LY completely blocked the Rg1-induced phosphorylation of Akt and upregulated phosphorylation of p38 MAPK. The p38 MAPK inhibitor SB inhibited only the p38 MAPK pathway without affecting PI3K and Akt. In addition to directly inhibiting p38 MAPK by Rg1, Rg1-induced activation of PI3K/Akt also contributed to downregulation of p38 MAPK [30]. The downregulation of p38 MAPK, together with activation of PI3K/Akt, contributed to both angiogenesis and attenuation of inflammation-induced LV myocardial fibrosis.
Previous studies suggested that assembly of the fibronectin and collagen network correlated with suppression of angiogenesis [34, 35]. Increased number of capillaries in the peri-infarct area can alleviate inadequate oxygenation and nutrient supply after MI, reduce progressive collagen deposition, and contribute to prevention of ECM remodeling. The degradation of the ECM facilitates new capillaries sprouting from existing vessels and improves angiogenesis.
To the best of our knowledge, our study is the first to report that Rg1 enhances angiogenesis and attenuates LV myocardial fibrosis via cross talk between p38 MAPK and PI3K/Akt signaling pathways (Fig. 6). Thus, Rg1 treatment contributed to long-term improvement of regional and global myocardial contractile function, not only by improving angiogenesis in the heart but also by attenuating LV remodeling. However, our study contains several limitations. First, despite a significant improvement in LV function, Rg1 treatment did not completely halt the progression of LV remodeling. Whether a larger dose of Rg1 or a combination of Rg1 and other medications may achieve better therapeutic effects awaits further investigations. Second, although p38 MAPK and PI3K/Akt signaling pathways were examined in the present studies, the therapeutic effects of Rg1 may involve multiple pathways and further studies are required to elucidate additional pathways and downstream mediators mediating Rg1 treatment.
Schematic diagram of the effects of Rg1 on angiogenesis and fibrosis. Rg1 enhanced VEGF-induced angiogenesis and attenuated TNF-α-induced myocardial fibrosis via activating PI3K/Akt, inhibiting p38 MAPK, and cross talk between the two signaling pathways. Solid lines indicate positive effects whereas dashed lines represent negative effects
In summary, Rg1 treatment provides notable benefits in preservation of cardiac structure and function by enhancing angiogenesis and attenuating LV myocardial fibrosis in a rat model of myocardial infarction. Thus, our findings pave a novel and promising approach to the treatment of ischemic heart disease.
References
Engel FB, Hsieh PC, Lee RT, Keating MT (2006) FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA 103:15546–15551
Swynghedauw B (1999) Molecular mechanisms of myocardial remodeling. Physiol Rev 79:215–262
Urabe A, Izumi T, Abe Y, Taniguchi I, Mochizuki S (2006) Effects of eplerenone and salt intake on left ventricular remodeling after myocardial infarction in rats. Hypertens Res 29:627–634
Leung KW, Pon YL, Wong RN, Wong AS (2006) Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem 281:36280–36288
Chan LS, Yue PY, Mak NK, Wong RN (2009) Role of microRNA-214 in ginsenoside-Rg1-induced angiogenesis. Eur J Pharm Sci 38:370–377
Cheung LW, Leung KW, Wong CK, Wong RN, Wong AS (2011) Ginsenoside-Rg1 induces angiogenesis via non-genomic crosstalk of glucocorticoid receptor and fibroblast growth factor receptor-1. Cardiovasc Res 89: 419-425
Lu MC, Lai TY, Hwang JM, Chen HT, Chang SH, Tsai FJ, Wang HL, Lin CC, Kuo WW, Huang CY (2009) Proliferation- and migration-enhancing effects of ginseng and ginsenoside Rg1 through IGF-I- and FGF-2-signaling pathways on RSC96 Schwann cells. Cell Biochem Funct 27:186–192
Jia L, Zhao Y (2009) Current evaluation of the millennium phytomedicine—ginseng (I): etymology, pharmacognosy, phytochemistry, market and regulations. Curr Med Chem 16:2475–2484
Lu H, Xu X, Zhang M, Cao R, Brakenhielm E, Li C, Lin H, Yao G, Sun H, Qi L, Tang M, Dai H, Zhang Y, Su R, Bi Y, Zhang Y, Cao Y (2007) Combinatorial protein therapy of angiogenic and arteriogenic factors remarkably improves collaterogenesis and cardiac function in pigs. Proc Natl Acad Sci USA 104:12140–12145
Blazquez C, Gonzalez-Feria L, Alvarez L, Haro A, Casanova ML, Guzman M (2004) Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res 64:5617–5623
Mottet D, Michel G, Renard P, Ninane N, Raes M, Michiels C (2003) Role of ERK and calcium in the hypoxia-induced activation of HIF-1. J Cell Physiol 194:30–44
Issbrucker K, Marti HH, Hippenstiel S, Springmann G, Voswinckel R, Gaumann A, Breier G, Drexler HC, Suttorp N, Clauss M (2003) p38 MAP kinase—a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J 17:262–264
Wu CF, Bi XL, Yang JY, Zhan JY, Dong YX, Wang JH, Wang JM, Zhang R, Li X (2007) Differential effects of ginsenosides on NO and TNF-alpha production by LPS-activated N9 microglia. Int Immunopharmacol 7:313–320
Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL (2001) Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 104:826–831
Manning AM, Davis RJ (2003) Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov 2:554–565
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5:749–759
Li M, Georgakopoulos D, Lu G, Hester L, Kass DA, Hasday J, Wang Y (2005) p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation 111:2494–2502
Kanda S, Kanetake H, Miyata Y (2007) Role of Src in angiopoietin 1-induced capillary morphogenesis of endothelial cells: Effect of chronic hypoxia on Src inhibition by PP2. Cell Signal 19:472–480
Ravingerova T, Matejikova J, Neckar J, Andelova E, Kolar F (2007) Differential role of PI3K/Akt pathway in the infarct size limitation and antiarrhythmic protection in the rat heart. Mol Cell Biochem 297:111–120
Yamaguchi Y, Higashi M, Kobayashi H (1997) Effects of ginsenosides on maze performance and brain choline acetyltransferase activity in scopolamine-treated young rats and aged rats. Eur J Pharmacol 329:37–41
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Yin H, Zhang J, Lin H, Wang R, Qiao Y, Wang B, Liu F (2008) p38 mitogen-activated protein kinase inhibition decreases TNFalpha secretion and protects against left ventricular remodeling in rats with myocardial ischemia. Inflammation 31:65–73
Wei HJ, Yang HH, Chen CH, Lin WW, Chen SC, Lai PH, Chang Y, Sung HW (2007) Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial injection in a rat model with infarcted myocardium. J Control Release 120:27–34
Bozkurt B, Kribbs SB, Clubb FJ Jr, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL (1998) Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation 97:1382–1391
Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP (1999) Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol 17:331–367
Chang YS, Seo EK, Gyllenhaal C, Block KI (2003) Panax ginseng: a role in cancer therapy? Integr Cancer Ther 2:13–33
Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS (2002) Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 51:1851–1858
Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RN, Sasisekharan R, Fan TP (2004) Modulating angiogenesis: the yin and the yang in ginseng. Circulation 110:1219–1225
Risau W (1997) Mechanisms of angiogenesis. Nature 386:671–674
Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC (2001) Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem 276:30359–30365
Bondeson J (1997) The mechanisms of action of disease-modifying antirheumatic drugs: a review with emphasis on macrophage signal transduction and the induction of proinflammatory cytokines. Gen Pharmacol 29:127–150
Monden Y, Kubota T, Inoue T, Tsutsumi T, Kawano S, Ide T, Tsutsui H, Sunagawa K (2007) Tumor necrosis factor-alpha is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am J Physiol Heart Circ Physiol 293:H743–H753
Heeneman S, Cleutjens JP, Faber BC, Creemers EE, van Suylen RJ, Lutgens E, Cleutjens KB, Daemen MJ (2003) The dynamic extracellular matrix: intervention strategies during heart failure and atherosclerosis. J Pathol 200:516–525
Ingber DE (2002) Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91:877–887
Rundhaug JE (2005) Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9:267–285
Acknowledgment
This work was supported by the National 973 Basic Research Program of China (no. 2010CB732605). the National High-tech Research and Development Program of China (no. 2006AA02A406), the State Program of National Natural Science Foundation of China for Innovative Research Group (81021001), the State Key Program of National Natural Science of China (no. 60831003), the National Natural Science Foundation of China (nos. 30700301, 30971096, and 30972809), the Major Project of Fujian Medical University (09ZD019), the Foundation for Excellent Young Scientists of Shandong Province (BS2009SW026, 2008BS03017), and the Natural Science Foundation of Shandong Province (ZR2010HQ012, ZR2009CZ003, and Q2006C12).
Disclosures
None declared.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article has been retracted upon request of the authors. The retraction has been made due to duplication of blots in Figure 1a and Figure 3 in this paper. The authors deeply apologize for any inconvenience this may have caused to the readers.
The retraction note to this article can be found online at http://dx.doi.org/10.1007/s00109-013-1018-0.
Huiqiu Yin and Zhaoqiang Liu contributed equally to this work.
About this article
Cite this article
Yin, H., Liu, Z., Li, F. et al. RETRACTED ARTICLE: Ginsenoside-Rg1 enhances angiogenesis and ameliorates ventricular remodeling in a rat model of myocardial infarction. J Mol Med 89, 363–375 (2011). https://doi.org/10.1007/s00109-011-0723-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0723-9