Abstract
VEGF-C is a newly identified proangiogenic protein playing an important role in vascular disease and angiogenesis. However, its role in myocardial ischemia/reperfusion (I/R) injury remains unknown. The objective of this study was to determine the role and mechanism of VEGF-C in myocardial ischemia–reperfusion injury. Rat left ventricle myocardium was injected with recombinant human VEGF-C protein (0.1 or 1.0 µg/kg b.w.) 1 h prior to myocardial ischemia–reperfusion (I/R) injury. 24 h later, the myocardial infarction size, the number of TUNEL-positive cardiomyocytes, the levels of creatine kinase (CK), CK-MB, cardiac troponin, malondialdehyde (MDA) content, and apoptosis protein Bax expression were decreased, while Bcl2 and pAkt expression were increased in VEGF-C-treated myocardium as compared to the saline-treated I/R hearts. VEGF-C also improved the function of I/R-injured hearts. In the H2O2-induced H9c2 cardiomyocytes, which mimicked the I/R injury in vivo, VEGF-C pre-treatment decreased the LDH release and MDA content, blocked H2O2-induced apoptosis by inhibiting the pro-apoptotic protein Bax expression and its translocation to the mitochondrial membrane, and consequently attenuated H2O2-induced decrease of mitochondrial membrane potential and increase of cytochrome c release from mitochondria. Mechanistically, VEGF-C activated Akt signaling pathway via VEGF receptor 2, leading to a blockade of Bax expression and mitochondrial membrane translocation and thus protected cardiomyocyte from H2O2-induced activation of intrinsic apoptotic pathway. VEGF-C exerts its cardiac protection following I/R injury via its anti-apoptotic effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial ischemia or infarction is one of the main causes of heart failure. Myocardial infarction (MI) is usually initiated by myocardial ischemia resulting from the narrowing of coronary arteries due to atherosclerosis. Clinically, a number of cardiovascular intervention techniques such as thrombolytic therapy, percutaneous coronary intervention, and cardiac bypass surgery can restore myocardial perfusion. However, if the coronary flow is not restored within 1~2 h after the MI, reperfusion itself may cause harmful effects in the ischemic heart, a phenomenon known as reperfusion injury (I/R) [1, 2]. One of the factors causing damage is the over-production of reactive oxygen species (ROS). The ROS release subsequently causes extensive cardiomyocyte death and acute heart dysfunction [3]. ROS-caused apoptotic damages include membrane lipid peroxidation, protein cross-linking and degradation, DNA nicking, and mitochondria damage [4]. Blockade of oxidative stress-induced injury is therefore beneficial for the protection of cardiomyocytes.
Vascular endothelial growth factor (VEGF) family comprises five isoforms A, B, C, D, and E, and is known to promote vasculogenesis and angiogenesis [5]. In addition to angiogenesis, VEGF-A and its receptor play important roles in repairing ischemic or infracted heart through inducing cardiac stem cell differentiation and migration [6]. VEGF-C,an important member of VEGF family [7], has been characterized as a lymphangiogenic and angiogenic growth factors. VEGF-C functions through three VEGF-C receptor subtypes, VEGFR-2 (KDR/Flk-1), VEGFR-3 (Flt-4), and NRP-2 [8–10]. VEGF-C is significantly up-regulated in both early and late stages of MI [11]. However, it is unknown if VEGF-C plays a role in ROS-induced cardiomyocyte injury.
Two distinct apoptotic pathways are reported to direct cell apoptosis: a death receptor-mediated pathway and an intrinsic pathway in which mitochondria play a central role in assimilating and affecting various apoptotic signals [8]. H2O2 is reported to induce cardiomyocyte apoptosis through mitochondria-dependent pathway [4]. In the present study, we found that VEGF-C promoted rat cardiomyocyte survival through VEGFR-2 under oxidative stress conditions. The underlying molecular mechanism of VEGF-C involves the activation of PI3k/Akt signaling and blockade of mitochondria apoptotic pathway.
Materials and methods
All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals recommended by the National Institutes of Health of USA. All protocols of animal studies were approved by the Institutional Animal Care and Use Committee of Hubei University of Medicine.
Heart ischemia–reperfusion injury model
Myocardial ischemia–reperfusion injury (I/R) in rats was performed as previously described [12]. Briefly, Sprague–Dawley rats (240 ± 35 g, male) were randomly divided into four groups as follows: sham group, I/R group, VEGF-C (0.1 µg/kg b.w.) group, and VEGF-C (1.0 µg/kg b.w.) group. These rats were anesthetized with ketamine (50 mg/kg, b.w., ip) and xylazine (10 mg/kg b.w., ip) and ventilated during surgery using a Columbus ventilator (HX-300, Taimeng Instruments, China). 1 h after pre-treatment with VEGF-C (local injection of myocardium), the hearts were ligated on the left anterior descending coronary artery (LAD) for 1 h before reperfusion for 24 h. Buprenorphine hydrochloride (0.05 mg/kg, sc) was administered one time after the procedure.
Measurement of hemodynamics
Hemodynamic measurement was carried out using protocol published in our previous study [12]. Briefly, 24 h after reperfusion, left carotid artery was exposed. The heparinized (10 U/ml) catheter was inserted into the left ventricle to monitor hemodynamic parameters including left ventricle systolic pressure (LVSP), left ventricle end-diastolic pressure (LVEDP), and rates of the rise or fall of left ventricular pressure (±dp/dt max) through signal conversion of pressure transducer (Chengdu taimeng Technology Co., Ltd, China) linked into BL-420S (Chengdu taimeng Technology Co., Ltd, China) for 15 min.
Measurement of the infarcted sizes
The infarcted sizes were analyzed as previously described [6]. Briefly, each heart was cut into 5 sections with a thickness of 2–5 mm each section. 1 % 2, 3, 5-triphenyltetrazolium chloride (TTC, Sigma, USA) was used to stain the heart sections at 37 °C for 15 min. The infracted size was calculated and expressed as a percentage of the area of infarcted LV myocardium unstained by TTC in the total area of LV myocardium stained and unstained by TTC.
Biochemical detection
Measurement of creatine kinase (CK), CK-MB activity, cardiac troponin T (cTnT), malondialdehyde (MDA), and lactate dehydrogenase (LDH) levels were performed as described in detail previously [12, 13]. Briefly, 24 h after treatment, blood samples were centrifuged at 3500 rpm for 15 min at 4 °C, then serum was collected. CK activities (JianCheng Bioengineering Institute, China), CK-MB activity (Rapidbio, USA), and cTnT (Rapidbio, USA) levels were analyzed using corresponding commercial kits by following the manufacture’s instruction. MDA (an oxidative damage marker of myocardium) and LDH (an indicator of cellular injury) were detected by using commercial kits (JianCheng Bioengineering Institute, China).
ROS detection
The levels of \({\text{O}}_{2}^{ - }\) in cells were detected with oxidation-sensitive fluorescent probe dihydroethidium (DHE) (Invitrogen, USA) and measured with fluorescent microscopy as described previously [13].
Culture of H9c2 rat cardiomyocytes
The rat embryonic ventricular myocardial cell line H9c2 was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). H9c2 cells were cultured in DMEM (Invitrogen, USA) with 5 g/l glucose supplemented with 15 % fetal bovine serum (FBS, Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., China) in 10 cm cell culture dish at 37 °C with an atmosphere of 5 % CO2. When the 70–80 % confluence of cells was reached, the cells were passaged or used for experiments. For inducing apoptosis, the cells were exposed to H2O2 (400 µM, Sigma-Aldrich, St Louis, MO, USA) for different times following VEGF-C treatment (20 ng/ml, Peprotech, USA).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA of rat H9c2 cells was extracted by using Trizol Reagent (Invitrogen). RNA concentration was measured by UV spectrophotometry. qRT-PCR was performed using THUNDERBIRD SYBR qPCR Mix (TOYOBO, Japan) on a real-time PCR detection system (Slan, Hongshi) with the following cycles: 95 °C for 1 min, followed by 95 °C for 15 s, 58 °C for 20 s, and 72 °C for 20 s for 40 cycles. β-actin was used as an internal control. Primer sequences were as follows: flk-1: 5′-ACC TAT CAA CTT ACT TAC GGG GC-3′ (forward), 5′-TCT GAC TGC TGG TGA TGC TGT-3′ (reverse); flt-4: 5′-TGA AAG ACG GCA CAC GAA TG-3′ (forward), 5′-CCT CGC TTT AGG GTC TCC AG-3′(reverse); nrp-2: 5′-GGC AGG TTT CTC CCT ACG CT-3′ (forward), 5′-TTT ACA GTC TCC TTC CCC CAC-3′ (reverse); and β-actin: 5′-CGT TGA CAT CCG TAA AGA CCT C-3′ (forward), 5′-TAG GAG CCA GGG CAG TAA TCT-3′ (reverse).
Immunofluorescent staining of VEGF-C receptors
To detect VEGF-C receptors in H9c2 cells, cells were cultured to 50–60 % confluence in the 8-well chamber slide (Lab-Tek® Chamber Slide™ System, NUNC, USA). Cells were fixed with 4 % paraformaldehyde for 1 min, washed three times with PBS followed by permeabilization with 0.2 % Triton X-100 for 5 min, and blocked using 10 % goat serum dissolved in PBS for 30 min at room temperature. Cells were then incubated with polyclonal anti-Flk-1, anti-Flt-4, and anti-neuropilin-2 IgG (diluted 1:50 in blocking buffer, Santa Cruz, USA) at 4 °C overnight followed by incubation with FITC-conjugated secondary antibodies (1:100, Santa Cruz) at room temperature for 1 h. After washing twice, cells were stained with Hoechst 33258 (2.5 μg/ml, sigma) in a dark chamber at room temperature for 20 min. The immunostaining of VEGF receptors was observed using a fluorescence microscope.
Cell viability and apoptosis assay
Cell viability was determined using Cell Counting Kit-8 as described previously [14]. H9c2 cell apoptosis was detected using Annexin V and Propidium Iodide (PI) binding Assay (Bender MedSystems, Austria) as described previously [12]. The apoptotic H9c2 cells were observed using a fluorescence microscope. In vivo cardiomyocyte apoptosis was detected using an In Situ Apoptosis Detection Kit (MM_NF-S7165#, Millipore, UEA) as described previously [4].
Measurement of mitochondrial membrane potential
Mitochondrial transmembrane potential was analyzed using a sensitive fluorescent probe, JC-1 (Invitrogen, USA) as described previously [15]. In brief, H9c2 cells were cultured in the 8-well chamber slide (Lab-Tek® Chamber Slide™ System, NUNC, USA) followed by appropriate treatments. The cells were then incubated with 5 µm of JC-1 in fresh culture medium at 37 °C for 15 min. JC-1 fluorescence was immediately detected and imaged under a fluorescent microscope.
Detection of intracellular cytochrome C localization
Cytochrome C release from the mitochondria was analyzed using MitoTracker Red CMXRos dye (Molecular Probes, USA) as described previously [16]. In brief, H9c2 cells were cultured in the 8-well chamber slide (Lab-Tek® Chamber Slide™ System, NUNC, USA) followed by appropriate treatments. The cells were incubated with 20 nM MitoTracker Red CMXRos dye at 37 °C for 15 min, fixed with 4 % paraformaldehyde for 15 min, washed three times with PBS followed by permeabilizing with 0.2 % Triton X-100 for 5 min, and blocked using 10 % goat serum dissolved in PBS at room temperature for 30 min. Cells were then incubated with monoclonal anti-cytochrome C IgG (diluted 1:50 in blocking buffer, Santa Cruz) at 4 °C overnight followed by incubation with FITC-conjugated goat anti-mouse IgG (1:100, Santa Cruz) at room temperature for 1 h. After washing twice, cells were observed under a fluorescence microscope.
Detection of Bax N-terminal exposure
Bax N-terminal epitope in mitochondrial membrane was analyzed using anti-Bax N-terminal (N20) IgG as described previously [16]. The cells were cultured in the 8-well chamber slide (Lab-Tek® Chamber Slide™ System, NUNC, USA) similarly as described in the detection of cytochrome C release. Cells were then incubated with anti-Bax N-terminal antibody (1:50, Santa Cruz) at 4 °C overnight followed by incubation with FITC-conjugated anti-rabbit IgG (1:50, Santa Cruz) at room temperature for 1 h. Bax conformational changes were observed under a fluorescence microscope.
Western blot
Western blot analysis was carried out as described previously [6]. The following primary antibodies were used: rabbit anti-Akt (#9272s, Cell Signaling Technology, 1:1000), rabbit anti-phospho-Akt (#9271s, Cell Signaling Technology, 1:1000), rabbit anti-Bax (#2772s, Cell Signaling Technology, 1:1000), rabbit anti-Bcl2 (#2876s, Cell Signaling Technology, 1:1000), or mouse anti-α-Tubulin antibody (Sigma, 1:5000). The corresponding horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies (1:10,000 dilution; Santa Cruz) were used. The immunoblots were detected by enhanced chemiluminescence reaction (Sigma).
Statistical analysis
All results are expressed as mean ± SD. Differences of parameters between two groups were evaluated by unpaired t test. Differences of parameters among more than two experimental groups were evaluated by one-way analysis of variance. P values <0.05 were considered statistically significant.
Results
VEGF-C reduced infarct size and improved heart function
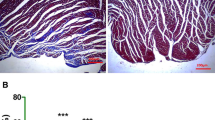
To observe the effect of VEGF-C on myocardial infarct size in I/R model in vivo. 1 % TTC staining was used to evaluate infarct size of rat hearts undergoing I/R injury with or without VEGF-C treatment. We found that 0.1 and 1.0 μg/kg b.w. of VEGF-C treatment obviously decreased infarct size compared to the I/R-injured heart with vehicle treatment. 1.0 μg/kg b.w. of VEGF-C achieved a greater decrease of infarct size than 0.1 μg/kg b.w. of VEGF-C (Fig. 1a, b). Consistent with the reduction of the infarct size, left ventricle function in VEGF-C-treated animals was significantly improved. It was apparent that the higher dose of VEGF-C produced a much greater effect on the increase of LVSP and ±dp/dt max along with the decrease of LVEDP than the lower dose of VEGF-C (Fig. 1c–f).
VEGF-C improved heart function impaired due to I/R injury. a The TTC staining showing the infarcted myocardium 24 h after reperfusion. b Quantitative analysis of infarction size. *P < 0.01 versus sham group; # P < 0.01 versus I/R group, n = 6. (c–f) Quantitative analysis of heart function including LVSP (c), LVEDP (d), ±dp/dt max (e, f). *P < 0.01 versus sham group; # P < 0.05 versus I/R; & P < 0.05 versus hearts with the injection of 0.1 μ kg b.w. of VEGF-C, n = 6
VEGF-C suppressed the activities of CK and CK-MB, reduced the levels of cTnT and MDA, and inhibited I/R-mediated cardiocytes apoptosis through PI3K/Akt signaling
To explore the role of VEGF-C in myocardium ischemia reperfusion (I/R) injury, CK and CK-MB activities as well as cTnT and MDA levels were detected to evaluate the myocardial injury. As shown in Fig. 2a–d, although I/R injury increased the CK and CK-MB activities and the cTnT and MDA levels, VEGF-C treatment significantly reversed the effect of I/R injury.
To determine how VEGF-C protects heart from I/R injury, cells apoptosis was first detected 24 h after VEGF-C treatment. As shown in Fig. 3a, b, VEGF-C significantly decreased the apoptosis rate of cardiomyocytes in I/R-injured hearts. Bcl-2, a marker for the survival of cardiocytes, and Bax, a marker of cardiocytes apoptosis [17, 18], were then used to evaluate the effect of VEGF-C on myocardium protection. Bcl-2 was markedly induced, and Bax was significantly inhibited in VEGF-C-treated hearts compared to the I/R-injured hearts with saline injection (P < 0.05). Importantly, Akt signaling as shown by Akt phosphorylation was obviously increased in VEGF-C-treated hearts (Fig. 3c–e). The higher dose of VEGF-C appeared to induce a much greater activation of the Akt signaling. These data suggest that VEGF-C may inhibit I/R-caused cardiomyocyte apoptosis through PI3K/Akt signaling.
VEGF-C inhibited I/R-mediated cardiomyocyte apoptosis through PI3K/Akt signaling. a Cardiomyocyte apoptosis in heart I/R model was detected by TUNNEL staining. Red fluorescence apoptotic cells; Blue fluorescence nuclei. b Quantitative analysis of cell apoptosis rate in (a). *P < 0.01 versus sham group; # P < 0.05 versus I/R group; & P < 0.05 versus hearts with the injection of 0.1 μ kg b.w. of VEGF-C, n = 6. c Bcl-2, Bax and pAkt levels were detected by Western blot. d–f Quantification of Bcl-2, Bax, and pAkt expression by normalizing to α-Tubulin. *P < 0.01 versus sham group; # P < 0.05 versus I/R group; & P < 0.05 versus hearts with the injection of 0.1 μ kg b.w. of VEGF-C, n = 6
VEGF-C protected H9c2 cells from H2O2 damage
Since ROS production is a major factor in I/R-induced myocardial infarction, we evaluated the protective effect of VEGF-C on H2O2-treated H9c2 cells. The H9c2 cell viability was gradually decreased when H2O2 concentration was increased (Fig. 4a). 50 or 100 ng/ml of VEGF-C significantly decreased the cell viability as shown by the CCK-8 assay (Fig. 4b). H2O2 has been shown to alter H9c2 cell morphology from the well organized and rod-like cell morphology to irregular morphology [19]. However, VEGF-C treatment restored the morphology of H9c2 cells (Fig. 4c). In addition,
VEGF-C restored H2O2-induced alteration of H9C2 cell morphology while inhibiting H2O2-induced LDH release and MDA production. a Cell viability with H2O2 treatments was measured by CCK-8 assay. *P < 0.01 versus 0 μM; # P < 0.01 versus 200 μM; & P < 0.01 versus 400 μM; @ P < 0.01 versus 800 μM H2O2-treated groups, n = 3. b Cell viability with VEGF-C treatment was measured by CCK-8 assay. *P < 0.01 versus 0–20 ng/ml; # P < 0.05 versus 50 ng/ml VEGF-C-treated group. c Alterations of cell morphology were observed under light microscope 24 h after VEGF-C treatment (5–20 ng/ml) prior to treatment with 400 μM H2O2. d The condensed nuclei in apoptotic cells were stained with Hoechst 33258 and visualized under a fluorescence microscopy. e Cell apoptosis was detected by Annexin V/PI staining. f Quantitative analysis of cell apoptosis in (e). g VEGF-C reduced LDH levels. h VEGF-C decreased MDA levels. For f–g: *P < 0.01 versus control (CTRL) for 2 % FBS-treated group; # P < 0.05 versus H2O2 group; & P < 0.05 versus 5 ng/ml of VEGF-C treatment group; @ P < 0.05 versus 10 ng/ml VEGF-C treatment group. n = 6
VEGF-C prevented H9c2 cells from producing the condensed nuclei induced by H2O2 (Fig. 4d). Meanwhile, VEGF-C attenuated the H2O2-induced phosphatidylserine (PS) production as shown by the Annexin V staining (Fig. 4e, f). The appearance of PS in the outer leaflet of the phospholipid bilayer without disrupted integrity of the membrane is one of the earliest characteristics of apoptotic process [15]. These data suggest that VEGF-C inhibited H2O2-induced apoptosis.
LDH level is considered as a typical marker for cellular damage, and MDA is an indicator of cell oxidative injury. Compared to normal cells, LDH releases and MDA contents were obviously increased by oxidative injury (Fig. 4g, h). VEGF-C treatment, however, dose dependently decreased H2O2-induced LDH release and MDA contents (Fig. 4g, h).
To further test the role of VEGF-C in protecting H2O2-caused damage to cardiomyocytes, we treated H9c2 cells with VEGF-C for 6, 12, and 24 h and found that VEGF-C restored H9c2 cell morphology (Fig. 5a), decreased ROS production (Fig. 5b), repaired the condensed nuclei (Fig. 5c), reduced the PS production (Fig. 5d), decreased the LDH release and MDA contents (Fig. 5e, f), and increased SOD expression (Fig. 5g) that was altered by H2O2 in time-dependent manners. These data further demonstrated that VEGF-C is able to protect cardiomyocytes from ROS-induced injury.
Time-course protective effect of VEGF-C on H2O2-induced H9c2 cell apoptosis. a Effect of VEGF-C on H9c2 cell morphology. b \({\text{O}}_{2}^{ - }\) levels were detected by fluorescent microscopy. Red color was indicated as the changes of \({\text{O}}_{2}^{ - }\) levels in cells detected with oxidation-sensitive fluorescent probe dihydroethidium (DHE), Blue color was indicated as cell nucleus by DAPI staining. c The condensed nuclei in apoptotic cells were visualized by Hoechst 33258. d Cell apoptosis was detected by Annexin V/PI staining. e VEGF-C reduced LDH levels. f VEGF-C reduced MDA levels. g VEGF-C restored SOD levels. *P < 0.01 versus 2 % FBS (CTRL) group; # P < 0.05 versus H2O2 treatment group; & P < 0.05 versus pre-treatment with VEGF-C for 6 h; @ P > 0.05 versus pre-treatment with VEGF-C for 12 h; n = 6 for each assay
VEGF-C restored H2O2-blocked mitochondrial membrane potential
The mitochondrial membrane potential plays a crucial role in sustaining the entirety of mitochondria and subsequent regulation of apoptosis in response to injury stimuli. Loss of mitochondrial membrane potential activates the cytochrome c-initiated pathway to induce cell apoptosis [16]. JC-1, a specific indicator for mitochondrial membrane potential, can be used to assess mitochondria function by its different aggregations due to the switch between red and green color. Normal growing H9c2 cells exhibited a bright red fluorescence with the JC-1 incubation (Fig. 6a). H2O2 treatment reduced the red aggregates and generated significantly more JC-1 monomers as shown by the green fluorescence, indicating dissipation of the mitochondrial membrane potential. VEGF-C treatment, however, attenuated the H2O2-induced effects (Fig. 6a), suggesting that VEGF-C inhibits H2O2-induced apoptosis by restoring mitochondrial membrane potential.
VEGF-C restored H2O2-induced reduction of mitochondrial membrane potential via VEGFR2. a The lipophilic cationic probe JC-1 was used to analyze the alterations of mitochondrial transmembrane potential (MTP). JC-1 aggregates in mitochondria show red color, JC-1 monomers display green signal, indicator of the loss of MTP. b Quantitative analysis of MTP in (a). *P < 0.01 versus 2 % FBS (CTRL) group; # P < 0.05 versus H2O2 treatment group; &, $ P < 0.05 versus VEGF-C pre-treatment group; @ P > 0.05 versus VEGF-C pre-treatment group; n = 6. VEGFR2-I: VEGFR2 inhibitor; VEGFR3-I: VEGFR3 inhibitor
VEGFR-2 mediated VEGF-C-induced mitochondria-protective effects
VEGF-C functions by activating its cognate tyrosine kinase receptors VEGFR2 (Flk-1), VEGFR3 (Flt-4), and/or the nontyrosine kinase receptor neuropilin-2 (NRP-2). We found that H9c2 cells mainly express Flk-1 with a low level expression of Flt-4 (Supplemental Figure S1). To determine whether Flk-1 or Flt4 is important for VEGF-C function, we blocked these two receptors in VEGF-C- and H2O2-treated H9c2 cells. As shown in Fig. 6a, b. Flk1 inhibitor, but not Flt4 inhibitor, blocked VEGF-C function and thus retained H2O2-induced reduction of red/green JC-1 aggregate ratio. These data indicated that VEGF-C functions through VEGFR-2 to exert the mitochondria-protective effects.
VEGF-C regulated Bcl2/Bax expression via PI3/Akt signaling
Apoptosis is mediated by numerous signaling pathways such as PI3K/Akt and p38 MAPK [20, 21]. To determine if VEGF-C affects PI3K/Akt signaling, we examined the activation of PI3K/Akt signaling. VEGF-C increased Akt phosphorylation that was reduced by H2O2 but did not affect its expression, which was blocked by Flk1 inhibitor, but not Flt4 inhibitor, suggesting that VEGF-C induced the increase of pAkt levels through VEGFR2 (Fig. 7a, b). Subsequently, PI3K-specific inhibitor wortmannin or LY294002 was used to analyze VEGF-C-mediated activation of PI3K/Akt signaling, we found that both wortmannin and LY294002 could abolish the specific effects of VEGF-C on Akt phosphorylation (Fig. 7c, d). Since cell survival regulators Bcl2 and Bax are regulated by PI3K/Akt signaling, we tested if VEGF-C regulates Bcl2 and Bax expression. As shown in Fig. 7e, f, VEGF-C reversed the effect of H2O2 and thus markedly increased Bcl-2, while decreased Bax expression suggesting that VEGF-C protected H9c2 cells from H2O2-induced apoptosis via augmenting Bcl-2 while suppressing Bax expression. Wortmannin or LY294002 blocked VEGF-C-mediated Bcl2 and Bax expression, suggesting that VEGF-C regulates Bcl2/Bax expression through PI3/Akt signaling pathway.
VEGF-C regulated Bcl2/Bax expression via PI3/Akt signaling. a VEGF-C restored H2O2-blocked Akt phosphorylation via PI3K signaling. Akt phosphorylation and expression were analyzed by Western blot. α-tubulin served as an internal control. b Quantitative analysis of Akt phosphorylation by normalized to the total Akt level. c Bcl2 and Bax protein expression was detected by Western blot. d Quantification of Bcl2 and Bax expression by normalized to α-Tubulin. *P < 0.01 versus 2 % FBS (CTRL) group; # P < 0.05 versus H2O2 treatment group; &, @ P < 0.05 versus VEGF-C pre-treatment group; n = 6
VEGF-C inhibited H2O2-induced reduction of mitochondrial membrane potential through Bax translocation
The pro-apoptotic protein Bax causes the loss of mitochondrial membrane potential and damages mitochondrial function by its translocation from cytoplasm to mitochondrial membrane [22]. To determine if VEGF-C protects H2O2-induced damage via Bax mitochondrial translocation, we observed the exposure of Bax N-terminal domain on mitochondrial membrane by immunostaining. In normal cells, no Bax N-terminal was present on mitochondria. H2O2 treatment induced a significant exposure of Bax N-terminal domain as shown by green fluorescent signal (Fig. 8a). However, this effect was clearly inhibited by VEGF-C treatment (Fig. 8a). Interestingly, the effect of VEGF-C on Bax translocation was blocked by PI3 kinase-specific inhibitor Wortmannin or LY294002, suggesting that VEGF-C recovered mitochondrial function through blocking Bax translocation, which was mediated by PI3K signaling.
VEGF-C inhibited H2O2-induced reduction of MTP through Bax translocation. a VEGF-C alleviated Bax translocation from cytoplasm to mitochondrial membrane induced by H2O2. b MTP was assessed by JC-1 aggregates. c Quantitative analysis of membrane potential in (b). *P < 0.01 versus control (CTRL) group; # P < 0.05 versus H2O2 treatment group; & P < 0.05 versus VEGF-C pre-treatment group; @ P < 0.05 versus VEGF-C pre-treatment group; n = 6
To further explore the involvement of mitochondria in VEGF-C function, we also tested if the effect of VEGF-C on mitochondrial membrane potential involves PI3K signaling by immunostaining the JC-1. As shown in Fig. 8b, c, VEGF-C evidently increased the JC-1 aggregates shown in red and decreased JC-1 monomers in the cytoplasm, and these special effects were abolished by LY294002 or Wortmannin, suggesting that PI3K signaling plays a critical role in VEGF-C-mediated restoration of mitochondrial function such as mitochondrial membrane potential in H9c2 cells that were impaired by ROS.
Together, these data demonstrated that VEGF-C protects cardiomyocytes from ROS-induced injury through inhibiting cardiomyocyte apoptosis, which is achieved by blocking Bax mitochondrial translocation via PI3 kinase signaling pathway.
VEGF-C inhibited H2O2-induced cytochrome c release via PI3K/Akt Signaling
Cytochrome c Release from mitochondria is another indicator of intracellular apoptotic signaling activation and subsequent cell death in response to injury stimuli [23]. H2O2 treatment induced cytochrome c release from mitochondria, as shown by the loss of co-localization between cytochrome c and mitochondria (Fig. 9a). The effect of H2O2 was alleviated by pre-treatment with VEGF-C (Fig. 9a). However, when PI3 kinase signaling was blocked by LY294002 or Wortmannin, VEGF-C-inhibited apoptosis shown by Annexin V staining was diminished, confirming that VEGF-C inhibited H2O2-induced cytochrome c release through activating PI3k signaling (Fig. 9b, c).
VEGF-C restored H2O2-induced cytochrome c release via PI3k signaling. a VEGF-C reduced cytochrome c release from mitochondria in response to H2O2. Red signal indicates mitochondria by mitotracker staining, green signal shows cytochrome c staining, and yellow in merged images indicates localization of cytochrome c in mitochondria. b Cell apoptosis was measured by Annexin V/PI staining. c Quantitative analysis of apoptosis cell in b. *P < 0.01 versus control (CTRL) group; # P < 0.05 versus H2O2 treatment group; & P < 0.05 versus VEGF-C pre-treatment group; @ P < 0.05 versus VEGF-C pre-treatment group; n = 6
Discussion
Myocardial I/R injury causes complications such as decreased cardiac contractile function and arrhythmias [24]. Oxidative stress is one of the major factors causing I/R injury, and many of the therapies targeting the reduction of this stress in vivo are proven to be of clinically benefit [25, 26]. VEGF-C is increased in the infarcted heart in both early and late stages of MI [11]. In the present study, we demonstrate for the first time that VEGF-C protects cardiomyocytes from oxidative stress-induced apoptosis in vitro and I/R-induced apoptosis in vivo, suggesting that the VEGF-C induced during the MI is for the protection of the infracted heart. VEGF-C appears to prevent the activation of mitochondria apoptotic pathway through activating PI3k/Akt signaling pathway.
VEGF regulates the function of cardiovascular system through binding in an overlapping pattern to three receptor tyrosine kinases (RTKs), known as VEGF receptor-1, -2, and -3 (VEGFR1-3), as well as co-receptors NRP2 [27, 28]. VEGF-C appears to exert its protective effect by binding to VEGFR2 because only VEGFR2 is expressed at a high level in H9c2 cell, while other receptors or NRP-2 is barely detectable. Moreover, blocking VEGFR2 by its inhibitor significantly inhibits VEGF-C protective effect, demonstrating that VEGFR2 mediates VEGF-C function in cardiomyocytes.
Previous studies have suggested that mitochondrion plays an important role in the determination of cells fates such as cells survival or apoptosis in response to stress [26]. Herein, VEGF-C maintains cardiomyocyte survival by reversing ROS-induced nuclear condensation, disruption of membrane integrity, loss of mitochondrial membrane potential, and release of cytochrome c. Mechanistically, VEGF-C prevents the activation of the intrinsic pathway of apoptosis by preventing H2O2-induced mitochondria localization and activity of the pro-apoptotic Bax protein, leading to mitochondrial membrane potential restoration and inhibition of subsequent cytochrome c release. These results implied that VEGF-C blocks apoptosis in cardiomyocyte through inhibiting the mitochondria apoptotic pathway.
Previous studies have also shown that VEGF has a pro-survival function of cells [29]. Herein, VEGF-C increases Akt activation in cardiomyocytes both in vitro and in vivo, and these special effects are abolished by PI3K inhibitors, suggesting that the protective effect of VEGF-C on cardiomyocytes is dependent of PI3/Akt signaling. Interestingly, PI3K inhibitors wortmannin and LY294002 have been shown to cause endothelial cell apoptosis by significantly increasing caspase-3 activity in the presence of exogenous VEGF-A [28, 30]. Furthermore, a critical pathway of caspase activation is the cytochrome c-initiated pathway [31]. In this study, the effects of VEGF-C on Bcl2/Bax expression, mitochondria membrane potential, and cytochrome c release in cardiomyocytes are all abolished by wortmannin or LY294002, demonstrating that VEGF-C prevents the activation of the intrinsic apoptotic pathway through PI3K/Akt signaling pathway.
Importantly, consistent with the effect of VEGF-C in H2O2-induced apoptosis of cardiomyocytes in vitro, VEGF-C induces an increase in Bcl-2 level and a decrease in Bax level, suggesting that heart following I/R in vivo may be benefited more by VEGF-C treatment than the factors only affecting a single apoptosis-related protein.
Interestingly, 50 ng and 100 ng/ml of VEGF-C reduces H9c2 cells viability, suggesting that high dose of VEGF-C can cause damage to cardiomyocytes. Indeed, VEGF-C has been found to promote hypertrophy of cardiomyocyte [32], and hypertrophying cardiomyocytes exhibit lower cell viability [33]. Therefore, overdosing VEGF-C may decrease cell viability by inducing cells hypertrophy.
Although VEGF-C promotes cardiomyocyte hypertrophy and survival [32, 34], but the molecular mechanisms remain to be studied. Our studies show that VEGF-C markedly decreases myocardiocyte apoptosis and the infarction size in I/R hearts, significantly improved LVSP and ±dp/dt max, and reduced LVDEP. Collectively, our results demonstrate that VEGF-C exerts a protective effect against I/R-induced and ROS-mediated apoptosis of cardiomyocytes by preventing activation of the mitochondrial-dependent intrinsic apoptotic pathway, via the activation of PI3K signaling.
References
Ibáñez B, Heusch G, Ovize M, Van de Werf F (2015) Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 65:1454–1471
Reiter R, Swingen C, Moore L, Henry TD, Traverse JH (2012) Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ Res 110:105–110
Hausenloy DJ, Yellon DM (2013) Myocardial ischemia–reperfusion injury: a neglected therapeutic target. J Clin Investig 123:92–100
von Harsdorf R, Li PF, Dietz R (1999) Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 99:2934–2941
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Tang JM, Wang JN, Zhang L (2011) VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res 91:402–411
Kajdaniuk D, Marek B, Foltyn W (2011) Vascular endothelial growth factor (VEGF): part 1: in physiology and pathophysiology. Endokrynol Pol 62:444–455
Dias S, Choy M, Alitalo K (2002) Vascular endothelial growth factor (VEGF)-C signaling through FLT-4 (VEGFR-3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood 99:2179–2184
Karpanen T, Heckman CA, Keskitalo S (2006) Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J 20:1462–1472
Muders MH, Zhang H, Wang E (2009) Vascular endothelial growth factor-C protects prostate cancer cells from oxidative stress by the activation of mammalian target of rapamycin complex-2 and AKT-1. Cancer Res 69:6042–6048
Zhao T, Zhao W, Chen Y (2013) Differential expression of vascular endothelial growth factor isoforms and receptor subtypes in the infarcted heart. Int J Cardiol 167:2638–2645
Huang GQ, Wang JN, Tang JM, Zhang L, Zheng F, Yang JY, Guo LY, Kong X, Huang YZ, Liu Y, Chen SY (2011) The combined transduction of copper, zinc-superoxide dismutase and catalase mediated by cell-penetrating peptide, PEP-1, to protect myocardium from ischemia–reperfusion injury. J Transl Med 9:73
Mishra V, Baines M, Wenstone R (2005) Markers of oxidative damage, antioxidant status and clinical outcome in critically ill patients. Ann Clin Biochem 42:269–276
Hou G, Xue L, Lu Z (2007) An activated mTOR/p70S6 K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett 253:236–248
Shin EJ, Schram K, Zheng XL (2009) Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol 221:490–497
Eguchi M, Liu Y, Shin EJ (2008) Leptin protects H9c2 rat cardiomyocytes from H2O2-induced apoptosis. FEBS J 275:3136–3144
Cook SA, Sugden PH, Clerk A (1999) Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes: association with changes in mitochondrial membrane potential. Circ Res 85:940–949
Valks DM, Kemp TJ, Clerk A (2003) Regulation of Bcl-xL expression by H2O2 in cardiac myocytes. J Biol Chem 278:25542–25547
Lee YS, Kang YJ, Kim HJ (2006) Higenamine reduces apoptotic cell death by induction of hemeoxygenase-1 in rat myocardial ischemia–reperfusion injury. Apoptosis 11:1091–1100
Zhang L, Dong XW, Wang JN (2012) PEP-1-CAT-transduced mesenchymal stem cells acquire an enhanced viability and promote ischemia-induced angiogenesis. PLoS One 7:e52537
Desbiens KM, Deschesnes RG, Labrie MM, Desfossés Y, Lambert H, Landry J, Bellmann K (2003) c-Myc potentiates the mitochondrial pathway of apoptosis by acting upstream of apoptosis signal-regulating kinase 1 (Ask1) in the p38 signalling cascade. Biochem J 372:631–641
Hou Q, Hsu YT (2005) Bax translocates from cytosol to mitochondria in cardiac cells during apoptosis: development of a GFP-Bax-stable H9c2 cell line for apoptosis analysis. Am J Physiol Heart Circ Physiol 289:H477–H487
Sun B, Sun GB, Xiao J (2012) Isorhamnetin inhibits H (2) O (2)-induced activation of the intrinsic apoptotic pathway in H9c2 cardiomyocytes through scavenging reactive oxygen species and ERK inactivation. J Cell Biochem 113:473–485
Fröhlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ (2013) Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J 34:1714–1722
Li R, Yan G, Li Q (2012) MicroRNA-145 protects cardiomyocytes against hydrogen peroxide (H2O2)-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS One 7:e44907
Crow MT, Mani K, Nam YJ (2004) the mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res 95:957–970
Shibuya M (2013) Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153:13–19
Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273:30336–30343
Fujio Y, Nguyen T, Wencker D (2000) Akt promotes survival of cardiomyocytes in vitro and protects against ischemia–reperfusion injury in mouse heart. Circulation 101:660–667
Fujio Y, Walsh K (1999) Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem 274:16349–16354
Jiang X, Wang X (2004) Cytochrome C-mediated apoptosis. Annu Rev Biochem 73:87–106
Zhao T, Zhao W, Meng W, Liu C, Chen Y, Gerling IC, Weber KT, Bhattacharya SK, Kumar R, Sun Y (2015) VEGF-C/VEGFR-3 pathway promotes myocyte hypertrophy and survival in the infarcted myocardium. Am J Transl Res 7:697–709
van Wamel AJ, Ruwhof C, van der Valk-Kokshoom LE, Schrier PI, van der Laarse A (2001) The role of angiotensin II, endothelin-1 and transforming growth factor-beta as autocrine/paracrine mediators of stretch-induced cardiomyocyte hypertrophy. Mol Cell Biochem 218:113–124
Taimeh Z, Loughran J, Birks EJ, Bolli R (2013) Vascular endothelial growth factor in heart failure. Nat Rev Cardiol 10:519–530
Acknowledgments
This study was supported by grants from the National natural Science Foundation of China (81170095 to J.M.T, 81328002 to S.Y.C), Hubei Department Science (2014CFB644 to J.M.T), Hubei Education Department Science Foundation (T201112 to J.M.T), and the National Institutes of Health (HL123302, HL119053, and HL107526 to S.Y.C.).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xu-guang Chen and Yan-xia Lv are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Xg., Lv, Yx., Zhao, D. et al. Vascular endothelial growth factor-C protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. Mol Cell Biochem 413, 9–23 (2016). https://doi.org/10.1007/s11010-015-2622-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2622-9