Abstract
Partial resuscitative balloon occlusion of the aorta (P-REBOA) has emerged as an alternative technique to complete aortic occlusion (REBOA). As it is currently utilized, REBOA is a simple “all-or-none” phenomenon. The balloon is inflated causing exclusion of downstream tissue beds from blood flow. P-REBOA differs from REBOA in that it allows a limited amount of blood flow beyond the aortic occlusion balloon in order to mitigate the negative effects of complete aortic occlusion. P-REBOA is guided by the underlying concept that lowering the pressure and flow to a hemorrhaging tissue bed will allow clot to form around the source of hemorrhage. This clot formation, along with vasospasm, will minimize ongoing bleeding. Furthermore, the small amount of blood flow beyond the balloon may lessen the ischemic burden and allow aerobic metabolism within distal tissue beds, while simultaneously mitigating the supraphysiologic proximal blood pressures observed during conventional REBOA. This variation of REBOA may serve to extend the tolerable duration of intervention. This chapter will discuss the technique of P-REBOA including the physiology of partial aortic occlusion, translational and early clinical data of P-REBOA, as well as future considerations for continued refinement of the technique.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
7.1 Introduction
Resuscitative endovascular balloon occlusion of the aorta (REBOA) has emerged as a feasible technique for modern hemorrhage control and trauma resuscitation [1, 2]. REBOA results in decreased bleeding below the level of complete occlusion and augmentation of blood pressure and blood flow to the heart, lungs, and brain [3]. While initially advocated for trauma patients in extremis as an alternative to emergency thoracotomy, the indications for REBOA have continued to expand [4]. Its use has been described as a prophylactic hemorrhage control adjunct in major elective surgery, for the management of postpartum hemorrhage, and in nontraumatic cardiac arrest [5,6,7,8,9,10].
Despite its increasing utilization and broadening applications, REBOA carries several negative physiologic consequences primarily related to the profound distal ischemia created by complete aortic occlusion. Additionally, REBOA can produce severe supraphysiologic proximal hypertension and cardiac afterload, which may have detrimental effects [11, 12]. One proposed alternative to complete aortic occlusion is a technique of partial REBOA (P-REBOA) [13]. The application of partial aortic occlusion allows a limited amount of blood flow beyond the occlusion balloon. This technique maintains the benefits of increased perfusion created above the level of occlusion, while simultaneously allowing some blood flow to areas distal to the level of occlusion. The purpose of P-REBOA is to minimize distal ischemia and limit proximal hypertension, while simultaneously limiting downstream bleeding. This may serve to extend the duration of intervention, allowing prolonged application in scenarios where hemorrhage control will be delayed beyond the ischemia threshold of complete REBOA (Figs. 7.1, 7.2, 7.3, 7.4, 7.5, and 7.6, Table 7.1).
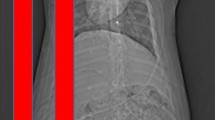
Establishing arterial access is key for P-REBOA. Distal pressure can be transduced through the femoral sheath through which a REBOA catheter is placed, or though the contralateral femoral artery. Proximal pressure can be transduced through a radial arterial line or directly from more modern occlusion catheters such as the ER-REBOA™. The black arrow denotes arterial sheath with REBOA (note integrated arterial pressure port). The white arrow denotes contralateral femoral access
Effects of REBOA, P-REBOA, and no occlusion on proximal blood pressure during intervention in a translational animal model of trauma. Note supraphysiologic pressures created by REBOA with more physiologic blood pressures in the P-REBOA group. The vertical red line at 90 min indicates balloon deflation. The horizontal red line indicates mean arterial pressure of 60
This chapter will discuss the technique of P-REBOA including the physiology of partial aortic occlusion, translational and early clinical data of P-REBOA, as well as future considerations for continued refinement of the technique.
7.2 Physiology
7.2.1 The Effects of Complete Aortic Occlusion
Occlusion of the descending aorta is associated with a complex hemodynamic response with several intended and unintended physiologic consequences.
It creates a relative arterial hypertension proximal to the level of occlusion with a redistribution of blood flow in order to preserve perfusion to vital proximal organs [14]. Although some scant collateral arterial blood flow remains, this is insufficient to maintain organ viability over prolonged periods [15]. Therefore, aortic occlusion creates an “on the clock” phenomenon, with progressive ischemia as a function of the duration of occlusion [16]. Ultimately, this can result in irreversible ischemia once a critical time threshold has been passed [17]. Additionally, once aortic blood flow is restored, these distal tissue beds experience a reperfusion injury with a washout of ischemic metabolites such as potassium and lactic acid. This ischemia-reperfusion injury is quite dramatic and is the primary reason that aortic occlusion is a time-sensitive modality [18]. Notably, patients experience worse outcomes the longer aortic occlusion is maintained due to this phenomenon [19].
The primary intended consequence of aortic occlusion is a near instantaneous increase in blood pressure proximal to the level of occlusion with a resultant increase in blood flow to organs cranial to the level of occlusion. In the shock state, this has the ability to promote and maintain perfusion to critical organs such as the heart, lungs, and brain [20]. The development of arterial hypertension is the most consistent and dramatic finding that occurs during aortic occlusion. This increase in blood flow has the desired effect of maintenance of perfusion; however, often this dramatic increase in pressure can create an additional burden on the heart and may exacerbate injuries such as traumatic brain injury or pulmonary contusions [21, 22].
The two primary physiologic limitations of aortic occlusion are ischemia and reperfusion. This pathologic condition is characterized by an initial restriction of blood supply followed by subsequent restoration of perfusion and oxygenation. The ischemic period is associated with impaired endothelial cell barrier function with a concomitant increase in blood vessel permeability and leakage. In the short term, this causes a cellular transition to anaerobic metabolism in the affected tissues with inhibition of key enzymes that require oxygen as a cofactor. This is associated with alterations to transcription and gene expression. As the duration of ischemia is increased, activation of cell death (apoptosis) occurs. This in turn causes a release of proteases, lysosomes, and key intracellular electrolytes (i.e., potassium) into the extracellular compartment. These ischemic metabolites will continue to collect as the duration of ischemia increases. Eventually, an organ reaches an ischemic threshold of irreversibility beyond which recovery cannot occur [23]. Reperfusion then reintroduces blood flow into the area. This creates an abrupt “washout” of these ischemic metabolites to the systemic circulation. If the duration of ischemia is short, this may be of limited physiologic consequence. However, if the duration is longer, there may be severe consequences such as cardiac arrest or multiorgan failure. Resistance to ischemic injury is dependent on the organ type and its metabolic requirements for oxygen (i.e., skeletal muscle is more resistant and kidneys are less resistant) [24].
7.2.2 The Effects of Partial REBOA
The physiologic rationale for P-REBOA is to decrease distal ischemia by allowing limited blood flow beyond the level of occlusion, while simultaneously reducing proximal hypertension and cardiac afterload. This limited downstream blood flow created by P-REBOA generates a “relative” ischemia as opposed to “complete” ischemia created by REBOA, which serves to extend the duration of intervention and limit the reperfusion injury that occurs at the time of full balloon deflation. Furthermore, P-REBOA may mitigate the “washout” of ischemic metabolites experienced during reperfusion by continuously clearing some metabolites through continued low volume distal flow. Additionally, P-REBOA may lessen the potentially detrimental effects of proximal arterial hypertension and increased afterload associated with complete aortic occlusion. In theory, this will decrease the strain on the heart, and may reduce the potential for pulmonary or cerebral edema.
In the context of uncontrolled injury, P-REBOA does create the potential for ongoing bleeding by perpetuating blood flow to injured vascular beds. This is inherently unpredictable and influenced by factors such as the extent and location of injury, tamponade and thrombus formation at the injury site, and overall coagulation status. It is conceivable that hemorrhage may ensue, particularly from injuries involving large blood vessels such as the aorta and its primary branches. However, this bleeding may occur at tolerable levels that can be met by ongoing resuscitation efforts. This concept is supported by the notion of permissive hypotension after traumatic injury [25]. The theory is that low arterial blood pressures may support some coagulation and clot formation, which is not easily dislodged by low flow and low arterial pressures. Therefore, P-REBOA may carry a benefit over other REBOA alternatives such as intermittent REBOA that periodically restores high-pressure arterial blood flow.
7.3 Development of the Technique
The complications of ischemia-reperfusion caused by aortic occlusion prompted practitioners to find alternative endovascular solutions to REBOA. One technique considered was intermittent balloon deflation that was extrapolated from other techniques in trauma such as the Pringle maneuver or intermittent aortic cross-clamping. The theory was that intermittently providing blood flow to distal organs could extend the duration of tolerable ischemia. This technique is essentially an “all-or-none” approach of complete occlusion alternating with brief periods of no occlusion. An alternative to the binary nature of intermittent occlusion was to provide continuous, low volume, distal perfusion through partial inflation of an occlusion balloon catheter. By simply changing the volume of fluid in the balloon, varying degrees of arterial occlusion and thus downstream blood flow can be achieved. However, the term P-REBOA is not ubiquitous and can imply any degree of aortic occlusion. This simple fact makes comparison of scientific studies challenging and makes discussion about P-REBOA problematic. The fact that this process is dynamic is a source of strength and also a disadvantage. The inherent advantage of P-REBOA is that the degree of occlusion can be proportionate to the patient’s physiology at a given time point. At times complete occlusion may be appropriate, whereas at others no occlusion may be required. However, the physiologic processes that determine aortic blood flow are complex. Determining and maintaining a consistent degree of aortic occlusion is difficult due to the interaction of multiple physiologic, mechanical, and metabolic factors. Therefore, the technique of P-REBOA is somewhat variable from patient to patient and user to user. However, we use the following strategies as guidelines when utilizing a technique of P-REBOA [13].
The first step for P-REBOA is a brief period of complete occlusion. This brief period allows for clot development and hemostasis and provides a brief period for initial volume resuscitation to occur. Next, we establish proximal and distal arterial pressure lines to measure the arterial pressure gradient that will occur across the balloon. During this time, we also use a three-way stopcock on the REBOA balloon with a 30-cm3 “coarse” balloon syringe for initial balloon inflation and a 5-cm3 “fine” adjustment syringe to enable precise balloon deflation. Once proximal hemodynamics are in the acceptable range, distal reintroduction of flow is considered. We typically aim for a 7–10 mmHg increase of distal pressure above the baseline distal pressure measurement with the balloon at full occlusion. In other words, if the distal pressure is 10 mmHg at full occlusion, we would target a pressure of 17–20 mmHg. Alternatively, we aim for the beginning of the return to a pulsatile waveform. Ideally, this should look like a dampened arterial pressure waveform with minimal pulsatility. If the pressure spikes become too high, there is a risk of clot destabilization or hemodynamic collapse. If the patient decompensates, the balloon can rapidly be reinflated to allow for resuscitation to catch up. Additional attempts at P-REBOA can always be re-established when improved hemodynamics are achieved. Once hemostasis has been achieved, the degree of P-REBOA support can be progressively decreased to allow for even more distal perfusion. However, practitioners should be aware that if hemostasis is not ongoing or has not been achieved, there is a risk of disruption of a clot that may have formed with the consequence of resuming hemorrhage. In our experience, the range of desired distal flow occurs over a very narrow range of balloon volume manipulation and requires vigilance and frequent balloon volume titrations. Therefore, when using the technique of P-REBOA a dedicated provider should be available whose sole focus is the balloon occlusion catheter.
7.4 Preclinical and Translational Studies of P-REBOA
Early large animal studies attempted to determine if P-REBOA offered an advantage over complete aortic occlusion. One of the first large animal studies to evaluate the effects of partial aortic occlusion compared REBOA, P-REBOA, and control groups in swine who had undergone a controlled hemorrhage over a 90-min intervention period. P-REBOA was defined as a 50% proximal to distal pressure gradient in this initial work. This study demonstrated feasibility of P-REBOA, demonstrating lower markers of ischemia and less rebound hypotension at the time of balloon deflation compared to complete REBOA. Furthermore, P-REBOA resulted in a more modest blood pressure augmentation, avoiding the severe hypertension seen in the complete REBOA cohort [26].
A similar follow-up study compared complete occlusion to partial occlusion in a severe uncontrolled liver hemorrhage model using similar methodology. However, in this study, partial REBOA was defined as a 60–70% proximal to distal arterial pressure gradient. Complete occlusion again demonstrated supraphysiologic proximal arterial blood pressures, whereas partial REBOA restored near physiologic pressures. However, study animals in the complete occlusion group had a longer mean survival time and experienced less blood loss. Notably, in this study the authors noted difficulty in maintaining a consistent pressure gradient across the balloon. This required frequent balloon volume titrations and resulted in significant fluctuations in blood flow beyond the balloon. This study highlighted the difficulty in maintaining consistent low-volume blood flow beyond the level of balloon occlusion [27].
In another study comparing the effects of REBOA vs. P-REBOA on the progression of traumatic brain injury in swine, the investigators noted that P-REBOA followed a similar pattern of more physiologic proximal hemodynamics and smaller increases in intracranial pressure compared to REBOA [28]. Additional animal studies have focused on the hemodynamic changes that occur during occlusion balloon volume titration. These studies have found that there is a point during P-REBOA at which there are large fluctuations in aortic blood flow despite very small changes in balloon volume [29]. One study noted that absolute arterial pressure distal to the level of balloon occlusion appeared to have a linear relationship with distal blood flow during early restoration of flow. This relationship was preserved at varying degrees of blood loss, suggesting that absolute distal arterial pressure may be the best surrogate for low-volume aortic flow [30]. Another study in sheep noted that, during balloon inflation, there appeared to be a linear relationship with balloon volume, proximal blood pressure, and distal blood pressure, but the authors did not comment on changes observed during deflation or after an ischemic period [31].
Alternative partial flow strategies have been investigated using unconventional experimental models [32]. In a novel experiment, an extracorporeal flow circuit and automation was used to precisely regulate aortic flow to low level in an uncontrolled hemorrhage model with a 90-min intervention period. This study was the first to utilize a flow-based strategy, termed Variable Aortic Control (VAC), where direct flow measurements were utilized to guide intervention. Unique to this study was a period of 20 min of complete occlusion to allow for some initial hemostasis, followed by distal flow rates of 100–300 mL/min in the VAC group based on preset proximal blood pressure ranges. In this experiment, survival was 90% in the VAC group and only 50% in the complete occlusion group. Additionally, the study again found that complete occlusion caused supraphysiologic arterial blood pressures, whereas VAC was able to maintain more physiologic pressures. VAC animals also experienced less rebound hypotension once complete aortic flow was restored and had faster lactate clearance and higher urine output despite requiring approximately half the volume of crystalloids for resuscitation during a critical care period [33]. The authors went on to develop an automated endovascular platform using conventional balloons, termed Endovascular Variable Aortic Control (EVAC), which was compared to REBOA in a 45-min controlled hemorrhage large animal study. Even at this shorter occlusion interval, EVAC resulted in similar physiologic benefits seen in prior studies, with less ischemia and lower resuscitation requirements during critical care.
Overall, animal studies have demonstrated that P-REBOA is feasible and creates more physiologic proximal arterial pressures compared to that of REBOA. P-REBOA is associated with more blood loss compared to REBOA. It is also difficult to achieve with manual balloon volume titration alone and this fluctuation has shown a higher mortality due to ongoing blood loss in large animal models. Nonetheless, these experimental models have not entirely recapitulated the nuances of an active resuscitation, where ready access to blood products may allow for continued hemorrhage seen with P-REBOA in favor of the progressive ischemic penalty incurred by prolonged complete REBOA. In the future, novel techniques such as EVAC, where automation is used to tightly regulate continuous low-volume flow, may overcome the challenges that exist currently with P-REBOA, striking a delicate balance between ischemic injury and ongoing hemorrhage and enabling prolonged interventions without the need for large volume blood product administration.
7.5 Clinical Evidence and Case Reports
Early clinical reports describing the use of P-REBOA have been generally positive. An early case report describing the use of P-REBOA in the United States noted a positive outcome, but also described the difficulty maintaining a consistent degree of aortic blood flow. The surgeon noted not only wide fluctuations in blood pressure during titration but also minimal blood loss distal to the balloon with rapid resolution of acidosis [34]. P-REBOA has been utilized successfully in cases of hemorrhage from ectopic pregnancy and placenta accreta [7, 35]. In these cases, REBOA was able to provide initial hemostasis and then intra-operatively provided some distal blood flow while definitive hemostasis efforts were ongoing. The authors noted that hemostasis was able to proceed at a much more methodical pace given that the degree of ongoing blood loss was minimal with P-REBOA as an adjunct.
P-REBOA has been noted to be the preferred method of REBOA in several centers in Japan [36]. A population-based study reported better hemodynamic responses and longer occlusion duration with P-REBOA compared to REBOA in 78 cases of P-REBOA vs. 63 cases of REBOA. However, there was no noted difference in 24-h or 30-day survival [37].
7.6 Feasibility
Due to the complex interplay of physiologic and mechanical factors, determining and maintaining the degree of P-REBOA is challenging. The benefits of regional hypoperfusion are extrapolated from the positive effects observed from permissive hypotension after traumatic injury. Translational research has shown that use of P-REBOA is effective as a treatment for noncompressible torso hemorrhage and carries advantages over REBOA for mitigation of ischemia-reperfusion.
P-REBOA is not ubiquitous and is difficult to reliably and reproducibly perform with current balloon catheter technology. However, with appropriate resources and a dedicated provider, P-REBOA has been successfully utilized with favorable outcomes. In the event of distal injury, P-REBOA may be associated with ongoing blood loss and a balance needs to be struck between minimizing ongoing hemorrhage and reducing distal ischemia.
7.7 Future Directions
Current use of P-REBOA is through proximal and distal pressure gradients across the balloon. However, it has been demonstrated that maintaining a consistent and reliable blood flow rate is difficult at best. Without a direct understanding of blood flow, it is almost impossible to determine the amount or rate of oxygen delivery or organ perfusion. This makes translational and preclinical studies extremely difficult to reproduce and compare to one another. In the future, balloon catheters will be designed that are able to either measure blood flow directly or dictate the exact amount of blood flow that is allowed past the balloon. Once we understand the amount of blood flow that is delivered the tissues, we can begin to understand how much blood flow is “enough” to maintain tissue viability and delay or prevent cell death. The ischemia threshold after hemorrhage appears to be much earlier than that described for patients with peripheral arterial disease. However, the relationship between minimum blood flow, ischemia, reperfusion, and cell death is not yet known in trauma. This relationship will help inform blood flow titration strategies which during P-REBOA or EVAC allow for comparisons between patients and studies. P-REBOA may also allow for CT scanning with contrast. Translational studies have shown that CT angiography is feasible in the setting of P-REBOA, which may allow for more accurate diagnosis of distal injuries with an occlusion catheter in place [38]. However, its utility for injury diagnosis is unclear at this point.
We envision that P-REBOA will be a feasible alternative to REBOA across multiple and variable applications. Already we have seen it utilized for high-risk pregnancy deliveries and high-risk oncologic resections. We also envision that P-REBOA may develop expanded indications such as a resuscitation adjunct. Practical applications for P-REBOA are likely to expand as balloon catheter technology develops and our understanding of the physiology of P-REBOA continues to grow.
7. Conclusion
P-REBOA is an alternative strategy for aortic occlusion which allows for some blood flow across the area of occlusion in the hope of minimizing ischemia and reperfusion injury. P-REBOA appears to mitigate supraphysiologic arterial blood pressures that can be observed with complete aortic occlusion. Future catheter designs aimed at improving the ability to perform manual control of P-REBOA are ongoing. Next-generation automated techniques such as EVAC, which build upon the concept of P-REBOA, have shown a survival benefit by carefully regulating low-volume continuous flow in the face of uncontrolled hemorrhage. Benefits of this strategy include reduced cognitive burden on the provider and early detection of hemodynamic deterioration. Future studies will focus on the amount of blood flow required to maintain tissue viability yet prevent ongoing hemorrhage.
References
Russo R, Neff LP, Johnson MA, Williams TK. Emerging endovascular therapies for non-compressible torso hemorrhage. Shock. 2016;46(12S):12–9.
White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011;150(3):400–9.
Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg. 2015;78(5):1054–8.
van der Burg BB, van Dongen TT, Morrison J, Joosten PH, DuBose J, Hörer TM, Hoencamp R. A systematic review and meta-analysis of the use of resuscitative endovascular balloon occlusion of the aorta in the management of major exsanguination. Eur J Trauma Emerg Surg. 2018;44:535–50.
Manning JE. Feasibility of blind aortic catheter placement in the prehospital environment to guide resuscitation in cardiac arrest. J Trauma Acute Care Surg. 2013;75(2):S173–S7.
Bodner LJ, Nosher JL, Gribbin C, Siegel RL, Beale S, Scorza W. Balloon-assisted occlusion of the internal iliac arteries in patients with placenta accreta/percreta. Cardiovasc Intervent Radiol. 2006;29(3):354–61.
Russo RM, Girda E, Kennedy V, Humphries MD. Two lives, one REBOA: hemorrhage control for urgent cesarean hysterectomy in a Jehovah’s Witness with placenta percreta. J Trauma Acute Care Surg. 2017;83:551.
Stensaeth KH, Sovik E, Haig INY, Skomedal E, Jorgensen A. Fluoroscopy-free resuscitative endovascular balloon occlusion of the aorta (REBOA) for controlling life threatening postpartum hemorrhage. PLoS One. 2017;12(3):e0174520.
Tsurukiri J, Akamine I, Sato T, Sakurai M, Okumura E, Moriya M, Yamanaka H, Ohta S. Resuscitative endovascular balloon occlusion of the aorta for uncontrolled haemorrahgic shock as an adjunct to haemostatic procedures in the acute care setting. Scand J Trauma Resusc Emerg Med. 2016;24:13.
Weltz AS, Harris DG, O’Neill NA, O’Meara LB, Brenner ML, Diaz JJ. The use of resuscitative endovascular balloon occlusion of the aorta to control hemorrhagic shock during video-assisted retroperitoneal debridement or infected necrotizing pancreatitis. Int J Surg Case Rep. 2015;13:15–8.
Dunn EL, Moore EE, Moore JB. Hemodynamic effects of aortic occlusion during hemorrhagic shock. Ann Emerg Med. 1982;11(5):238–41.
Diakos NA, Pozios I, Katsaros L, Vakrou S, Sventzouri S, Michelinakis N, Tseliou E, Bonios M, Malliaras K, Papalois A. Afterload‐induced left ventricular diastolic dysfunction during myocardial ischaemia and reperfusion. Exp Physiol. 2015;100(3):288–301.
Johnson MA, Neff LP, Williams TK, DuBose JJ, Group ES. Partial resuscitative balloon occlusion of the aorta (P-REBOA): clinical technique and rationale. J Trauma Acute Care Surg. 2016;81(5):S133–S7.
Gelman S, Khazaeli M, Orr R, Henderson T, Reves J. Blood volume redistribution during cross-clamping of the descending aorta. Anesth Analg. 1994;78(2):219–24.
Gelman MDPS. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82(4):1026–57.
Avaro J-P, Mardelle V, Roch A, Gil C, de Biasi C, Oliver M, Fusai T, Thomas P. Forty-minute endovascular aortic occlusion increases survival in an experimental model of uncontrolled hemorrhagic shock caused by abdominal trauma. J Trauma Acute Care Surg. 2011;71(3):720–6.
Hörer TM, Skoog P, Nilsson KF, Oikonomakis I, Larzon T, Norgren L, Jansson K. Intraperitoneal metabolic consequences of supraceliac aortic balloon occlusion in an experimental animal study using microdialysis. Ann Vasc Surg. 2014;28(5):1286–95.
Reva VA, Matsumura Y, Horer T, Sveklov DA, Denisov AV, Telickiy SY, Seleznev AB, Bozhedomova ER, Matsumoto J, Samokhvalov IM, et al. Resuscitative endovascular balloon occlusion of the aorta: what is the optimum occlusion time in an ovine model of hemorrhagic shock? Eur J Trauma Emerg Surg. 2018;44:511.
DuBose JJ, Scalea TM, Brenner M, Skiada D, Inaba K, Cannon J, Moore L, Holcomb J, Turay D, Arbabi CN. The AAST prospective aortic occlusion for resuscitation in trauma and acute care surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409–19.
de Figueiredo LFP, Peres CA, Attalah AN, Romaldini H, Miranda F, Francisco J, Burihan E. Hemodynamic improvement in hemorrhagic shock by aortic balloon occlusion and hypertonic saline solutions. Cardiovasc Surg. 1995;3(6):679–86.
Annecke T, Kubitz JC, Langer K, Hilberath JM, Kahr S, Krombach F, Bittmann I, Rehm M, Kemming GI, Conzen PF. Lung injury following thoracic aortic occlusion: comparison of sevoflurane and propofol anaesthesia. Acta Anaesthesiol Scand. 2008;52(7):977–86.
Dunn E, Prager RL, Fry W, Kirsh MM. The effect of abdominal aortic cross-clamping on myocardial function. J Surg Res. 1977;22(5):463–8.
Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17(11):1391.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317.
Kudo D, Yoshida Y, Kushimoto S. Permissive hypotension/hypotensive resuscitation and restricted/controlled resuscitation in patients with severe trauma. J Intensive Care. 2017;5(1):11.
Russo RM, Neff LP, Lamb CM, Cannon JW, Galante JM, Clement NF, Grayson JK, Williams TK. Partial resuscitative endovascular balloon occlusion of the aorta in a swine model of hemorrhagic shock. J Am Coll Surg. 2016;223(2):359–68.
Russo RM, Williams TK, Grayson JK, Lamb CM, Cannon JW, Clement NF, Galante JM, Neff LP. Extending the golden hour: partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA) in a highly lethal liver injury model. J Trauma Acute Care Surg. 2016;80:372.
Johnson MA, Williams TK, Ferencz SE, Davidson AJ, Russo RM, O’Brien WT Sr, Galante JM, Grayson JK, Neff LP. The effect of resuscitative endovascular balloon occlusion of the aorta, partial aortic occlusion and aggressive blood transfusion on traumatic brain injury in a swine multiple injuries model. J Trauma Acute Care Surg. 2017;83(1):61–70.
Davidson AJ, Russo RM, Ferencz S-AE, Cannon JW, Rasmussen TE, Neff LP, Johnson MA, Williams TK. Incremental balloon deflation following complete REBOA results in steep inflection of flow and rapid reperfusion in a large animal model of hemorrhagic shock. J Trauma Acute Care Surg. 2017;83(1):139–43.
Johnson MA, Davidson AJ, Russo RM, Ferencz S-AE, Gotlib O, Rasmussen TE, Neff LP, Williams TK. Small changes, big effects: the hemodynamics of partial and complete aortic occlusion to inform next generation resuscitation techniques and technologies. J Trauma Acute Care Surg. 2017;82(6):1106–11.
Reva VA, Matsumura Y, Samokhvalov IM, Pochtarnik AA, Zheleznyak IS, Mikhailovskaya EM, Morrison JJ. Defining degree of aortic occlusion for partial-REBOA: a computed tomography study on large animals. Injury. 2018;49:1058.
Williams TK, Neff LP, Johnson MA, Ferencz SA, Davidson AJ, Russo RM, Rasmussen TE. Extending resuscitative endovascular balloon occlusion of the aorta: endovascular variable aortic control in a lethal model of hemorrhagic shock. J Trauma Acute Care Surg. 2016;81(2):294–301.
Williams TK, Neff LP, Johnson MA, Russo RM, Ferencz SA, Davidson AJ, Clement NF, Grayson JK, Rasmussen TE. Automated variable aortic control versus complete aortic occlusion in a swine model of hemorrhage. J Trauma Acute Care Surg. 2017;82(4):694–703.
Davidson AJ, Russo RM, DuBose JJ, Roberts J, Jurkovich GJ, Galante JM. Potential benefit of early operative utilization of low profile, partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA) in major traumatic hemorrhage. Trauma Surg Acute Care Open. 2016;1(1):e000028.
Okumura E, Tsurukiri J, Oomura T, Tanaka Y, Oomura R. Partial resuscitative endovascular balloon occlusion of the aorta as a hemorrhagic shock adjunct for ectopic pregnancy. Am J Emerg Med. 2016;34:1917.
Matsumura Y, Matsumoto J, Kondo H, Idoguchi K, Funabiki T. Partial occlusion, conversion from thoracotomy, undelayed but shorter occlusion: resuscitative endovascular balloon occlusion of the aorta strategy in Japan. Eur J Emerg Med. 2018;25:348.
Matsumura Y, Matsumoto J, Kondo H, Idoguchi K, Ishida T, Kon Y, Tomita K, Ishida K, Hirose T, Umakoshi K. Fewer REBOA complications with smaller devices and partial occlusion: evidence from a multicentre registry in Japan. Emerg Med J. 2017;34:793–9.
Madurska MJ, Jansen JO, Reva VA, Mirghani M, Morrison JJ. The compatibility of computed tomography scanning and partial REBOA: a large animal pilot study. J Trauma Acute Care Surg. 2017;83(3):557–61.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Davidson, A.J., Williams, T.K. (2020). Partial REBOA. In: Hörer, T., DuBose, J., Rasmussen, T., White, J. (eds) Endovascular Resuscitation and Trauma Management . Hot Topics in Acute Care Surgery and Trauma. Springer, Cham. https://doi.org/10.1007/978-3-030-25341-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-25341-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25340-0
Online ISBN: 978-3-030-25341-7
eBook Packages: MedicineMedicine (R0)