Abstract
Purpose
The aim of this work was to determine predictive factors for gastroduodenal (GD) toxicity in hepatocellular carcinoma (HCC) patients who were treated with radiotherapy (RT).
Patients and methods
A total of 90 HCC patients who underwent esophagogastroduodenoscopy (EGD) before and after RT were enrolled. RT was delivered as 30–50 Gy (median 37.5 Gy) in 2–5 Gy (median 3.5 Gy) per fraction. All endoscopic findings were reviewed and GD toxicities related to RT were graded by the Common Toxicity Criteria for Adverse Events, version 3.0. The predictive factors for the ≥ grade 2 GD toxicity were investigated.
Results
Endoscopic findings showed erosive gastritis in 14 patients (16 %), gastric ulcers in 8 patients (9 %), erosive duodenitis in 15 patients (17 %), and duodenal ulcers in 14 patients (16 %). Grade 2 toxicity developed in 19 patients (21 %) and grade 3 toxicity developed in 8 patients (9 %). V25 for stomach and V35 for duodenum (volume receiving a RT dose of more than x Gy) were the most predictive factors for ≥ grade 2 toxicity. The gastric toxicity rate at 6 months was 2.9 % for V25 ≤ 6.3 % and 57.1 % for V25 > 6.3 %. The duodenal toxicity rate at 6 months was 9.4 % for V35 ≤ 5.4 % and 45.9 % for V35 > 5.4 %. By multivariate analysis including the clinical factors, V25 for stomach and V35 for duodenum were the significant factors.
Conclusion
EGD revealed that GD toxicity is common following RT for HCC. V25 for the stomach and V35 for the duodenum were the significant factors to predict ≥ grade 2 GD toxicity.
Zusammenfassung
Ziel
Ziel der Studie war es, Vorhersagefaktoren für Magen-Darm-(GD-)Toxizität bei Patienten mit Leberzellkrebs (HCC) zu bestimmen, die eine Strahlenbehandlung (RT) erhalten hatten.
Patienten und Methoden
In die Studie wurden insgesamt 90 HCC-Patienten aufgenommen, die vor und nach einer RT einer Esophagogastroduodenoskopie (EGD) unterzogen wurden. Es wurden RT-Dosen zwischen 30–50 Gy (Median 37,5 Gy) in Einzeldosen zu je 2–5 Gy (Median 3,5 Gy) verabreicht. Alle endoskopischen Ergebnisse wurden überprüft und die GD-Toxizität in Bezug auf die RT wurde entsprechend den Kriterien der „Common Toxicity Criteria for Adverse Events Version 3.0“ eingestuft. Untersucht wurde die Vorhersagefaktoren für die GD-Toxizität ≥Stufe 2.
Ergebnisse
Die endoskopischen Befunde zeigten eine erosive Gastritis bei 14 Patienten (16 %), Magengeschwüre bei 8 Patienten (9 %), erosive Duodenitis bei 15 Patienten (17 %) und Duodenalgeschwüre bei 14 Patienten (16 %). Eine Stufe-2-Toxizität entwickelte sich bei 19 Patienten (21 %), eine Stufe-3-Toxizität bei 8 Patienten (9 %). V25 für den Magen und V35 für den Zwölffingerdarm hatten die höchsten Vorhersagefaktoren bei einer Toxizität ≥Stufe 2. Die Magentoxizitätsrate bei 6 Monaten betrug 2,9 % für V25 ≤ 6,3 % und 57,1 % für V25 > 6,3 %. Die Zwölffingerdarmtoxizitätsrate bei 6 Monaten war 9,4 % für V35 ≤ 5,4 % und 45,9 % für V35 > 5,4 %. Bei multivariaten Analysen inklusive klinischen Faktoren waren V25 für den Magen und V35 für den Zwölffingerdarm signifikante Faktoren.
Schlussfolgerung
Die EGD hat gezeigt, dass die GD-Toxizität nach einer RT für HCC verbreitet ist. V25 für den Magen und V35 für den Zwölffingerdarm waren signifikante Faktoren zur Vorhersage einer GD-Toxizität ≥Stufe 2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Radiation therapy (RT) is seldom used for the treatment of hepatocellular carcinoma (HCC) due to a lack of experience and understanding of liver tolerance to RT and technical problems associated with the delivery of RT to part of the liver, as it moves during normal breathing. With recent advances in RT techniques, such as the development of three- or four-dimensional conformal RT and image-guided RT, many institutions have reported their experiences using RT for HCC [8, 9, 12, 15, 17, 19, 20, 21]. The RT dose–response relationship has been well established in HCC [16] and patients with responsive tumors have been shown to have better survival rates [8, 9, 21]. Higher doses of RT are needed for maximal tumor control, but the escalation of RT is limited by organ toxicity. In addition to the liver, gastrointestinal organs near the liver, such as the stomach or duodenum, are the major organs at risk (OAR). Gastroduodenal (GD) tolerance to RT has been investigated in abdominal malignancies [7, 13, 14, 22], but the application of these results to HCC patients requires caution because most HCC patients have liver cirrhosis (LC) and portal hypertension, contributing to the development of GD ulcers [1, 11, 18]. Thus, it is important to investigate predictive factors for GD toxicity in HCC patients.
Previously, we reported that the percentage of GD volume receiving a RT dose of more than 35 Gy (V35) was the most predictive factor for GD toxicity in patients with cirrhosis of the liver [10]. The development of GD toxicity was defined mainly based on patient symptoms. Thereafter, to strengthen our findings, we have tried to perform esophagogastroduodenoscopy (EGD) before and after RT based on the protocol for high-risk patients in whom the target volume is in close proximity to the GD. In the current study, we analyzed the predictive factors for GD toxicity based on EGD findings in HCC patients who were treated with radiotherapy.
Patients and methods
Patients
A total of 445 patients were treated with RT for HCC between October 2008 and December 2010 at our institution. For the current study, we selected 119 patients in whom the GD was located within 2 cm from the planning target volume (PTV). Of the 119 patients, 90 patients who underwent EGD before and after RT were ultimately enrolled in the current study.
Four-dimensional (4D) simulation and RT
All patients underwent 4D-CT simulation. Before simulation, each patient received respiratory training aided by a goggle display, which showed a visual prompting signal based on the prerecorded respiratory cycles for each patient. A CT scan with contrast enhancement was then obtained for the arterial and portal phase during quiet breathing. Images of respiration aided by a goggle display were acquired using the real-time position management (RPM) system (Varian Medical Systems, Palo Alto, CA, USA) to record the respiratory phase. The respiratory phase was divided into ten equal phases with 0 % as end inspiration and 50 % as end expiration (0–90 %). The images of 0, 30, 50, 80 % and the portal phase were used to delineate targets and organs at risk (OAR). The portal phase was chosen for dose calculation.
The gross tumor volumes (GTV), including the main tumors and/or portal vein tumor thrombosis (PVTT), were delineated at each phase and summed to determine the internal target volume (ITV). A 5 mm margin was added to the ITV to create the PTV. RT was delivered as 30–50 Gy (median 37.5 Gy) in 2–5 Gy (median 3.5 Gy) per fraction using a 6, 10, or 15 MV X-ray. All patients were educated for a minimum 2-h fast before simulation and treatment to minimize the variation of stomach volume.
Dosimetric analysis
GD was delineated from the esophagogastric junction to the second portion of the duodenum. The planning OAR volume (PRV), which was obtained from the sum of OAR at each respiratory phase, was chosen for dosimetric analysis in the stomach (S-PRV) and duodenum (D-PRV) to account for organ movement due to respiration. Dose–volume histograms (DVHs) of both S-PRV and D-PRV were calculated. The dosimetric parameters from DVHs were as follows: (1) Dmax: the maximum dose, (2) D3 ml, D5 ml and D10 ml: the irradiated dose to 3, 5 and 10 ml of volume, (3) Vdose: the percentage of volume receiving more than the irradiated dose and (4) aVdose: the absolute volume receiving more than the irradiated dose. All irradiated doses were converted to the biologically effective dose (BED) as described below.
Mathematical modeling of GD dose
The BED to the GD was calculated by considering the various doses per fraction. First, BED10 (α/β = 10) was calculated using linear-quadratic model (BED10 = n • d • (1 + d/10), where n is the fraction number and d is the daily dose) at each dose. Second, BED10 was converted to the 2-Gy equivalent dose (Gy2/10 = 2-Gy equivalent dose with α/β = 10), which was calculated by dividing BED10 by (1 + d/10), where d is 2 Gy. For example, a total dose of 30 Gy in 3 Gy per fraction was converted to 32.5 Gy2/10.
Endoscopic assessment
One endoscopist and one radiation oncologist reviewed the patients’ medical records, endoscopic findings before and after RT, and the RT field. In all patients, EGD was performed at median 2 months (range 1–6 months) after RT. GD toxicity as related to RT was defined as the new development of or the aggravation of endoscopic abnormalities such as an erosive gastroduodenitis or a GD ulcer in close proximity to the RT field following RT. For example, if a new gastric ulcer occurred in the fundus of stomach following RT and only the antrum of the stomach and duodenum were included in the RT field, we did not consider it to be GD toxicity as related to RT. Stomach and duodenum toxicity were evaluated separately and were graded by the Common Toxicity Criteria for Adverse Events, version 3.0.
Statistics
All dosimetric parameters were analyzed using a receiver operating characteristics (ROCs) curve. The area under the curve (AUC) was calculated to determine the best predictive parameters of ≥ grade 2 toxicity. The most predictive dosimetric factor and all clinical parameters including gender, age, the presence of LC, Child–Pugh class, the time interval between previous transcatheter arterial chemoembolization (TACE) and RT, the presence of main portal vein tumor thrombosis (PVTT), smoking history, a past history of GD ulcer, and the use of anti-ulcer drug during RT were analyzed by simple and multiple logistic regression. The analyses for the stomach and the duodenum were performed separately. P values less than 0.05 were considered to be statistically significant. SPSS 19.0 was used for analyses.
Results
Patients
The median age of all patients was 57 years (range 35–75 years). In all, 77 patients (86 %) had LC and 78 patients (87 %) had a classification of Child–Pugh A. The median time interval between RT and previous TACE was 17 days (range 12–782 days). Main PVTT was present in 43 patients (48 %). Patient characteristics are summarized in Tab. 1. There were 44 (49 %) never smokers, 35 (39 %) former smokers, and 11 (12 %) current smokers. A total of 13 patients had a past history of GD ulcer before RT. Among them, 8 patients used the anti-ulcer drugs during the course of RT.
Endoscopic findings related to RT
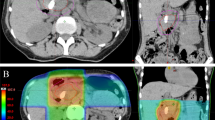
Findings in the stomach included erosive gastritis in 14 patients (16 %) and gastric ulcers in 8 patients (9 %). In comparison, findings in the duodenum included erosive duodenitis in 15 patients (17 %) and duodenal ulcers in 14 patients (16 %; Tab. 2). The median time to the development of GD toxicity was 3 months (range 1–6 months). Endoscopic findings of GD toxicities related to RT are demonstrated in Fig. 1. Grade 2 toxicity developed in 19 patients (21 %) and grade 3 toxicity developed in 8 patients (9 %; Tab. 2). The clinical characteristics of patients who experienced grade 3 toxicity are summarized in Tab. 3.
Endoscopic findings of gastroduodenal (GD) toxicities related to radiotherapy. a At 23 days after completion of RT with 35 Gy in 10 fractions, endoscopy showed an approximately 1 cm ulceration with whitish exudate on a pylorus ring and a 5 mm shallow ulceration on a duodenal bulb. Treatment with proton pump inhibitors was initiated. b At 107 days after completion of RT with 35 Gy in 10 fractions, endoscopy was performed as the patient had complained of melena. Diffuse mucosal hyperemia with blood oozing on the antrum was observed. Treatment included argon plasma coagulation with two vials of thrombin spray, followed by a transfusion due to the low hemoglobin level of 6.6 g/dl
Dosimetric analysis and clinical factors
The results of ROC curve analysis for all dosimetric parameters are shown in Tab. 4. For the stomach, V25 for S-PRV was the most predictive factor for ≥ grade 2 toxicity. The cut-off value was 6.3 % and the gastric toxicity rate at 6 months was 2.9 % for V25 ≤ 6.3 % and 57.1 % for V25 > 6.3 % (Tab. 4). By multiple logistic regression analysis including the clinical factors, V25 was the only significant factor for gastric toxicity (Tab. 5).
For the duodenum, ROC analysis showed that V35 for D-PRV was the most predictive factor for ≥ grade 2 toxicity. The cut-off value was 5.4 % and the duodenal toxicity rate at 6 months was 9.4 % for V35 ≤ 5.4 % and 45.9 % for V35 > 5.4 %. (Tab. 4) By multiple logistic regression analysis including the clinical factors, V35 was the only significant factor for duodenal toxicity (Tab. 5).
Discussion
The current study is unique in several ways. First, we selected high-risk patients in whom the GD was in close proximity to the PTV. Second, all GD toxicities were determined by EGD findings. Third, all patients underwent four-dimensional simulation for treatment planning, introducing the concept of PRV for GD to account for organ movement. Our results show that ≥ grade 2 GD toxicity occurred in 30 % of patients and that the RT dose–volume effect for GD toxicity was shown. V25 and V35 were the most predictive factors for ≥ grade 2 GD toxicity for the stomach and duodenum, respectively.
The GD is the most important dose-limiting factor during RT delivery for the treatment of upper abdominal malignancy. The tolerance dose for gastric ulceration was demonstrated by Emami et al. [4]. For the whole stomach, 2/3 of the stomach, and 1/3 of the stomach, 50, 55, and 60 Gy, respectively, were suggested for TD5/5 (the probability of 5 % complication within 5 years). In the era of three-dimensional conformal RT (3D-CRT), dose–volume analyses using DVH parameters have been reported. In two recent studies, dosimetric parameters for predicting gastrointestinal toxicity were analyzed in patients with pancreatic cancer treated with CCRT. Huang et al. [7] suggested that limiting the V35 of the duodenum to ≤ 20 % may be important for the reduction of grade 3 GI toxicity. Nakamura et al. [14] reported that V50 of ≥ 16 cm3 may be the best predictor for ≥ grade 2 acute GI toxicity.
However, these data may not be applicable for HCC patients because most HCC patients have LC and/or portal hypertension, which are known to be predisposing factors for GD ulcers. The prevalence of GD ulcers in patients with LC is higher than in the general population [1, 11, 18]. Chon et al. [3] showed that the presence of LC was a risk factor for serious GD complications in HCC patients such as bleeding after CCRT. In the present study, Child–Pugh classification B was found to be a risk factor for duodenal toxicity on simple regression analysis. It has been shown that portal hypertension may contribute to an increased risk of GD ulcer as a result of impairment of the gastric mucosal defenses [11]. In an animal experiment, gastric mucosal damage was reduced by portal hypertensive treatment with propranolol [5]. These findings were also observed in endoscopy of cirrhotic patients [6].
Our previous report first showed a dose–volume analysis of GD toxicity in cirrhotic patients with HCC [10]. Grade 2 and 3 GD toxicity was observed in 27.4 and 12.3 % of patients, respectively. In addition, our results suggested that V35 of < 5 % could predict ≥ grade 3 GD toxicity. Thereafter, GD toxicity has been a concern in HCC patients treated with RT; thus, we tried to perform EGD in high-risk patients to detect the GD toxicity. In the current study, we confirmed the dose–volume effect for GD toxicity, but there were several differences compared to our previous study: GD toxicity was detected based on EGD findings, a BED equivalent of 2 Gy per fraction was used to represent the various doses per fraction, and the concept of PRV was introduced. We found that V25 for the stomach and V35 for the duodenum were the most predictive factors for ≥ grade 2 GD toxicity.
Although the overall incidence of GD toxicity after 3D-CRT has been reported to be between 5.7 and 23.1 % [2, 12, 16], there is a lack of data on the evaluation of GD toxicity based on EGD findings. Chon et al. [3] recently reported their EGD findings following CCRT for HCC patients. They showed RT-related gastritis of 40.7 %, duodenitis of 34.1 %, gastric ulcer of 26.0 %, and duodenal ulcer of 16.3 %, which were higher values than in our data (Tab. 2). The rate of GD bleeding (10.6 %) was similar to our results (9 %). The use of concurrent chemotherapy, differences in RT volume and prescribed dose, and selection bias for the study population may explain the higher rates of modest complications in the study by Chon et al.
Our study has several limitations. First, we used the concept of PRV as the GD volume in our analysis. However, PRV is not able to represent the true irradiated volume during RT because the stay time of each respiratory phase during the “beam-on” phase of RT could vary. If respiratory-gated RT had been used, the specific phase could be used for dose–volume analysis. In addition, PRV could not guarantee all variations of organ volume despite of a minimum 2-h fast. Second, we used a 2 Gy equivalent dose per fraction using α/β = 10 because of various dose per fraction as our institutional protocol. Despite this, it may be difficult to generalize our data because of the limitations of the linear-quadratic model in calculating the BED. Third, since this was a retrospective study conducted at one institution, selection bias may influence the determination of the cut-off values for dosimetric factors. If more patients were enrolled, the values might change. Thus, a larger study is necessary to verify our results.
Conclusion
EGD revealed that GD toxicity is a common complication following RT for HCC when the GD is in close proximity to the target volume. It is essential to reduce such GD toxicity in order to improve the quality of life for HCC patients. V25 for the stomach and V35 for the duodenum are the predictive factors for GD toxicity.
References
Chen LS, Lin HC, Hwang SJ et al (1996) Prevalence of gastric ulcer in cirrhotic patients and its relation to portal hypertension. J Gastroenterol Hepatol 11:59–64
Cheng SH, Lin YM, Chuang VP et al (1999) A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol 14:1025–1033
Chon YE, Seong J, Kim BK et al (2011) Gastroduodenal complications after concurrent chemoradiation therapy in patients with hepatocellular carcinoma: endoscopic findings and risk factors. Int J Radiat Oncol Biol Phys 81:1343–1351
Emami B, Lyman J, Brown A et al (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21:109–122
Geoffroy P, Duchateau A, Thiefin G et al (1987) Effects of propranolol and sucralfate on ethanol-induced gastric mucosal damage in chronic portal hypertensive rats. J Hepatol 5:162–166
Hosking SW, Kennedy HJ, Seddon I et al (1987) The role of propranolol in congestive gastropathy of portal hypertension. Hepatology 7:437–441
Huang J, Robertson JM, Ye H et al (2012) Dose-volume analysis of predictors for gastrointestinal toxicity after concurrent full-dose gemcitabine and radiotherapy for locally advanced pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 83:1120–1125
Huang YJ, Hsu HC, Wang CY et al (2009) The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 73:1155–1163
Kim DY, Park W, Lim DH et al (2005) Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer 103:2419–2426
Kim H, Lim do H, Paik SW et al (2009) Predictive factors of gastroduodenal toxicity in cirrhotic patients after three dimensional conformal radiotherapy for hepatocellular carcinoma. Radiother Oncol 93:302–306
Kitano S, Dolgor B (2000) Does portal hypertension contribute to the pathogenesis of gastric ulcer associated with liver cirrhosis? J Gastroenterol 35:79–86
Li B, Yu J, Wang L et al (2003) Study of local three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for patients with stage III hepatocellular carcinoma. Am J Clin Oncol 26:e92–e99
Milano MT, Chmura SJ, Garofalo MC et al (2004) Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 59:445–453
Nakamura A, Shibuya K, Matsuo Y et al (2012) Analysis of dosimetric parameters associated with acute gastrointestinal toxicity and upper gastrointestinal bleeding in locally advanced pancreatic cancer patients treated with gemcitabine-based concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys 84:369–375
Oh D, Lim do H, Park HC et al (2010) Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol 33:370–375
Park HC, Seong J, Han KH et al (2002) Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 54:150–155
Seong J, Lee IJ, Shim SJ et al (2009) A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int 29:147–152
Siringo S, Burroughs AK, Bolondi L et al (1995) Peptic ulcer and its course in cirrhosis: an endoscopic and clinical prospective study. J Hepatol 22:633–641
Tokuuye K, Sumi M, Kagami Y et al (2000) Radiotherapy for hepatocellular carcinoma. Strahlenther Onkol 176:406–410
Yamada K, Izaki K, Sugimoto K et al (2003) Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 57:113–119
Yoon SM, Lim YS, Won HJ et al (2012) Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys 82:2004–2011
Zamboglou C, Messmer MB, Becker G et al (2012) Stereotactic radiotherapy in the liver hilum. Basis for future studies. Strahlenther Onkol 188:35–41
Conflict of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hyunsik Yoon and Dongryul Oh contributed equally to the article.
Rights and permissions
About this article
Cite this article
Yoon, H., Oh, D., Park, H. et al. Predictive factors for gastroduodenal toxicity based on endoscopy following radiotherapy in patients with hepatocellular carcinoma. Strahlenther Onkol 189, 541–546 (2013). https://doi.org/10.1007/s00066-013-0343-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-013-0343-0