Abstract
Introduction
Resuscitative endovascular balloon occlusion of the aorta (REBOA) increases proximal arterial pressure, but may also induce life-threatening distal ischemia. Partial REBOA (P-REBOA) is thought to mitigate distal ischemia during aortic occlusion. However, feasible indicators of the degree of P-REBOA remain inconsistent. We hypothesised percent balloon volume could be a substitute for pressure measurements of gradients during P- REBOA. This study aimed to compare balloon volume and arterial pressure gradient, and analysed with intra-balloon pressure and balloon shape.
Methods
Proximal (carotid) and distal (femoral) arterial pressures were recorded and a 7-Fr REBOA catheter was placed in four swine. Total REBOA was defined as a cessation of distal pulse pressure and maximum balloon volume was documented. The balloon volume was titrated by 20% increments of maximum capacity to adjust the degree of P-REBOA. The distal/proximal arterial pressure gradient and the intra-balloon pressures were also recorded. The changes in shape and the cross-sectional area of the balloon were evaluated with computed tomography (CT) images.
Results
The proximal mean arterial pressure (MAP) plateaued after 60% balloon volume; meanwhile, distal pulse pressure was still left. The balloon pressure was traced with proximal MAP before contact with aortic wall. The balloon shape changed unevenly from “cone” to “spindle” shape, although the balloon cross-sectional area of the mid-segment linearly increased.
Conclusion
Monitoring distal pressure and titrating percent balloon volume is feasible to manage P-REBOA. In this experiment, 60% balloon volume was enough inflation to elevate central pressure allowing distal perfusion. The intra-balloon pressure was not reliable due to the strong influence of proximal MAP and uneven change of the balloon shape.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is recognised as feasible as a supplementary tool in certain conditions in haemorrhagic shock [1,2,3,4]. REBOA increases proximal blood pressure through the minimally invasive aortic occlusion, but can induce distal ischemia in visceral organs and the lower extremities, and may be life- or limb-threatening [5,6,7,8].
Partial REBOA (P-REBOA) may extend the golden hour [9] or survival period [10,11,12,13] by mitigating distal ischemia in animal experiments. The compatibility and feasibility of computed tomography (CT) in the evaluation of partial REBOA was reported in a swine model [14]. A previous translational study defined the widest cross-sectional area of the balloon (and percentage occlusion of the aorta) as the degree of P-REBOA. The results demonstrated a correlation between percentage occlusion and balloon pressure, femoral mean arterial pressure, and distal/proximal pressure gradient [15].
Despite recent research, the definition and evaluation of the degree of P-REBOA remain inconsistent. In previous studies, the balloon was inflated until pulsatile distal aortic pressure was lost as complete REBOA (100%) [11, 16]. P-REBOA was defined as 50% [16] or 60–70% [11] proximal to distal aortic pressure gradient, presence of pulsatile flow on a distal pressure monitor [14] in translational research, or proximal systolic blood pressure > 90 mmHg in a clinical setting [9]. The degree of P-REBOA was evaluated using the incremental change in balloon volume according to aortic flow [17, 18] or widest cross-sectional area of the balloon [15]. However, neither of which can be evaluated in clinical practice. The aortic pressure gradient [11, 15, 16], balloon volume [15, 17], and balloon pressure [15] were also evaluated as potential indicators of the degree of P-REBOA, but the interpretation remains inconsistent.
We hypothesised that percent balloon volume (100% balloon volume is defined as the balloon volume at extinguishment of distal aortic pulsatile pressure) could be a substitute for pressure measurements of gradients during P- REBOA. This study aimed to evaluate the changes in arterial pressure gradients, intra-balloon pressure, and CT images at different percent inflation. The primary experimental outcome was proximal and distal arterial pressure, and the secondary variable was intra-balloon pressure. Since swine has similar anatomy to human, we employed a swine model to observe physiological response, which cannot be found in the simulators.
Methods
Overview
This study was conducted in an accredited animal research laboratory (Centre for Development of Advanced Medical Technology, Jichi Medical University, Tochigi, Japan). Institutional Animal Experiment Committee approval was obtained before beginning the study (authorisation number 17045-01). Five healthy female non-pregnant domestic pigs were obtained from Sanesu Breeding Co., Ltd. (Chiba, Japan) for this study. The first animal was used for model verification to minimise the technical error and effects of subjective bias and the remaining four animals were used for analysis. The sample size was estimated with the reproducibility of time–density curve analysis of CT data in the repeated scan of a model verification animal and determined to minimise the number of experimental animals in the Institutional Animal Experiment Committee. Animals were housed in quarantine for a minimum of 7 days and they were fasted for 24 h with free access to water prior to enrolment in the study protocol. At the time of experimentation, animals were between 3 and 4 months of age, weighing 35–45 kg.
Animal preparation
Animals were pre-medicated with 0.06 mg/kg medetomidine (Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan) and 0.3 mg/kg midazolam (Astellas Pharma Inc., Tokyo, Japan), and received 0.08 mg/kg atropine (Mitsubishi Tanabe Pharma Corporation, Osaka, Japan) intramuscularly. After confirmation of sedation and endotracheal intubation, maintenance anaesthesia consisted of 3% sevoflurane in 100% oxygen. Animals were mechanically ventilated with tidal volumes of 7–10 mL/kg and a respiratory rate of 10–15 breaths per minute to maintain end-tidal CO2 at 40 ± 5 mmHg and FIO2 at 0.4, unless hypoxia was observed. The pigs were placed on a warming blanket set at 39 °C.
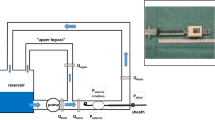
After induction of general anaesthesia, the right neck was exposed. An arterial catheter was placed in the right carotid artery for proximal pressure monitoring and blood sampling, with a central venous catheter in the right jugular vein. Both groins were exposed and a 9-Fr sheath was placed in the right femoral artery for insertion of a 7-Fr polyurethane REBOA catheter (Rescue Balloon®, Tokai Medical Products, Aichi, Japan; maximum diameter of 40 mm with inflation volume of 42 mL). An arterial line was placed in the left femoral artery for distal pressure monitoring. A large sheath size was chosen to ensure atraumatic balloon catheter removal, allowing for repeated use. Heparinised saline alone was used for flushing of sheaths and no systemic anticoagulation was administered. Acetated Ringer’s solution was infused to ensure stroke volume variation of 13%. The animal was transferred to the CT scanner (SOMATOM Definition AS + [128-slice], Siemens Healthcare GmbH, Erlangen, Germany) under general anaesthesia. The REBOA catheter was deployed in the thoracic aorta and balloon position was verified above the diaphragm with a CT scout view (Fig. 1). The REBOA catheter was sutured to the skin and the balloon was gradually inflated with normal saline under close monitoring of distal pressure. Total REBOA was defined when the distal pulse pressure entirely ceased; thus, it is not correlated with the size of the aorta. The balloon volume needed to obtain total REBOA was recorded in each animal. At total REBOA inflation, increased proximal pressure was confirmed, and catheter position was ensured to prevent downstream migration.
Scan range of computed tomography. Scout view; balloon positioning. Non-contrast scan confirm catheter and balloon position. Non-enhanced, 3-mm slice, supra-diaphragm to entire abdomen. Contrast-enhanced scan; diameter and shape of inflated balloon and aorta. 150 s, 3-mm slice, balloon to upper abdomen
Once an inflation cycle from 0 to 100% was completed, the study was terminated, and the animals were euthanised with the maximum dose of sevoflurane and intravenous injection of 1 mg/kg of potassium chloride.
Proximal or distal arterial pressure and intra-balloon pressure
The proximal (carotid) and distal (femoral) artery pressures were measured during incremental changes of balloon volume in P-REBOA. The distal/proximal pressure gradient was also calculated. The intra-balloon pressure was also measured at every incremental degree of occlusion. The balloon inflation lumen was connected to the proximal pressure line via a 3-way stopcock and balloon pressure was recorded after enhanced scanning at each degree of occlusion.
CT imaging of balloon shape during P-REBOA
The contrast-enhanced CT images of the animals were obtained for each increment of maximum balloon volume by 20% of total REBOA (0, 20, 40, 60, 80, 100%) to evaluate the cross-sectional diameter and shape of the balloon during P-REBOA. A scan cycle at each degree of occlusion included non-contrast and contrast-enhanced images. Non-contrast images were obtained to verify the balloon level at zone 1 (above the diaphragm) during P-REBOA. For contrast enhancement, a 600-mg iodine/kg bolus of iopamidol (300 mg iodine/mL Iopaque®, Fuji Pharma, Tokyo, Japan) was administered intravenously through the right jugular vein over 30 s followed by flushing with 10 cc of 0.9% saline; then, the animal was scanned at 150 s after initiation of injection. The balloon was deflated soon after the scan to minimise ischemia (approximately 150 s/scan, performed six times). The scan range was from the balloon top to the upper abdomen to evaluate balloon shape at each degree of occlusion (Fig. 1). The images were reconstructed and analysed analysed with a 3D workstation (Ziostation2® PLUS Classic S, Ziosoft Inc., Tokyo, Japan). The balloon shape was evaluated in 3D volume rendering images. We then measured the cross-sectional area of the aorta and balloon. Acetated Ringer’s solution was infused continuously to wash out the contrast between every scan cycle of approximately 30 min.
Anatomical and physiologic evaluation
Physiologic and laboratory parameters were recorded. Arterial pressure-based cardiac output was measured with EV1000® (Edwards Lifesciences, Irvine, CA, USA) through the femoral artery. Arterial blood specimens were analysed with a blood gas analyser (iSTAT® blood gas analyser, CG4 +, CG8 +, EC4 +, Abbott Point of Care Inc., Princeton, NJ, USA).
Statistical analysis
Data were presented as mean ± standard deviation (SD). Linear regression analysis was performed using GraphPad Prism 6.07 for Windows (GraphPad Software Inc., La Jolla, CA, USA).
Results
All experimental animals were anaesthetised and underwent surgical procedures safely. No adverse event was observed throughout the entire experiment. This experiment used four animals for the analysis. The mean (± SD) length of the animals was 107.3 ± 5.9 cm and weight was 37.8 ± 4.2 kg. The balloon volume needed to obtain total REBOA was 10, 10, 9.0, and 5.0 (8.5 ± 2.4) mL, respectively (Supplemental Table 1). Pre-occlusion arterial pressures, water balance, the data of blood gas analysis remained stable throughout the experiment (Supplemental Tables 2 and 3).
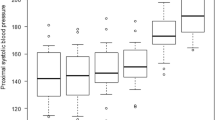
The proximal pressure increased then plateaued after 60% balloon volume. The distal MAP and the pressure gradient dropped sharply, then declined gradually after 60% balloon volume (Fig. 2). The balloon pressure was traced with proximal MAP then rose steeply after 60%, which is felt as tactile feedback with the aortic wall (Fig. 3).
Proximal and distal arterial pressures and distal/proximal gradient. The proximal pressure reached a plateau at 60% of balloon volume (MAP, 164 ± 14 mmHg). The distal pulse pressure decreased as occlusion increased. The distal pressure decreased and almost reached bottom at 60% of balloon volume (116 ± 15, 84 ± 35, 59 ± 14, 36 ± 8, 31 ± 10, 27 ± 2.7 mmHg, respectively). The distal/proximal pressure gradient decreased from 0 to 60% rapidly, then gradually to 100% (1.04 ± 0.06, 0.65 ± 0.24, 0.39 ± 0.10, 0.22 ± 0.06, 0.19 ± 0.06, 0.17 ± 0.02, respectively)
The balloon shape did not change uniformly. It changed from “cone” to “spindle” by increasing the contact area with the aortic wall (Fig. 4). As the balloon inflated, the aortic cross-sectional area above the balloon expanded, while the area below the balloon shrank. The balloon cross-sectional area of the mid-segment linearly increased (Y = 0.016 × X + 0.50, R2 = 0.99, P = 0.0006) (Fig. 5).
3D images of incremental balloon inflation during P-REBOA. The caudal side of the balloon was initially inflated and formed a “cone” shape; the balloon then became “spindle” shaped. The widest cross-sectional balloon area occurred upon contact with the aortic wall at 40%; the contact area then widened as the balloon volume increased. Motion artefact was not negligible at 0 or 20% occlusion, probably because the balloon was not in contact with the aortic wall
The changes in cross-sectional area of the aorta and balloon. The proximal aorta has a larger area (2.12 ± 0.37 cm2) than that of the distal aorta (1.43 ± 0.32 cm2) before inflation. The aortic area above and at the balloon level was increased as the balloon inflated and plateaued at 60% occlusion (2.36 ± 0.46 cm2 at 60%). The aortic area below the balloon shrank according to the balloon inflation (0.75 ± 0.14 cm2 at 100% occlusion). The cross-sectional area at mid-segment of the balloon linearly increased as the balloon inflated (Y = 0.016 × X + 0.50, R2 = 0.99, P = 0.0006) and it reached the same area as the aorta at 2.19 ± 0.56 cm2
Discussion
The present study compared percent balloon volume with proximal and distal arterial pressures and analysed with balloon shape. An animal experiment was necessitated to investigate physiologic response during REBOA, because the patients undergoing REBOA/P-REBOA has high morbidity and mortality. The proximal MAP plateaued after 60% balloon volume; meanwhile, distal pulse pressure was still left. The balloon pressure was influenced with proximal MAP. The balloon shape changed unevenly from “cone” to “spindle” shape although the balloon cross-sectional area of the mid-segment linearly increased.
The proximal MAP reached its plateau after 60% balloon volume, while the distal MAP, pulse pressure and distal/proximal gradient declined gradually after 60% (Fig. 2). The distal MAP and pulse pressure dropped regardless of the plateau of the proximal MAP. It means that 60% balloon volume was the minimal inflation to reach plateau proximal pressure, while leaving distal pressure. Total REBOA is required to control distal arterial exsanguination; on the other hand, 60% balloon volume was enough to elevate central pressure allowing distal perfusion. Previously reported REBOA algorithms [19, 20] were based on haemodynamic instability and haemorrhage location diagnosed with X-ray and FAST examination, but did not include the concept of P-REBOA. Many Japanese centres have utilised P-REBOA, which is simply titrated to maintain the proximal SBP over 90 mmHg [2]. Our results added value to the current algorithm and would recommend that monitoring distal pressure to define 100% balloon volume and titrating at 60% to manage P-REBOA allowing distal perfusion.
The balloon is often adjusted with tactile feedback; thus, the analysis of balloon pressure is necessary to manage P-REBOA safely and to avoid over inflation. The volume–pressure curve precisely traced that of proximal MAP from 0 to 60% of the balloon. Namely, the intra-balloon pressure was strongly influenced by proximal MAP before contact with the aortic wall. The aortic flow pushes down the broadest segment; thus, the balloon forms “cone” shape first then turned “spindle” shape. The combination of compliant occlusion balloon and non-compliant “spine” balloon may be beneficial to avoid uneven changes in balloon shapes [21]. Afterwards, the balloon pressure increased steeply (Fig. 3) due to irregular changes in balloon shape by increasing the contact area with the aortic wall (Fig. 4). The balloon pressure was affected by compliance, proximal MAP, and contact with the aortic wall, which is not reliable in the management of P-REBOA. The balloon area at the mid-segment increased linearly according to volume (Fig. 5), which is not clinically measurable. Future balloon design would be helpful to titrate P-REBOA precisely. A newer catheter which has non-compliant “spine” balloon was developed for P-REBOA and has recently suggested in the swine model [14].
This research has several limitations. First, this animal model did not mimic haemorrhagic shock status. Although 100% balloon volume defined by distal pulse pressure is not correlated with the size of the aorta, arterial pressures or aortic area may be altered by hypovolemia. Second, the arterial pressure may not indicate organ perfusion, which should ideally be evaluated to avoid ischemic complications. Third, the relationship to aortic flow was not assessed in this study. Despite these limitations, the analysis of percent balloon volume and arterial pressure would contribute to a better understanding of P-REBOA.
Conclusion
Monitoring distal pressure and titrating percent balloon volume is feasible to manage P-REBOA. In this experiment, 60% balloon volume was enough inflation to elevate central pressure allowing distal perfusion, while total REBOA controls distal arterial exsanguination. The intra-balloon pressure was not reliable due to the strong influence of proximal MAP and uneven change of the balloon shape.
References
Sadeghi M, Nilsson KF, Larzon T, Pirouzram A, Toivola A, Skoog P, et al. The use of aortic balloon occlusion in traumatic shock: first report from the ABO trauma registry. Eur J Trauma Emerg Surg. 2018;44(4):491–501. https://doi.org/10.1007/s00068-017-0813-7.
Matsumura Y, Matsumoto J, Kondo H, Idoguchi K, Funabiki T, investigators D-I. Partial occlusion, conversion from thoracotomy, undelayed but shorter occlusion: resuscitative endovascular balloon occlusion of the aorta strategy in Japan. Eur J Emerg Med. 2018;25(5):348–54. https://doi.org/10.1097/MEJ.0000000000000466.
Brenner M, Inaba K, Aiolfi A, DuBose J, Fabian T, Bee T, et al. Resuscitative endovascular balloon occlusion of the aorta and resuscitative thoracotomy in select patients with hemorrhagic shock: early results from the american association for the surgery of Trauma's aortic occlusion in resuscitation for trauma and acute care surgery registry. J Am Coll Surg. 2018;226(5):730–40. https://doi.org/10.1016/j.jamcollsurg.2018.01.044.
Lendrum R, Perkins Z, Chana M, Marsden M, Davenport R, Grier G, et al. Pre-hospital resuscitative endovascular balloon occlusion of the aorta (REBOA) for exsanguinating pelvic haemorrhage. Resuscitation. 2019;135:6–13. https://doi.org/10.1016/j.resuscitation.2018.12.018.
Manzano-Nunez R, Orlas CP, Herrera-Escobar JP, Galvagno S, DuBose J, Melendez JJ, et al. A meta-analysis of the incidence of complications associated with groin access after the use of resuscitative endovascular balloon occlusion of the aorta in trauma patients. J Trauma Acute Care Surg. 2018;85(3):626–34. https://doi.org/10.1097/TA.0000000000001978.
Davidson AJ, Russo RM, Reva VA, Brenner ML, Moore LJ, Ball C, et al. The pitfalls of resuscitative endovascular balloon occlusion of the aorta: risk factors and mitigation strategies. J Trauma Acute Care Surg. 2018;84(1):192–202. https://doi.org/10.1097/TA.0000000000001711.
Taylor JR 3rd, Harvin JA, Martin C, Holcomb JB, Moore LJ. Vascular complications from resuscitative endovascular balloon occlusion of the aorta: life over limb? J Trauma Acute Care Surg. 2017;83(1 Suppl 1):S120–S123123. https://doi.org/10.1097/TA.0000000000001514.
Joseph B, Zeeshan M, Sakran JV, Hamidi M, Kulvatunyou N, Khan M, et al. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in Civilian Trauma. JAMA Surg. 2019. https://doi.org/10.1001/jamasurg.2019.0096.
Matsumura Y, Matsumoto J, Kondo H, Idoguchi K, Ishida T, Kon Y, et al. Fewer REBOA complications with smaller devices and partial occlusion: evidence from a multicentre registry in Japan. Emerg Med J. 2017;34(12):793–9. https://doi.org/10.1136/emermed-2016-206383.
Reva VA, Matsumura Y, Horer T, Sveklov DA, Denisov AV, Telickiy SY, et al. Resuscitative endovascular balloon occlusion of the aorta: what is the optimum occlusion time in an ovine model of hemorrhagic shock? Eur J Trauma Emerg Surg. 2018;44(4):511–8. https://doi.org/10.1007/s00068-016-0732-z.
Russo RM, Williams TK, Grayson JK, Lamb CM, Cannon JW, Clement NF, et al. Extending the golden hour: Partial resuscitative endovascular balloon occlusion of the aorta in a highly lethal swine liver injury model. J Trauma Acute Care Surg. 2016;80(3):372–8. https://doi.org/10.1097/TA.0000000000000940(discussion 8–80).
Kuckelman J, Barron M, Moe D, Derickson M, Phillips C, Kononchik J, et al. Extending the golden hour for zone 1 reboa: improved survival and reperfusion injury with intermittent versus continuous reboa in a porcine severe truncal hemorrhage model. J Trauma Acute Care Surg. 2018. https://doi.org/10.1097/TA.0000000000001964.
Sadeghi M, Horer TM, Forsman D, Dogan EM, Jansson K, Kindler C, et al. Blood pressure targeting by partial REBOA is possible in severe hemorrhagic shock in pigs and produces less circulatory, metabolic and inflammatory sequelae than total REBOA. Injury. 2018;49(12):2132–41. https://doi.org/10.1016/j.injury.2018.09.052.
Madurska MJ, Jansen JO, Reva VA, Mirghani M, Morrison JJ. The compatibility of computed tomography scanning and partial REBOA: a large animal pilot study. J Trauma Acute Care Surg. 2017;83(3):557–61. https://doi.org/10.1097/TA.0000000000001574.
Reva VA, Matsumura Y, Samokhvalov IM, Pochtarnik AA, Zheleznyak IS, Mikhailovskaya EM, et al. Defining degree of aortic occlusion for partial-REBOA: a computed tomography study on large animals. Injury. 2018;49(6):1058–63. https://doi.org/10.1016/j.injury.2018.04.021.
Russo RM, Neff LP, Lamb CM, Cannon JW, Galante JM, Clement NF, et al. Partial resuscitative endovascular balloon occlusion of the aorta in swine model of hemorrhagic shock. J Am Coll Surg. 2016;223(2):359–68. https://doi.org/10.1016/j.jamcollsurg.2016.04.037.
Johnson MA, Neff LP, Williams TK, DuBose JJ, Group ES. Partial resuscitative balloon occlusion of the aorta (P-REBOA): Clinical technique and rationale. J Trauma Acute Care Surg. 2016;81(5 Suppl 2):S133–S137137. https://doi.org/10.1097/TA.0000000000001146.
Williams TK, Neff LP, Johnson MA, Ferencz SA, Davidson AJ, Russo RM, et al. Extending resuscitative endovascular balloon occlusion of the aorta: endovascular variable aortic control in a lethal model of hemorrhagic shock. J Trauma Acute Care Surg. 2016;81(2):294–301. https://doi.org/10.1097/TA.0000000000001075.
Brenner M, Hoehn M, Pasley J, Dubose J, Stein D, Scalea T. Basic endovascular skills for trauma course: bridging the gap between endovascular techniques and the acute care surgeon. J Trauma Acute Care Surg. 2014;77(2):286–91. https://doi.org/10.1097/TA.0000000000000310.
Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg. 2015;78(5):1054–8. https://doi.org/10.1097/TA.0000000000000609.
Rasmussen TE. Update on Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) Remote Damage Control Resuscitation Symposium. 2016. https://rdcr.org/wp-content/uploads/2016/07/13-Rasmussen-20116-RDCR-Symposium-V2.pdf. Accessed 15 Nov 2019
Acknowledgements
We appreciate Tomomasa Ttateno RT(R), Sho Kawai RT(R), Fumiharu Jimbo RT(R), Ryo Owada RT(R) for taking and processing CT images. We thank all members of the Centre for Development of Advanced Medical Technology, Jichi Medical University.
Funding
Grant-in-Aid for Research Activity Start-up, Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number, 17H06567.
Author information
Authors and Affiliations
Contributions
YM, a corresponding author, was responsible for drafting, editing, and submission of the manuscript. YM, AH, SH, and YI conducted the animal experiment and collected the data. HK, VR, SO, and JM critically appraised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Yosuke Matsumura was a Clinical Advisory Board Member at Tokai Medical Products. Other authors declare no conflict of interest.
Ethical approval
This study was conducted in an accredited animal research laboratory (Centre for Development of Advanced Medical Technology, Jichi Medical University, Tochigi, Japan). Institutional Animal Experiment Committee approval was obtained before beginning the study (authorisation number 17045-01).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsumura, Y., Higashi, A., Izawa, Y. et al. Distal pressure monitoring and titration with percent balloon volume: feasible management of partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA). Eur J Trauma Emerg Surg 47, 1023–1029 (2021). https://doi.org/10.1007/s00068-019-01257-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-019-01257-4