Abstract
Purpose
Partial breast irradiation using intraoperative radiotherapy (IORT) after breast-conserving surgery could be sufficient for a selected group of breast cancer patients. We report the results of a cohort of patients from a single center treated as part of the randomized phase-3 TARGIT-A trial.
Methods
Patients (≥50 years) with cT1 cN0 cM0 and invasive ductal histology on biopsy were randomized between IORT with 20 Gy (arm-A) or postoperative whole-breast RT (WBRT) up to 56 Gy in 2 Gy fractions (arm-B). Postoperatively, patients in arm-A with multifocality, lymphovascular invasion, nodal invasion, extensive intraductal component, invasive lobular carcinoma, or resection margins <1 cm received additional postoperative WBRT.
Results
Between 2002 and 2012, 184 patients were randomized, of whom 90 in arm-A and 90 in arm-B were evaluated. Median follow-up was 8.5 years. The 5‑year overall survival was 94.4% in arm-A and 93.3% in arm-B (p = 0.73). Two local recurrences were observed: one at 70.3 months in an arm-A patient who received IORT + WBRT and another at 4.5 months in an arm-B patient who refused all forms of adjuvant treatment, thus resulting in a 5-year local recurrence of 0% in arm-A and 1.1% in arm-B. The 5‑year in-breast recurrence (outside of the index quadrant) was 0% in arm-A and 1.2% in arm-B. Salvage mastectomy was performed successfully in all patients with relapse.
Conclusion
Long-term follow-up of this single-center cohort consolidates the earlier reports of low local recurrence rates after single-dose IORT. Our results are in line with non-inferiority of risk-adapted IORT for selected patients with early breast cancer.
Zusammenfassung
Ziel
Eine Teilbrustbestrahlung mittels intraoperativer Strahlentherapie (IORT) nach einer brusterhaltenden Operation könnte für eine ausgewählte Gruppe von Brustkrebspatientinnen ausreichend sein. Wir berichten über die Ergebnisse der Kohorte von Patientinnen unseres Zentrums, die im Rahmen der randomisierten Phase-3-TARGIT-A-Studie behandelt wurden.
Methoden
Patienten (≥50 Jahre) mit dem Stadium cT1 cN0 cM0 und invasiver duktaler Histologie eines Mammakarzinoms in der Biopsie wurden zwischen IORT mit 20 Gy (Arm A) oder postoperativer Ganzbrust-RT (WBRT) bis zu einer Dosis von 50 Gy in 2‑Gy-Fraktionen (Arm B) randomisiert. Postoperativ erhielten Patientinnen in Arm A bei Multifokalität, lymphovaskulärer Invasion, Lymphknotenmetastasen, umfangreicher intraduktaler Komponente, invasivem lobulärem Karzinom oder Resektionsrändern <1 cm eine zusätzliche postoperative WBRT.
Ergebnisse
Zwischen 2002 und 2012 wurden 184 Patientinnen randomisiert. Hiervon konnten 90 Patientinnen in Arm A und 90 in Arm B ausgewertet werden. Die mediane Nachbeobachtungszeit betrug 8,5 Jahre. Das 5‑Jahres-Gesamtüberleben betrug 94,4% in Arm A und 93,3% in Arm B (p = 0,73). Zwei Lokalrezidive wurden beobachtet: eines nach 70,3 Monaten bei einer Patientin im Studienarm A, die eine IORT und eine WBRT erhalten hat, sowie eines nach 4,5 Monaten bei einer Arm-B-Patientin, die alle Formen der adjuvanten Behandlung ablehnte. Damit beträgt die 5‑Jahres-Lokalrezidivrate 0% in Arm A und 1,1% in Arm B. Die 5‑Jahres-In-Brust-Rezidivrate (außerhalb des Indexquadranten) beträgt 0% in Arm A und 1,2% in Arm B. Bei allen Patientinnen mit Rezidiv konnte eine Salvage-Mastektomie erfolgreich durchgeführt werden.
Schlussfolgerung
Die Langzeitnachbeobachtung dieser Single-Center-Kohorte konsolidiert die früheren Berichte einer niedrigen Lokalrezidivrate nach IORT. Unsere Ergebnisse stimmen mit den vorhandenen Ergebnissen der Nichtunterlegenheit einer risikoadaptierten IORT für ausgewählte Patientinnen mit frühem Brustkrebs überein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of adjuvant radiotherapy has long been established as a central part of breast-conserving therapy. Not only does it improve local control, it also has a positive impact on overall survival [1, 2]. Whole-breast radiotherapy (WBRT) has always been the standard of care for adjuvant treatment. Still, the search for a more risk-adapted radiotherapy approach where WBRT would be replaced with partial breast irradiation (PBI) never stopped. The challenge facing clinicians is finding a working combination of PBI volume, radiation dose, and technique (external beam, intraoperative, or brachytherapy) that produces similar clinical results to WBRT while sparing certain patient groups the need for a protracted and potentially “over” treatment. The TARGIT-A trial is one of the largest international randomized trials that addressed the possibility of further individualizing adjuvant breast irradiation for early breast cancer patients. Using a risk-adapted approach, the use of intraoperative radiotherapy (IORT) to the tumor bed (with or without addition of external beam adjuvant WBRT according to risk profile) was randomized against WBRT alone. The early results of the international trial have already been published [3, 4]. In this report, we present long-term results of the local patient collective treated in a single center as part of the randomized phase-3 TARGIT-A trial.
Patients and methods
The international TARGIT-A trial design is described under Clinicaltrials.gov NCT00983684 [5]. The trial started recruitment in March 2000. Our center joined the trial in June 2002 after approval by the ethics committee and continued recruitment till the end of the trial in June 2012. After approval by the international steering committee, minor modifications of the inclusion and exclusion criteria were used in our center in comparison to the international protocol due to national regulations (Bundesamt für Strahlenschutz [BfS]). The updated criteria were as follows:
Recruitment criteria
Female patients with newly diagnosed invasive breast cancer, at least 50 years old, with no history of previous management of the current cancer were primarily selected. Included were patients with a unifocal cT1 (≤2 cm in mammography/ultrasonography) cN0 cM0 tumors and a biopsy negative for extensive intra-ductal component (EIC <25%) and lympho-vascular invasion. Excluded were patients with lobular cancer, bilateral breast cancer at diagnosis, or patients who underwent neoadjuvant systemic treatments. A written, informed consent had to be provided before randomization.
Treatment
The patients were randomized between IORT to the tumor bed at the time of breast-conserving surgery using 20 Gy prescribed to the applicator surface (arm-A) and adjuvant WBRT (arm-B) to 56 Gy in 2 Gy daily fractions over 5.5 weeks. IORT on a second instance after primary surgical resection was not allowed.
Postoperative evaluation
After complete pathological staging and analysis, patients randomized into arm-A with clear resection margins below 1 cm as required by the BfS, multifocal tumors, EIC ≥25%, or lympho-vascular invasion received an additional WBRT with 46 Gy in 2 Gy daily fractions over 4.5 weeks. Patients with positive resection margins underwent re-resection followed by WBRT with 50 Gy in 2 Gy daily fractions over 5 weeks. Similarly, patients with node-positive disease received WBRT with or without lymphatic irradiation with 50 Gy in 2 Gy daily fractions over 5 weeks.
Additional WBRT after IORT started within the 4th–6th week postoperatively. If adjuvant chemotherapy was indicated, patients were allowed to receive the planned chemotherapy first. Endocrine treatment was allowed to start with or during or after radiotherapy.
Technical aspects
IORT was delivered using a mobile 50 KeV x‑ray generator (Intrabeam®; Carl-Zeiss Meditec AG, Oberkochen, Germany) that was brought to the designated operating theater on the day of surgery. Only spherical applicators between 4 and 5 cm were allowed to be used as required by the BfS. Treatment time varied with the applicator size between approximately 30 and 50 min. 20 Gy was applied after complete tumor resection as confirmed by frozen section examination. Superficial tumors with less than 1 cm of tissue separating the applicator surface from the skin received no IORT and were designated as technically unsuitable and therefore treated with WBRT similar to arm-B with 56 Gy.
Follow-up
All patients were monitored at regular intervals (at least once a year) after the end of treatment where clinical examination and revision of latest radiological imaging (annual mammography/ultrasonography) of the breast were performed.
Data analysis
Local recurrence (LR) was defined as tumor recurrence in the index quadrant. The ipsilateral in-breast tumor recurrence (IBTR) was defined as tumor recurrence outside the index quadrant. Both were measured from the date of breast-conserving surgery ± IORT until the date of first recurrence or the final follow-up visit if recurrence was not detected during the follow-up period. Overall survival (OAS) was measured from the date of breast-conserving surgery ± IORT until the date of death from any cause or date of last follow-up. The Kaplan–Meier method and the log-rank test or the Breslow test were used to calculate survival curves and compare differences between the curves, respectively. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, NY, USA), and p < 0.05 was considered statistically significant.
Results
Between June 2002 and April 2012, 184 patients were randomized, 91 into arm-A and 93 into arm-B. One patient in arm-A and three patients in arm-B never showed for follow-up and were removed from further analysis. The median follow-up for the whole patient group was 8.5 years (range 0.4–15 years), arm-A: 8.3 years, arm-B: 8.5 years. Further patient characteristics according to study arm are shown in Table 1. Tumor-related characteristics reported in this table refer to the final pathological status after tumor resection and not the characteristics revealed by biopsy at the time of randomization. No characteristics were significantly different between the two study arms except for Her2/neu-positive tumors (p = 0.009), with more Her2/neu-positive tumors in the experimental arm (arm-A).
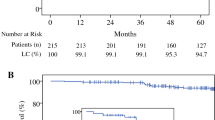
According to intention-to-treat analysis, the 5‑year risk of local recurrence (LR) was 0% in the experimental arm-A and 1.1% in the standard arm-B (p = 0.317; Fig. 1a), with one recurrence in each treatment arm at 70.3 and 4.5 months after surgery, respectively. The 5‑year risk of ipsilateral in-breast recurrence (IBTR) was 0% in arm-A and 1.2% in arm-B, with one recurrence in each arm at 86.5 and 43.9 months after surgery (p = 0.315; Fig. 1b). The 5‑year risk of distant metastases was 3.4% in arm-A and 2.3% in arm-B (p = 0.68; Fig. 1c). The 5‑year overall survival (OAS) was 94.4% in arm-A and 93.3% in arm-B (p = 0.73; Fig. 1d).
The patient suffering from local recurrence in arm-A was diagnosed almost 6 years after surgery, a premenopausal 52-year-old patient with invasive ductal carcinoma pT1c N0 R0 L0 V0 ER/PR+ Her2/neu+ who received IORT + WBRT (WBRT was added to IORT due to an uncertain resection margin with a non-invasive ductal part of the tumor despite re-resection) but refused any form of adjuvant systemic treatment. One year before the diagnosis of recurrence, the patient received hormone replacement therapy to treat menopausal symptoms. Salvage mastectomy was performed successfully to a 15 mm recurrence. The other patient suffering from local recurrence 4.5 months after surgery was randomized into arm-B but refused all forms of adjuvant treatment including radiotherapy. The patient developed distant metastases and died 2 months later. Two other patients, one in arm-A and one in arm-B, developed in-breast recurrences (out of index quadrant) 86.5 and 43.9 months after surgery. Both patients had received WBRT and endocrine therapy as part of their adjuvant treatment. Salvage mastectomy was performed successfully in both patients. Table 2 describes the patient distribution according to treatment received. Fifteen patients in arm-A were deemed unsuitable for IORT during surgery (10/15 due to small distance from the applicator surface to the skin, 6/15 due to large resection cavity, and 1 for unknown reasons, whereas 2 patients had more than one cause). In arm-A, 38 patients showed high-risk factors in final histology, indicating additional WBRT according to protocol (16/38 small resection margins, 5/38 re-resection of residual tumor, 6/38 lymphovascular invasion, 10/38 node positive, 5/38 lobular histology, 5/38 tumor >2 cm, 2/38 margins positive for DCIS, whereas 11 patients had more than one cause). Four patients in arm-B received IORT as a boost followed by WBRT at their request after the result of the randomization was revealed on the day before surgery; however, they were still included in the intention-to-treat analysis. Causes of death in each randomization arm are described in Table 3.
Discussion
In this report, we provide long-term tumor control results of the TARGIT-A trial patients treated in our center. The median follow-up of the studied group was 8.5 years, allowing sufficient time to detect most of the tumor-related events, especially local recurrences. In general, these events were very few. A total of five breast cancer-related deaths, two local recurrences, and two in-breast recurrences were recorded in 180 patients.
The use of intraoperative radiotherapy (IORT) within a risk-adapted approach for treatment of early invasive breast cancer has been offered to our patients within clinical trials since 2002. The presence of one or more of the pre-defined tumor- and treatment-related risk factors was considered an indication for adding WBRT to IORT as a safety net for patients at higher risk of recurrence. This approach ensured the required amount of flexibility to treat the patients safely throughout the trial period that spanned more than 10 years. In this study, 41% of the patients in the experimental arm successfully completed the de-escalated form of treatment with excellent outcome.
Other clinical trials reporting the use of kV-IORT in early breast cancer treatment have shown a similarly low incidence of local recurrence. In the US retrospective analysis, the TARGIT-R study, the 537 patients who were treated with primary IORT had an ipsilateral in-breast recurrence rate of 2.4% at almost 2 years of follow-up, with only 0.9% in the combined IORT + WBRT groups [6]. Early reports of the TARGIT-E trial, a single-arm phase‑2 trial offering IORT for elderly patients >70 years with selection criteria and design similar to the experimental arm of the TARGIT-A trial, revealed one ipsilateral in-breast recurrence (1/478, 0.3%) with a median follow-up of 2 years [7]. The results of the international multicenter TARGIT-A trial including 3451 patients showed a comparatively wider diversity between the results in the pre- and post-pathology subgroups. The 5‑year risk of local recurrence was 3.3% in arm-A vs. 1.3% in arm-B for the whole cohort, whereas in the pre- and post-pathology groups the rates were 2.1% vs. 1.1% and 5.4% vs. 1.7%, respectively, clearly favoring IORT at first instance.
Differences between the local and international versions of the study protocol regarding selection criteria included smaller tumors (max. 2 vs. 3.5 cm) and age limit (min. 50 vs. 45 years). A resection margin less than 1 cm necessitated adding WBRT to IORT in arm-A in the local protocol, whereas only a free surgical margin was required in the international version to omit WBRT. IORT on second instance (post-pathology) was not allowed as per local protocol. When comparing patient and tumor characteristics between the local and international analysis, our local cohort had a tendency towards less favorable prognostic factors, with Her2/neu positivity reaching 28.8% vs. 10%, tumors >T1 were 13.9% vs. 11.5%, tumors >N0 were 80.5% vs. 83.9%, G3 tumors were 18% vs 15%, lymphovascular invasion was 21.6% vs. 13%, and patients >70 years of age were 21.1% vs. 15%. It is, however, difficult to speculate on the reason why our local cohort tended to have better results based on these observations alone, since the relatively tighter selection criteria did not produce a prognostically more favorable patient cohort in this case.
The concept of accelerated partial breast irradiation (APBI) has certainly gained more approval in the past few years within the different oncological societies. The GEC-ESTRO, ASTRO, and DEGRO guidelines [8,9,10] consider APBI suitable in patients with criteria similar to those used in the TARGIT-A trial.
Patients with a low-risk profile for local recurrence have always been candidates for randomized clinical de-escalation trials testing the possibility of omitting breast radiotherapy. Earlier trials like the Milan-III, NSABP-B21, and CALBG-9343, as well as more recent trials PRIME-II and BASO-II, have consistently confirmed the effectiveness of WBRT in reducing local recurrences even when combined with anti-hormone therapy [11,12,13,14,15]. The CALBG-9343 selected patients with highly favorable prognosis (>70 years, T1, N0, ER positive) and reported 2% local recurrences at 10 years in the WBRT + TAM arm and 9% in the TAM-only arm [12]. The BASO-II trial with similarly strict selection criteria (<70 years, T1, N0, G1, L0, mostly ER positive) reported no recurrences at 10 years in the combined WBRT + TAM arm compared to 7% in the TAM-only arm [11]. A recent meta-analysis of five randomized clinical trials has shown how in this low-risk situation, the addition of adjuvant radiotherapy to TAM reduces local recurrence by an absolute 9–14% in 10 years [16]. In our study, the 37 patients with the lowest risk profile who received IORT with no further WBRT had no loco-regional recurrences at 5 years. In this group of patients, 76% were <70 years of age, 19% had G3, 19% were ER negative, 46% Her2/neu-positive, one T2, one had L1 tumors, and the median follow-up reached 9.6 years.
Evaluation of cosmetic outcome as well as late radiation side effects has shown better results with IORT. An earlier evaluation after 1 and 2 years revealed a statistically significant twofold increase in the odds of having an acceptable cosmetic outcome for the IORT patients relative to WBRT [17]. A more recent analysis revealed a better cosmetic outcome of IORT compared with EBRT patients at year 5, with 90% and 68.4% having acceptable cosmesis, respectively, (p = 0.042) [18]. In the analysis by Sperk et al., the hazard ratio to develop higher-grade toxicities or any retraction or any telangiectasia in TARGIT arm-A (IORT) was 0.46 (95% CI 0.26–0.83, p = 0.010) as compared to TARGIT arm-B (WBRT) [19]. Wound-related complications were reported to be similar in the international TARGIT-A trial at 0.55% vs. 0.45% (p = 0.599) [5].
This analysis reflects the results of a single center, a limitation where local experience could affect the reported outcome in both positive and negative ways. The small sample size analyzed is a further limitation that has been overcome, in our point of view, through long follow-up after randomization.
Based on the data of this analysis and our long experience in breast IORT, we believe that the TARGIT strategy is sufficient to safely treat low-risk breast cancer patients in a risk-adapted approach rather than using a “one size fits all” approach.
Conclusion
Long-term follow-up of this single-center cohort consolidates the earlier reports from the TARGIT-A trial of low local recurrence rate after single-dose IORT. Our results are in line with the non-inferiority of risk-adapted IORT for selected patients with early breast cancer.
References
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y, Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366(9503):2087–2106. https://doi.org/10.1016/S0140-6736(05)67887-7
Early Breast Cancer Trialists’ Collaborative G, Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R (2011) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378(9804):1707–1716. https://doi.org/10.1016/S0140-6736(11)61629-2
Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, Alvarado M, Flyger HL, Massarut S, Eiermann W, Keshtgar M, Dewar J, Kraus-Tiefenbacher U, Sutterlin M, Esserman L, Holtveg HM, Roncadin M, Pigorsch S, Metaxas M, Falzon M, Matthews A, Corica T, Williams NR, Baum M (2010) Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 376(9735):91–102. https://doi.org/10.1016/S0140-6736(10)60837-9
Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, Flyger HL, Massarut S, Alvarado M, Saunders C, Eiermann W, Metaxas M, Sperk E, Sutterlin M, Brown D, Esserman L, Roncadin M, Thompson A, Dewar JA, Holtveg HM, Pigorsch S, Falzon M, Harris E, Matthews A, Brew-Graves C, Potyka I, Corica T, Williams NR, Baum M, TARGIT trialists’ group (2014) Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5‑year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 383(9917):603–613. https://doi.org/10.1016/S0140-6736(13)61950-9
Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Saunders C, Brew-Graves C, Potyka I, Morris S, Vaidya HJ, Williams NR, Baum M (2016) An international randomised controlled trial to compare TARGeted Intraoperative radioTherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial). Health Technol Assess 20(73):1–188. https://doi.org/10.3310/hta20730
Valente SA, Tendulkar RD, Cherian S, O’Rourke C, Greif JM, Bailey L, Uhl V, Bethke KP, Donnelly ED, Rudolph R, Pederson A, Summer T, Lottich SC, Ross DL, Laronga C, Loftus L, Abbott AM, Kelemen P, Hermanto U, Friedman NB, Bedi GC, Joh JE, Thompson WA 3rd, Hoefer RA, Wilson JP, Kang SK, Rosen B, Ruffer J, Bravo L, Police A, Escallon JM, Fyles AW, McCready DR, Graves GM, Rohatgi N, Eaker JA, Graves J, Willey SC, Tousimis EA, Collins BT, Shaw CM, Riley L, Deb N, Kelly T, Andolino DL, Boisvert ME, Lyons J, Small W Jr., Grobmyer SR (2016) TARGIT-R (retrospective): north American experience with intraoperative radiation using low-kilovoltage X‑rays for breast cancer. Ann Surg Oncol 23(9):2809–2815. https://doi.org/10.1245/s10434-016-5240-1
Neumaier C, Elena S, Grit W, Yasser AM, Uta KT, Anke K, Axel G, Marc S, Frederik W (2012) TARGIT-E(lderly)—prospective phase II study of intraoperative radiotherapy (IORT) in elderly patients with small breast cancer. BMC Cancer 12:171. https://doi.org/10.1186/1471-2407-12-171
Correa C, Harris EE, Leonardi MC, Smith BD, Taghian AG, Thompson AM, White J, Harris JR (2017) Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol 7(2):73–79. https://doi.org/10.1016/j.prro.2016.09.007
Polgar C, Van Limbergen E, Potter R, Kovacs G, Polo A, Lyczek J, Hildebrandt G, Niehoff P, Guinot JL, Guedea F, Johansson B, Ott OJ, Major T, Strnad V, group G‑Ebcw (2010) Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol 94(3):264–273. https://doi.org/10.1016/j.radonc.2010.01.014
Sautter-Bihl ML, Budach W, Dunst J, Feyer P, Haase W, Harms W, Sedlmayer F, Souchon R, Wenz F, Sauer R (2007) DEGRO practical guidelines for radiotherapy of breast cancer I: breast-conserving therapy. Strahlenther Onkol 183(12):661–666. https://doi.org/10.1007/s00066-007-1811-1
Blamey RW, Bates T, Chetty U, Duffy SW, Ellis IO, George D, Mallon E, Mitchell MJ, Monypenny I, Morgan DA, Macmillan RD, Patnick J, Pinder SE (2013) Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer 49(10):2294–2302. https://doi.org/10.1016/j.ejca.2013.02.031
Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, Muss HB, Smith BL, Hudis CA, Winer EP, Wood WC (2013) Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 31(19):2382–2387. https://doi.org/10.1200/JCO.2012.45.2615
Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM, PRIME II investigators (2015) Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 16(3):266–273. https://doi.org/10.1016/S1470-2045(14)71221-5
Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, Margolese RG, Nesbitt L, Paik S, Pisansky TM, Wolmark N (2002) Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol 20(20):4141–4149. https://doi.org/10.1200/JCO.2002.11.101
Veronesi U, Marubini E, Mariani L, Galimberti V, Luini A, Veronesi P, Salvadori B, Zucali R (2001) Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol 12(7):997–1003
Matuschek C, Bolke E, Haussmann J, Mohrmann S, Nestle-Kramling C, Gerber PA, Corradini S, Orth K, Kammers K, Budach W (2017) The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer—a meta-analysis of randomized trials. Radiat Oncol 12(1):60. https://doi.org/10.1186/s13014-017-0796-x
Keshtgar MR, Williams NR, Bulsara M, Saunders C, Flyger H, Cardoso JS, Corica T, Bentzon N, Michalopoulos NV, Joseph DJ (2013) Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat 140(3):519–525. https://doi.org/10.1007/s10549-013-2641-8
Corica T, Nowak AK, Saunders CM, Bulsara MK, Taylor M, Williams NR, Keshtgar M, Joseph DJ, Vaidya JS (2018) Cosmetic outcome as rated by patients, doctors, nurses and BCCT.core software assessed over 5 years in a subset of patients in the TARGIT-A Trial. Radiat Oncol 13(1):68. https://doi.org/10.1186/s13014-018-0998-x
Sperk E, Welzel G, Keller A, Kraus-Tiefenbacher U, Gerhardt A, Sutterlin M, Wenz F (2012) Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat 135(1):253–260. https://doi.org/10.1007/s10549-012-2168-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Abo-Madyan reports personal fees from Carl-Zeiss Meditec, personal fees from Merck-Serono, and non-financial support from Elekta outside the submitted work. G. Welzel reports non-financial support from Carl-Zeiss Meditec and personal fees from Roche outside the submitted work. E. Sperk reports non-financial support from Carl-Zeiss Meditec outside the submitted work. S. Clausen reports personal fees from Carl-Zeiss Meditec and personal fees from Medical Solutions outside the submitted work. F. Schneider reports personal fees from Carl-Zeiss Meditec outside the submitted work. M. Ehmann reports personal fees from Carl-Zeiss Meditec, personal fees from Elekta, and personal fees from Siemens outside the submitted work. M. Sütterlin reports personal fees and non-financial support from Carl-Zeiss Meditec outside the submitted work. F. Wenz reports grants, personal fees, and non-financial support from Carl-Zeiss Meditec during the conduct of the study; personal fees and non-financial support from Elekta, grants and non-financial support from IBA outside the submitted work. C. Neumaier and A. Keller declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Abo-Madyan, Y., Welzel, G., Sperk, E. et al. Single-center long-term results from the randomized phase-3 TARGIT-A trial comparing intraoperative and whole-breast radiation therapy for early breast cancer. Strahlenther Onkol 195, 640–647 (2019). https://doi.org/10.1007/s00066-019-01438-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01438-5