Abstract
Purpose
The aim of this work was to evaluate the clinical efficacy and safety of simultaneous integrated boost-intensity modulated radiation therapy (SIB-IMRT) in patients with inoperable hepatocellular carcinoma (HCC).
Methods and materials
A total of 53 patients with inoperable HCC underwent SIB-IMRT using two dose-fractionation schemes, depending on the proximity of gastrointestinal structures. The 41 patients in the low dose-fractionation (LD) group, with internal target volume (ITV) < 1 cm from gastrointestinal structures, received total doses of 55 and 44 Gy in 22 fractions to planning target volume 1 (PTV1) and 2 (PTV2), respectively. The 12 patients in the high dose-fractionation (HD) group, with ITV ≥ 1 cm from gastrointestinal structures, received total doses of 66 and 55 Gy in 22 fractions to the PTV1 and PTV2, respectively.
Results
Overall, treatment was well tolerated, with no grade > 3 toxicity. The LD group had larger sized tumors (median: 6 vs. 3.4 cm) and greater frequencies of vascular invasion (80.6 vs. 16.7 %) than patients in the HD group (p < 0.05 each). The median overall survival (OS) was 25.1 mKonzept ist machbar und sicheronths and the actuarial 2-year local progression-free survival (LPFS), relapse-free survival (RFS), and OS rates were 67.3, 14.7, and 54.7 %, respectively. The HD group tended to show better tumor response (100 vs. 62.2 %, p = 0.039) and 2-year LPFS (85.7 vs. 59 %, p = 0.119), RFS (38.1 vs. 7.3 %, p = 0.063), and OS (83.3 vs. 44.3 %, p = 0.037) rates than the LD group. Multivariate analysis showed that tumor response was significantly associated with OS.
Conclusion
SIB-IMRT is feasible and safe for patients with inoperable HCC.
Zusammenfassung
Zielsetzung
Ziel der Arbeit war es, die klinische Wirksamkeit und die Sicherheit der intensitätsmodulierten Radiotherapie mit simultanem integriertem Boost (SIB-IMRT) für Patienten mit einem inoperablen hepatozellulären Karzinom (HCC) zu evaluieren.
Methode und Material
Bei 53 Patienten mit inoperablem HCC wurden zwei unterschiedliche Dosierungskonzepte je nach Lagebeziehung des internen Target-Volumens (ITV) zum gastrointestinalen (GI-)Trakt eingesetzt: Hochdosiskonzept (HD) und Niedrigdosiskonzept (LD). Bei 41 Patienten in der LD-Gruppe mit < 1 cm Abstand des ITV zum GI-Trakt wurden auf PTV1 55 Gy und auf PTV2 44 Gy in 22 Fraktionen appliziert. Bei 12 Patienten in der HD-Gruppe mit ≥ 1 cm Abstand wurden 66 und 55 Gy in 22 Fraktionen auf PTV1 und PTV2 gegeben.
Ergebnisse
Die Behandlung wurde gut vertragen und es wurden keine Toxizitäten > Grad 3 beobachtet. In der LD-Gruppe waren die Tumoren größer und hatten häufiger eine Gefäßinfiltration als in der HD-Gruppe (Median 6,0 vs. 3,4 cm und 80,6 vs. 16,7 %; jeweils p < 0,05). Die mediane Gesamtüberlebenszeit (mOS) betrug 25,1 Monate. Die 2-Jahres-Überlebensraten des lokalen progressionsfreien Überlebens (2J-LPFS), des rezidivfreien Überlebens (2J-RFS) und des 2J-OS lagen bei jeweils 67,3, 214,7 und 54,7 %. Die HD-Gruppe zeigte ein besseres Ansprechen (100 vs. 62,2 %, p = 0,039) und 2J-LPFS (85,7 vs. 59 %, p = n.s.), 2J-RFS 38.1 vs. 7,3 %, p = 0,063) und 2J-OS (83,3 vs. 44,3 %, p = 0,037) im Vergleich zur LD-Gruppe. Die multivariate Analyse zeigte, dass die Ansprechsrate signifikant mit dem OS korrelierte.
Schlussfolgerung
Das vorgestellte SIB-IMRT-Konzept ist machbar und sicher für Patienten mit einem inoperablen HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
HCC is closely associated with hepatitis B and C virus (HBV and HCV) infection, and most HCC patients have liver cirrhosis (LC), a condition that limits treatment options. Surgical resection and liver transplantation are restricted to fewer than 20 % of patients due to the multifocality of HCC development in cirrhotic livers, advanced tumor stage and/or poor hepatic function at diagnosis, and a shortage of graft donors. Ablative interventional techniques, including radiofrequency ablation (RFA) and percutaneous ethanol injection (PEI), are not suitable for patients with large tumors, bleeding tendencies, or unfavorable anatomic tumor locations. Transcatheter arterial chemoembolization (TACE) is one of the most popular and effective nonsurgical treatments, especially for multifocal tumors [1], but its radical effects are limited histopathologically [2]. Recently, targeted agents have shown activity in patients with HCC [3, 4], but are unlikely to be associated with cure in the absence of local treatments. Thus, there is a need for effective and less invasive local treatment modalities.
The technical advances of radiotherapy (RT) planning systems using computed tomography (CT) and computer technology, such as 3-dimensional conformal RT (3D-CRT), have made possible the conformal delivery of radiation to the focal tumor, thus, reducing the risk of toxicity. In addition, RT has been shown effective in treating HCC [5–8] and has, therefore, been incorporated into practice guidelines for the nonsurgical management of HCC [9]. It recommended that RT could be applied for HCC patients with or without PVTT who were treated incompletely by TACE and other local treatments or were not suitable [9]. Intensity-modulated radiotherapy (IMRT) is a type of RT in which radiation beams can be modulated to deliver a high dose to the tumor, while reducing the dose to the surrounding normal tissues. Conceptually, besides the conformal dose distribution, IMRT can exploit the potential biological advantages of accelerated forms of RT, known as SIB-IMRT (simultaneous integrated boost IMRT), in which different doses can be delivered to different targets at the same time. That is, a higher dose can be delivered to the gross tumor volume (GTV), while a lower dose is delivered to areas of subclinical disease at the same time. The potential advantage of this accelerated fractionation is to improve tumor control by reducing the accelerated repopulation of tumor clonogenic cells by shortening overall treatment time. Based on this background, patients with inoperable HCC have been treated at our institution by RT using the SIB-IMRT technique since May 2010. This study was designed to retrospectively analyze the clinical outcomes of SIB-IMRT in these patients and to evaluate the clinical efficacy and safety of this method.
Patients and methods
Patients
This study included 53 patients with inoperable HCC who underwent SIB-IMRT between May 2010 and April 2012. The inclusion criteria were (1) pathologically (n = 11) or clinically (n = 42) diagnosed with HCC, based on the guidelines of the Korean Liver Cancer Study Group and the National Cancer Center [9] ([i] histological confirmation; [ii] the presence of risk factors including HBV, HCV, or LC, a serum ɑ-fetoprotein [AFP] level ≥ 200 IU/ml, and an HCC-compatible radiological feature on one or more imaging modalities, such as CT, magnetic resonance imaging [MRI], and/or angiography; or [iii] the presence of risk factors including HBV, HCV, or LC, a serum AFP < 200 IU/ml, and an HCC-compatible radiological feature on two or more modalities), (2) naïve tumor to treatment, or had recurrent or residual tumor after treatment, which other treatments such as surgery, TACE, and RFA were considered unsuitable or ineffective, (3) no previous or concurrent malignancy, and (4) no evidence of distant metastasis. Portal vein tumor thrombosis (PVTT), present in 26 patients, was identified on contrast-enhanced CT by the presence of a low-attenuation intraluminal filling defect adjacent to the primary tumor. HCCs were classified according to the modified International Union Against Cancer staging classification [10]. The study was performed in accordance with the guidelines of our institutional review board, which deemed that informed consent was not required because the study was retrospective.
Pretreatment evaluation and treatment planning
All patients underwent blood tests, including measurements of blood cell counts, liver and renal function tests, titers of HBV and HCV and AFP. Liver dynamic enhanced CT and/or MRI was used to evaluate the extent of HCC. For RT planning, patients were placed in the treatment position (generally, supine with their arms above their head) and immobilized using an arm-up holder to improve setup reproducibility. CT images were acquired over ten respiratory phases, with 2.5 mm slice thicknesses, under shallow respiration using a four-dimensional CT simulator (Light-Speed RT, GE Healthcare, Waukesha, WI, USA). All CT images were transferred to a treatment planning system (Eclipse, version 8.0; Varian Medical System, Palo Alto, CA, USA), and contours for targets and organs at risk were drawn. The definition of target volumes are illustrated in Fig. 1. The gross tumor volume (GTV) included all detectable tumors, as determined by CT and/or MRI. An internal target volume (ITV) was obtained by summing the GTVs of all respiratory motion phases. Because several clinicopathologic studies showed that microscopic satellite lesions of HCC could be detected 5–10 mm around the gross tumor[11–13], the planning target volume 1 (PTV1) and 2 (PTV2) included the ITV and PTV1 plus a 5 mm margin in all directions, respectively, and an additional 2–5 mm margin to both PTVs in the craniocaudal direction was included to compensate for uncertainties resulting from respiratory liver motion. In case that the PTV1 was close to the gastrointestinal structures, the gastrointestinal structures should be spared at least 5 mm from the PTV1 to avoid acute gastrointestinal toxicity. SIB-IMRT planning was performed using five coplanar or non-coplanar beams of 6 MV photons. The treatment was designed so that at least 95 % of the PTV would receive 100 % of the prescribed dose, and such that a contiguous volume of no more than 2 cm3 inside the PTV would receive no more than 125 % of the prescribed dose. The equivalent dose in 2 Gy fraction (EQD2, Gy10), calculated using a linear quadratic model with ɑ/β ratios of 10 for acute effects on tumor and OARs, was used for normal tissue constraints. The maximum dose to the spinal cord could not exceed 45 Gy10; the relative volumes of the total and remaining normal liver that received doses of 30 Gy10 (TLV30 and RNLV30) were below 60 and 50 %, respectively; the absolute volumes of the esophagus and stomach that received at least 55 Gy10 were ≤ 2 cm3; and the absolute volumes of the small and large bowel that received at least 50 Gy10 were ≤ 2 cm3. Two SIB-IMRT dose-fractionation schemes were designed, depending on the closeness of gastrointestinal structures. The 41 patients in the low dose-fractionation (LD) group, with ITV < 1 cm from gastrointestinal structures, received total doses of 55 Gy (EQD2: 57.3 Gy10) to the PTV1 and 44 Gy (EQD2: 44 Gy10) to the PTV2 in 22 fractions, 5 fractions/week. The 12 patients in the high dose-fractionation (HD) group, with ITV ≥ 1 cm from gastrointestinal structures, received total doses of 66 Gy (EQD2: 71.5 Gy10) to the PTV1and 55 Gy (EQD2: 57.3 Gy10) to the PTV2 in 22 fractions, 5 fractions/week. At each treatment fraction, to verify the patients’ position and the isocenter, digital orthogonal fluoroscopic images were obtained and compared with treatment planning images by overlapping the diaphragm, bony landmarks, and/or internal markers (e.g., embolic iodine, surgical clip) (n = 50).
Follow-up and statistical considerations
During treatment, acute treatment-related toxicities were assessed weekly in all patients and scored using Common Terminology Criteria for Adverse Events software version 3.0 (CTCAE v3.0). After completion of SIB-IMRT, patients were followed up every 3 months for the first 2 years and every 6 months thereafter. Follow-up evaluations consisted of physical examination, complete blood count, liver-function testing, chest radiography, and liver dynamic enhanced CT or MRI.
Tumor responses were determined by comparison of CT scans before and 3 months after SIB-IMRT using the modified Response Evaluation Criteria in Solid Tumors criteria (mRECIST) [14]. Complete response (CR) was defined as the disappearance of the primary tumor. Partial response (PR) was defined as a decrease of at least 30 % in the longest diameter of the primary tumor. Progressive disease (PD) was defined as an increase of at least 20 % in the longest diameter of the primary tumor or the appearance of one or more new lesions. Stable disease (SD) was defined as a response that did not qualify as a PR or PD. Objective response rates were defined as the sum of the CR and PR rates. Recurrence was proven pathologically by surgical resection, biopsy, or cytology, and/or radiological findings, showing an increase in size over time. Local progression was defined as a regrowth or a new tumor within the treated volume; intrahepatic recurrence was defined as a regrowth or new intrahepatic tumor outside the target volume; and distant metastasis was defined as lymph node recurrence, peritoneal seeding, or metastasis to extra-abdominal sites. Local progression-free survival (LPFS), relapse-free survival (RFS), and overall survival (OS) were defined as the intervals from the date of the start of SIB-IMRT to the date of detection of local progression, any detection of recurrence, and death, respectively. Hematologic and nonhematologic toxicities occurring within and after 90 days of the start of treatment were defined as acute and late toxicity, respectively.
Fisher’s exact tests and Student’s t-tests were used to compare the distribution of clinical parameters between patients treated with the two dose-fractionation schemes. Survival rates were calculated using the Kaplan–Meier method. The propensity score matching was performed by modeling probability of receiving two different dose-fractionation groups. A logistic regression model was generated to predict probability of each individual patient based on tumor size and then the model was used to obtain a one-to-one match for the LD and HD group. Univariate analysis of parameters predicting OS were assessed with log rank tests, followed by multivariate analysis using Cox’s proportional hazard model with a stepwise forward procedure. All statistical analyses were two-sided and were performed using STATA software (version 9.0; Stata Corp., College Station, TX, USA). A p value < 0.05 indicated statistical significance.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. Not surprisingly, the patients in the LD group, with target volumes close to gastrointestinal structures, had larger sized tumors and greater frequencies of vascular invasion and advanced tumor stage than patients in the HD group (Table 1).
Failure patterns and tumor response
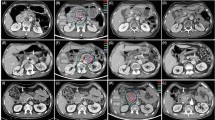
Primary tumor responses, evaluated at 3 months after treatment by imaging modalities, are summarized in Table 2. Of the 53 patients, 12 (22.6 %) achieved CR of the primary tumor, 21 (39.6 %), achieved PR, 18 (34 %) had SD, and 2 (3.8 %) had PD. Of the 41 patients in the LD group, 6 (14.6 %) achieved CR, 15 (36.6 %) achieved PR, 18 (43.9 %) had SD, and 2 (4.9 %) had PD (Fig. 2a–c), whereas of the 12 patients in the HD group, 6 (50 %) achieved CR and 6 (50 %) achieved PR (Fig. 1d–f, Table 2, p = 0.039). The objective response (CR + PR) rates of the primary tumors in all patients and in the LD and HD groups were 62.2 %, 51.2 %, and 100 %, respectively. Of the 26 patients with PVTT, 3 (11.5 %) achieved CR of the PVTT, 10 (38.5 %) achieved PR, 11 (42.3 %) had SD, and 2 (7.7 %) had PD. The objective response (CR + PR) rate of the PVTT in all patients was 50 %.
a–c Partial and d–f complete response of a primary tumor to simultaneous integrated boost-intensity modulated accelerated radiation therapy (SIB-IMRT). a Pretreatment CT scan showing the primary tumor (arrow), b The patient underwent SIB-IMRT using low dose-fractionation. c CT scan 3 months after SIB-IMRT. Note the tumor shrinkage (arrow). d Pretreatment CT scan showing the primary tumor (arrow). e The patient underwent SIB-IMRT using high dose-fractionation. f CT scan 3 months after SIB-IMRT. Note the complete remission of the primary tumor (arrow)
At the time of analysis, 22 patients had died from disease and 31 patients were alive. The median follow-up period for all patients was 18.9 months (range 3–37.8 months), and for living patients 21.2 months (range 14.9–37.8 months). Figure 3 illustrates the patterns of treatment failure in all patients and in the LD and HD groups. Of the 53 patients, 44 (83 %) developed disease recurrence, including 11 (20.8 %) with local recurrence, 40 (75.5 %) with intrahepatic recurrence, and 15 (28.3 %) with distant metastases. Of the 41 patients in the LD group, 10 (24.4 %) developed local recurrence, 38 (92.7 %) had intrahepatic recurrence, and 14 (34.1 %) had distant metastases. Of the 12 patients in the HD group, 1 (8.3 %) developed local recurrence, 8 (66.7 %) had intrahepatic recurrence, and 1 (8.3 %) had distant metastasis.
The median OS in all patients was 25.1 months. The actuarial 2-year LPFS, RFS, and OS rates were 67.3, 14.7, and 54.7 %, respectively. The 2-year LPFS (59 vs. 85.7 %, p = 0.119) and RFS (7.3 vs. 38.1 %, p = 0.063) rates tended to be lower in the LD than in the HD group, whereas the 2-year OS rate was significantly lower in the LD than in the HD group (44.3 vs. 83.3 %, p = 0.037).
Univariate and multivariate analyses were performed to identify parameters predicting OS (Table 3). Although univariate analyses showed that several factors, including the Child–Pugh score, AFP, tumor size, mUICC stage, concurrent sorafenib, dose-fractionation scheme and primary tumor response, were significantly associated with OS (p < 0.05 each), multivariate analysis showed that primary tumor response was the only factor independently associated with OS (p < 0.001).Primary tumor responders showed significantly higher actuarial 2-year (1-year) LPFS (78.3 % [96.9 %] vs. not reached (NR) [75.3 %], p < 0.001), RFS (23.7 % [50 %] vs. 0 % [5 %], p < 0.001), and OS (89.9 % [93.9 %] vs. 7.8 % [50 %], p < 0.001) rates than nonresponders (Fig. 4a–c). The 26 patients with PVTT had a median OS of 19.6 months and actuarial 2-year LPFS, RFS, and OS rates of 72.9, 11.5, and 24.3 %, respectively. PVTT responders showed higher actuarial 2-year (1-year) LPFS (84.6 % [84.6 %] vs. NR [70.3 %], p = 0.187), RFS (NR [30.8 %] vs. 0 % [7.7 %], p = 0.048), and OS (84.6 % [92.3 %] vs. 0 % [46.2 %], p = 0.001) rates than nonresponders (Fig. 4d–f).
a, d Local progression-free survival (LPFS), b, e relapse-free survival (RFS), and c, f overall survival (OS) curves according to primary tumor and portal vein tumor thrombosis (PVTT) response, respectively. Responder complete or partial response, Nonresponder stable or progressive disease. *log-rank test
As showed in Table 1, tumor size was significantly correlated with choice of LD and HD. Thus, to adjust for this bias between two groups, we performed the propensity score matching according to tumor size (Table 4). The distributions of patient characteristics between two groups are summarized in Table 4. Most of patient characteristics were well balanced between two groups, except for frequencies of vascular invasion and tumor stage due that the patients in LD group had tumor close to gastrointestinal structures and portal vein. The objective response (70 vs. 100 %, p = 0.211), the 2-year LPFS (70 vs. 83.3 %, p = 0.138), RFS (30 vs. 45.7 %, p = 0.380), and OS (45 vs. 90 %, p = 0.179) rates tended to be lower in the LD than in the HD group, but its differences were not significant due to the small number in this subgroup.
Toxicity
Overall, treatment was well tolerated, with no grade ≥ 3 toxicity. Within 3 months after SIB-IMRT, acute toxicities were transient, easily manageable, and caused no interruption in treatment course. Of the 53 patients, 47 (88.7 %) showed no change in Child–Pugh score, 4 (7.5 %) showed a 1-point decrease and 2 (3.8 %) showed a 1-point increase. Elevated ALT without evidence of tumor progression was observed in 8 (15.1 %) patients, 6 (11.3 %) with grade 1, and 2 (3.8 %) with grade 2 increases. Late gastrointestinal complications, defined as gastric or duodenal ulcers within the RT field, were observed in 5 (9.4 %) patients, 2 (1.9 %) with grade 1 and 3 (5.7 %) with grade 2. No treatment-related hepatic failure or treatment-related death was observed.
Discussion
A multicenter retrospective cohort study on the use of RT for HCC showed that various fractionation schedules and various RT techniques, such as 3D-CRT, IMRT, and stereotactic body radiation therapy (SBRT), have been used [15]. To date, most studies using conventional fractionated 3D-CRT for HCC have reported favorable outcomes, with response rates of 40–76 % and 45–46 % for primary tumors and PVTT, respectively, and median OS times of 10–25 months and 8–16 months for patients with and without PVTT, respectively [6, 15–20]. Recent results of hypofractionated 3D-CRT or SBRT [21–23] have shown higher tumor response rates (range 49–80 %) but similar median OS (range 17–23 months) compared with conventional fractionated 3D-CRT.The reported 2-year LPFS rates in HCC patients treated with hypofractionated 3D-CRT or SBRT ranged from 43–90 % [21–23]. In the present study on patients with inoperable HCC, the response rates to SIB-IMRT of primary tumors and PVTT were 62.2 and 50 %, respectively; and the median OS times in patients without and with PVTT were 26.6 months and 19.6 months, respectively. The 2-year LPFS rates for all patients and for the LD and HD groups were 67.3, 59, and 85.7 %, respectively. In the present study, the tumor response rate, LPFS rate, and median OS were at the higher end of the wide range of previously reported values [6, 15, 16, 21–23].
Technological advances in RT, including those in imaging and computer technology, have influenced the whole process of RT, from treatment planning to dose delivery. SIB-IMRT has become widely used for prostate and head and neck cancers but not for HCC. Despite the physical and biological advantages of SIB-IMRT relative to 3D-CRT, including improvements in tumor dose conformation, the avoidance of normal tissues, and preventing the accelerated repopulation of tumors, IMRT has not been utilized to treat HCC due to concerns about dose uncertainty resulting from liver motion during respiration. However, the inclusion of tumor motion when treating abdominal tumors with IMRT did not significantly degrade the target dose–volume histogram [24]. Because underdosed regions blur out as the number of treatments is increased, we utilized a relatively longer fractionation schedule, 22 fractions, rather than the fewer than 10 fractions utilized for hypofractionated RT or SBRT.
RT has shown a dose–response relationship with local tumor control in patients with HCC, with an increased RT dose resulting in improved local tumor response and OS. Similarly, despite differences in patient characteristics, such as tumor size and tumor stage, between our LD (57.3 Gy10) and HD (71.5 Gy10) groups, the present study showed that tumor response and 2-year LPFS, RFS, and OS rates tended to be higher in the HD than in the LD group. However, concerns have arisen about the safe delivery of high-dose radiation using 3D-CRT or SBRT to HCCs adjacent to critical normal organs. Generally, the stomach and duodenum are very radiosensitive, especially in HCC patients with LC and portal hypertension, which may result in impairments to the mucosal defense mechanism. Careful consideration during RT planning and delivery is therefore necessary when treating HCCs located near these structures. Although the overall incidence rates of gastrointestinal complications after RT for HCC are low, severe gastrointestinal complications, even perforation, can be observed when these organs are exposed to high RT doses [21, 22]. Therefore, smaller fractions with prolonged treatment times and risk-adapted RT doses may be indispensable in these clinical situations. We utilized two risk-adapted SIB-IMRT dose-fractionation schemes, based on the closeness of gastrointestinal structures, to avoid gastrointestinal complications. None of our patients developed grade ≥ 3 complications. However, because this study involved a relatively small number of patients, additional larger scaled studies are warranted to verify our findings.
The main pattern of failure among our patients was intrahepatic recurrence (90.9 %), similar to previous findings [6, 15, 16, 21–23]. The high intrahepatic recurrence rate is likely due to the multifocal nature of HCC in the cirrhotic liver and the advanced tumor stage among our patients (IVA, 62.3 %). However, although the 2-year LPFS rate of the HD group was 85.7 %, the 2-year LPFS rate of the LD group was only 59 %, which may not be high enough to cure local disease. Fortunately, multikinase inhibitors, such as sorafenib, have been found to improve survival in patients with HCC [3, 4] and may enhance tumor sensitivity to radiation [25]. Although the present study did not show the clinical benefits of multikinase inhibitors due to the small number of patients and selection bias, aforementioned studies suggest that sequential or concurrent use of multikinase inhibitors with SIB-IMRT may reduce the intrahepatic recurrence rate and improve local control in these patients.
Conclusion
We found that risk-adapted SIB-IMRT for inoperable HCC showed promising results, including tumor response and local tumor control with minimal toxicity. Additional larger studies are warranted.
Compliance with ethical guidelines
Conflict of interest
T.H. Kim, J.-W. Park, Y.-J. Kim, B.H. Kim, S.M. Woo, S.H. Moon, S.S. Kim, W.J. Lee, D.Y. Kim, and C.-M. Kim state that there are no conflicts of interest.
References
Llovet JM, Real MI, Montana X et al (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. The Lancet 359:1734–1739
Higuchi T, Kikuchi M, Okazaki M (1994) Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer 73:2259–2267
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Seong J, Keum KC, Han KH et al (1999) Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 43:393–397
Kim TH, Kim DY, Park JW et al (2006) Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol 29:568–575
Cheng JC, Chuang VP, Cheng SH et al (2000) Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 47:435–442
Meng MB, Cui YL, Lu Y et al (2009) Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol 92:184–194
Korean Liver Cancer Study G, National Cancer Center K (2009) Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol 15:391–423
Ueno S, Tanabe G, Nuruki K et al (2002) Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res 24:395–403
Okusaka T, Okada S, Ueno H et al (2002) Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 95:1931–1937
Ikeda K, Seki T, Umehara H et al (2007) Clinicopathologic study of small hepatocellular carcinoma with microscopic satellite nodules to determine the extent of tumor ablation by local therapy. Int J Oncol 31:485–491
Nakazawa T, Kokubu S, Shibuya A et al (2007) Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 188:480–488
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Seong J, Lee IJ, Shim SJ et al (2009) A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int 29:147–152
Hawkins MA, Dawson LA (2006) Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer 106:1653–1663
Kim YI, Park HC, Lim do H et al (2012) Changes of the liver volume and the Child–Pugh score after high dose hypofractionated radiotherapy in patients with small hepatocellular carcinoma. Radiat Oncol Journal 30:189–196
Wang PM, Hsu WC, Chung NN et al (2013) Radiotherapy with volumetric modulated arc therapy for hepatocellular carcinoma patients ineligible for surgery or ablative treatments. Strahlenther Onkol 189:301–307
Gong GZ, Yin Y, Xing LG et al (2012) RapidArc combined with the active breathing coordinator provides an effective and accurate approach for the radiotherapy of hepatocellular carcinoma. Strahlenther Onkol 188:262–268
Yoon H, Oh D, Park HC et al (2013) Predictive factors for gastroduodenal toxicity based on endoscopy following radiotherapy in patients with hepatocellular carcinoma. Strahlenther Onkol 189:541–546
Klein J, Dawson LA (2013) Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys 87:22–32
Bujold A, Massey CA, Kim JJ et al (2013) Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 31:1631–1639
Mizumoto M, Tokuuye K, Sugahara S et al (2008) Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys 71:462–467
Gierga DP, Chen GT, Kung JH et al (2004) Quantification of respiration-induced abdominal tumor motion and its impact on IMRT dose distributions. Int J Radiat Oncol Biol Phys 58:1584–1595
Yu W, Gu K, Yu Z et al (2013) Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer letters 5329:109–117
Acknowledgment
This study was supported by National Cancer Center Grant (NCC 1310080, 1340940 and 1410160). We greatly appreciate Dr. Joo-Young Kim, M.D., Sang-Jae Park, M.D., and Young-Hwan Koh, M.D., of National Cancer Center, Korea, for reviewing the manuscript and supporting the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, T., Park, JW., Kim, YJ. et al. Simultaneous integrated boost-intensity modulated radiation therapy for inoperable hepatocellular carcinoma. Strahlenther Onkol 190, 882–890 (2014). https://doi.org/10.1007/s00066-014-0643-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0643-z