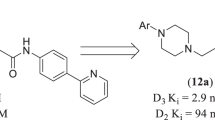
Abstract
In this manuscript we report a structure-activity relationship (SAR) study of analogs of 5/ 7-{[2-(4-Aryl-piperazin-1-yl)-ethyl]-propyl-amino}-5,6,7,8-tetrahydro-naphthalen-2-ol. Our study is focused on introduction of various bioisoteric and aromatic substitutions on the piperazine ring of the hybrid template to further probe into the accessory binding domains on dopamine D2/D3 receptors. Specifically, the goal behind this study is to delineate the nature of the binding pockets for such substitutions on the piperazine ring to determine their influence on binding affinity (Ki), as measured with tritiated spiperone and HEK-293 cells expressing either D2 or D3 receptors. Functional activity of selected compounds was assessed with the GTPγS binding assay. Our data indicates that various N-substitution with substituted and unsubstituted benzene sulfonyl group produced varied affinity and potency for D2/D3. Compound D-660 produced highest selectivity for the D3 receptor in the binding assay. In general, presence of hydroxyl group improved overall activity for both D2/D3 receptors. One such compound D-668 produced exceptional potencies for both the receptors. Overall, our results suggest that binding to the sites removed from the orthosteric binding sites contribute significantly to enhance functional potencies of ligands.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dopamine (DA) receptors have been targeted for drug development for a number of Central Nervous System (CNS) disorders, including drug abuse, schizophrenia, and Parkinson’s disease (PD) [1,2,3,4,5,6,7]. DA receptors are found throughout the CNS and periphery. Five subtypes of DA receptors have been identified and are classified as being either D1-like or D2-like [8]. These classifications are based on receptor pharmacology and function [9,10,11,12]. The D1 and D5 subtypes, known as D1-like, activate adenylate cyclase activity upon receptor activation. The D2-like receptors, which include the D2, D3, and D4 subtypes, inhibit adenylate cyclase activity. D3 receptors were found to have a different distribution in the brain from that of D2 receptors [13, 14]. Recent study on the brain distribution of D3 receptors indicated highest density in the nucleus accumbens. In addition D3 receptors are also expressed at a higher level compared to D2 receptors in the extrastriatal regions and also in the thalamus [14]. The D2 and D3 receptor subtypes possess 50% overall structural homology, and 75–80% in the agonist binding domains [2, 15].
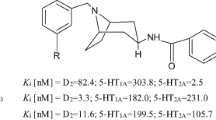
Many compounds have been developed with various selectivity for the D3 versus D2 receptor [16,17,18,19,20,21,22,23]. Due to high homology, development of selective agonists for D3 receptor is rather difficult as both receptors share nearly identical orthosteric active binding sites for agonist interaction [21, 24,25,26,27]. Some of the well known D3 selective agonists include ropinirole and pramipexole, and these agonists were shown to exhibit a 4- to 10-fold higher affinity for the D3 than D2 receptor [28]. In our own work, we have demonstrated development of some of the highly selective agonists for D3 receptors e.g. D-264, D-443 etc. (Fig. 1) that have been reported to date [20,21,22]. In comparison, a number of highly selective D3 antagonists have been developed. In the majority of these compounds there is a piperazine ring connected to a suitable benzamide-type moiety via a variable-size linker, such as in BP 897 (Fig. 1) [16, 17, 29,30,31].
In our previous structure activity relationship (SAR) study on our hybrid template developed earlier, we mapped out different aspects of structural alterations on affinity and selectivity for D3 receptor [21,22,23, 32, 33]. Some of those studies involved the incorporation of hydrophobic moieties on the distal part of the hybrid molecular template which led to enhancement of affinity and selectivity for D3 receptor in general [20, 34]. The compound D-264 (Fig. 1) is one such compound emerged from such studies. The compound D-264 also exhibited potent in vivo neuroprotection efficacy in the MPTP mouse model [4]. The part of the neuroprotection effect was attributed to its high affinity for the D3 receptor [4]. In our current study, we wanted to explore the effect of bio-isosteric replacement of N-substituted group on the piperazine ring in the highly D3 selective compound D-443 (Fig. 1). Impact on binding due to strongly electron withdrawing phenyl sulfone group on basicity of the piperazine nitrogen atom was evaluated. Additionally, we wanted to evaluate effect of similar N-substitution on phenyl aniline moiety with introduction of additional aromatic hydrophobic moiety.
Results and discussion
Chemistry
Scheme 1 describes synthesis of four final target molecules. The starting compound 1-(2-((tert-butyldimethylsilyl)oxy)-ethyl)piperazine was synthesized by following a procedure published by us [35]. Derivatization of the starting compound with 4-methoxy-benzenesulfonyl chloride followed by deprotection of the TBDMS group produced the intermediate 2 which on oxidation produced the intermediate 3. Reductive amination of 3 with either (S)-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine ((-)-DPAT) or (S)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine ((-)-Pramipexole) in presence of NaBH(OAc)3 produced compounds 4 (D-661) and 5 (D-663). The optically active amine intermediates with known absolute stereochemistry were previously synthesized by us [20, 36]. Demethylation of the methoxy group in 4 and 5 in presence of boron tribromide produced compounds 6 (D-660) and 7 (D-662).
The Scheme 2 describes the synthesis of the final compounds D-668, D-669 and D-672. The starting compound 1-(4-nitro-phenyl)-piperazine was N-alkylated with (2-bromoethoxy)(tert-butyl)dimethylsilane in presence of a base by following our earlier reported method to yield intermediate 8. Deprotection of the TBDMS group followed by oxidation yielded 10 which underwent reductive amination followed by reduction of the nitro group to produce 12. The compound 12 was transformed into 13 (D-669) by treatment with 4-methoxy-benzenesulfonyl chloride which on demethylation yielded 14 (D-668). The reaction of compound 12 with benzenesulfonyl chloride produced 15 which on demethylation yielded the final compound 16 (D-672).
Reagents and conditions: a (2-Bromo-ethoxy)-tert-butyl-dimethyl-silane, K2CO3, CH3CN, 90 °C, overnight; b n-Bu4NF, THF, 0 °C to rt, 2 h; c SO3.py, CH2Cl2:DMSO (2:1), Et3N, 0 °C to rt, 2 h; d (-)-DPAT, NaBH(OAc)3, CH2Cl2, rt, 48 h; e H2, Pd/C, 50 psi, MeOH/EtOAc, rt, overnight; f Et3N, CH2Cl2, 0 °C to rt, 3 h; g BBr3, CH2Cl2, −78 °C to rt, overnight
The Scheme 3 describes synthesis of hydroxy compound 21 (D-367). Reaction of Chloroacetylchloride with commercially available 4-methocy phenyl piperazine produced 18. N-alkylation of 5-methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine with 18 followed by reduction produced the intermediate 20. In the final step demethylation by refluxing with HBr yielded compound 21 (D-367).
Structure activity relationship study
Compounds D-661, D-663, D-660 and D-662 were designed as bio-isosteric mimics of compounds of highly selective D3 agonist D-440 where the amide moiety in D-440 is replaced by a sulfone group. It is expected that sulfone group should further lower the basicity of the piperazine N-atom it is attached to. The binding data (Table 1) indicates the effect of such replacement led to somewhat different outcomes. Compounds D-661 and D-663 exhibited low nanomolar potency for D3 while exhibiting moderate affinity for D2 (Ki; D2 = 382 & 592 nM and D3 = 7.75 & 4.59 nM, respectively for D-661 & D-663). Although the selectivity for D3 receptor turned out to be much less compared to D-264 and D-440. An interesting observation was made when demethylation of the methoxy group on the 4-methoxy-benzenesulfonyl moiety was carried out. The resultant compounds D-660 and D-662 exhibited very different profile from each other. The affinity of compound D-660 for D2/ D3 receptors went up significantly compared to the parent D-661 (Ki; D2 = 49.7 nM and D3 = 0.50 nM; D2/D3 = 99 for D-660). Thus, the compound D-660 exhibited more than fifteen-fold increase in affinity for D3 compared to D-661 with concomitant increase in selectivity for D3 receptor. However, no significant changes were observed for the corresponding D-662. The increase in binding affinity of D-660 for both D2/D3 receptors correlates with potent functional activity in the [35S]GTPγS binding assay which indicate sub-nanomolar potencies with full agonist activity (EC50; 0.7 and 0.36 nM for D2/D3 receptors for D-660, Table 2).
Our next series of compounds deals with further probing of electronic, hydrophobic and H-bonding on the distal part of the molecular template. To determine the effect of introduction of hydroxyl and amine functionalities on the aromatic ring, compounds D-367 and D-413 were designed and synthesized. Both phenolic and amine derivatives D-367 and D-413 exhibited high affinity for D2/D3 receptors (Ki; D2 = 17.5 & 9.56 nM and D3 = 1.46 & 0.35 nM, respectively for D-413 & D-367). The data indicates possible role of H-bonding interaction originating from the hydroxyl and amine functionalities. We further wanted to probe the basicity of the nitrogen atom in the amine group as well as any hydrophobic effect from derivatization with aromatic sulfone moiety. Addition of unsubstituted phenyl sulfone yielded D-672 which produced the comparable affinity for D2/D3 receptors as the parent D-413 (Ki; D2 = 20 nM and D3 = 0.7 nM, for D-672). However, a considerable loss of affinity was observed when 4-methoxy phenyl sulfone was introduced as shown in D-669 (Ki; D2 = 164 nM and D3 = 16.73 nM, for D-669). This could be due to unfavorable electronic effect of the methoxy group. Interestingly, high affinity was restored when demethylation of the methoxy group in D-669 was carried out to produce D-668 (Ki; D2 = 15.5 nM and D3 = 0.91 nM, for D-668). This clearly indicates the role of the hydroxyl group in enhancing activity. Indeed, in the functional assay compound D-668 produced exceptionally high potency for for both D2 and D3 receptors (EC50; 0.39 and 0.0104 nM for D2/D3 receptors for D-668, Table 2). A forty-fold increase of potency for D3 receptor took place with D-668 when compared to D-672 which does not contain hydroxyl substitution on the aromatic ring of phenyl sulfone group (EC50; 0.0104 vs 0.42 nM).
Conclusion
In conclusion, our current SAR studies on hybrid template shed additional light on the influence of H-bonding, basicity of N-atoms and hydrophobic effect on the distal part of the molecule for interaction with D2/D3 receptors. In general, presence of the methoxy substituent on the aromatic ring lowered the affinity for D2/D3 receptors. However, the restoration of activity except for compound D-662 upon replacement of methoxy by hydroxyl group might indicate possible involvement of H-bonding or favorable electronic effect. One of the lead compounds D-660 produced high selectivity for the D3 receptors. Selected compounds were found to have potent agonist activity in the functional assays. One of the compounds D-668 produced exceptional potencies for both D2 and D3 receptors. Overall, our results suggest that binding to the sites removed from the orthosteric binding sites contribute to enhance functional activity of the ligands significantly.
Experimental description
Reagents and solvents were purchased from commercial suppliers and used as received unless otherwise noted. Dry solvent was obtained following the standard procedure. All reactions were performed under N2 atmosphere unless otherwise indicated. Analytical silica gel 60 F254-coated TLC plates were purchased from EMD Chemicals, Inc. and were visualized with UV light or by treatment with phosphomolybdic acid (PMA), Dragendorff’s reagent, or ninhydrin. Whatman Purasil 60A silica gel 230–400 mesh was used for flash column chromatographic purifications. Proton nuclear magnetic resonance (1H NMR) spectra were measured on Varian 400 and 600 MHz NMR spectrometer (Pao Alto, California, USA), using tetramethylsilane (TMS) as an internal standard. The NMR solvent used was either CDCl3 or CD3OD unless otherwise indicated. Optical rotations were recorded on Autopol III automatic polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA). Melting points were recorded using a MEL-TEMP II (Laboratory Devices Inc., California, U.S.) capillary melting point apparatus. Purity of the compounds was determined by elemental analysis and was within ±0.4% of the theoretical value (≥95% purity). Elemental analyses were performed by Atlantic Microlab, Inc, GA, USA. Selected compounds were furher analyzed by reverse phase HPLC (Waters 2489 Alliance Integrated System, Massachusetts, USA) to check for purity.
Procedure A. 1-[2-(tert-Butyl-dimethyl-silanyloxy)-ethyl]-4-(4-methoxy-benzene-sulfonyl)-piperazine (1)
To a stirring solution of 1-(2-((tert-butyldimethylsilyl)oxy)-ethyl)piperazine (1.0 g, 4.09 mmol) in CH2Cl2 (10 mL), Et3N (2.57 mL, 18.41 mmol) and 4-methoxy-benzenesulfonyl chloride (1.01 g, 4.91 mmol) were added at 0 °C. The reaction mixture was stirred at the same temperature for 1 h after which it was quenched with saturated NaHCO3 solution and the aqueous phase was extracted with CH2C12 (3 × 30 mL). The organic portions were dried over Na2SO4 and rotary evaporated to dryness, which was purified by silica gel column chromatography (hexane:EtOAc = 7:3) to give compound 1 as white solid (1.4 g, 83%). 1H NMR (600 MHz, CDCl3): δ 7.68 (dd, J = 6.0, 1.8 Hz, 2H), 6.99 (dd, J = 6.0, 1.8 Hz, 2H), 3.87 (s, 3H), 3.68 (t, J = 6.0 Hz, 2H), 3.00 (s, 4H), 2.61 (t, J = 4.8 Hz, 4H), 2.51 (t, J = 6.0 Hz, 2H), 0.86 (s, 9H), 0.02 (s, 6H).
2-[4-(4-Methoxy-benzenesulfonyl)-piperazin-1-yl]-ethanol (2)
Into a stirring solution of compound 1 (1.2 g, 2.89 mmol) in THF (12 mL) was added n-tetrabutylammonium fluoride (4.34 mL, 4.34 mmol, 1.0 M solution in THF) at 0 °C. The reaction mixture was then stirred at room temperature for 2 h. THF was evaporated in vacuo, and the residue was diluted with CH2Cl2 (25 mL) and washed with a saturated solution of NaHCO3. The water layer was extracted with CH2Cl2 (3 × 40 mL). The combined organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure. The crude product was purified by silica gel column chromatography (EtOAc:MeOH = 19:1) to afford white solid 2 (0.71 g, 82%). 1H NMR (600 MHz, CDCl3): δ 7.69 (dd, J = 6.0, 1.8 Hz, 2H), 7.01 (dd, J = 6.0, 1.8 Hz, 2H), 3.88 (s, 3H), 3.57–3.55 (m, 2H), 3.01 (s, 4H), 2.58 (s, 4H), 2.53–2.51 (m, 2H).
[4-(4-Methoxy-benzenesulfonyl)-piperazin-1-yl]-acetaldehyde (3)
Into a stirring solution of compound 2 (0.35 g, 1.17 mmol) in CH2Cl2 (8 mL) and DMSO (4 mL), was added Et3N (1.14 mL, 8.16 mmol) at 0 °C. The reaction mixture was stirred for 5 min followed by addition of SO3.py complex (0.927 g, 5.83 mmol) at 0 °C. Ice bath was removed and the reaction mixture was stirred at room temperature for 2 h. The reaction mixture was quenched by addition of water and extracted with CH2Cl2 (3 × 30 mL). The combined organic layer was dried using Na2SO4, and the solvent was removed under reduced pressure. The crude product was purified by silica gel column chromatography with EtOAc as the eluent to give aldehyde 3 (0.32 g, 92%). The purified aldehyde was used immediately for next step. 1H NMR (600 MHz, CDCl3): δ 9.60 (s, 1H), 7.70–7.67 (m, 2H), 7.02–6.99 (m, 2H), 3.88 (s, 3H), 3.23–3.22 (m, 2H), 3.06 (s, 4H), 2.61–2.60 (m, 4H).
Procedure B. (S)-{2-[4-(4-Methoxy-benzenesulfonyl)-piperazin-1-yl]-ethyl}-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine (4) (D-661)
Into a stirring solution of aldehyde 3 (0.31 g, 1.04 mmol) in CH2Cl2 (15 mL) was added (S)-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine (0.23 g, 1.04 mmol). After the mixture was stirred for 1.5 h, NaBH(OAc)3 (0.44 g, 2.08 mmol) was added portion wise and the mixture was stirred for 48 h at room temperature. The reaction mixture was quenched with a saturated solution of NaHCO3 at 0 °C and extracted with CH2Cl2 (3 × 40 mL). The combined organic layer was dried over Na2SO4, and the solvent was removed under reduced pressure. Crude product was purified by column chromatography (hexane:EtOAc = 3:7) to afford compound 4 (0.45 g, 86%). 1H NMR (600 MHz, CDCl3): δ 7.68 (dd, J = 7.2, 1.8 Hz, 2H), 7.07 (t, J = 7.8 Hz, 1H), 6.98 (dd, J = 7.2, 1.8 Hz, 2H), 6.67 (d, J = 7.8 Hz, 1H), 6.64 (d, J = 7.8 Hz, 1H), 3.86 (s, 3H), 3.79 (s, 3H), 3.06–2.94 (m, 5H), 2.89–2.84 (m, 1H), 2.80–2.77 (m, 1H), 2.71–2.66 (m, 1H), 2.62–2.43 (m, 11H), 2.00–1.97 (m, 1H), 1.50 (td, J = 12, 5.4 Hz, 1H), 1.42 (sx, J = 7.2 Hz, 2H), 0.85 (t, J = 7.2 Hz, 3H); [α]D25 = −26.2 (c = 1.0 in CH2Cl2); Anal. Calcd for C27H43Cl2N3O5S: C, 54.72; H, 7.31; N, 7.09. Found: C, 54.43; H, 7.29; N, 6.92.
(S)-N 6-{2-[4-(4-Methoxy-benzenesulfonyl)-piperazin-1-yl]-ethyl}-N 6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine (5) (D-663)
Aldehyde 3 (0.265 g, 0.89 mmol) in CH2Cl2 (10 mL) was reacted with (S)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine (0.17 g, 0.80 mmol) and NaBH(OAc)3 (0.376 g, 1.78 mmol) according to procedure B. Crude product was purified by column chromatography (EtOAc/MeOH 19:1) to afford compound 5 (0.275 g, 70%). 1H NMR (600 MHz, CDCl3): δ 7.70–7.67 (m, 2H), 7.00–6.97 (m, 2H), 4.80 (bs, 2H), 3.87 (s, 3H), 3.02‒2.93 (m, 5H), 2.68–2.60 (m, 3H), 2.58‒2.52 (m, 6H), 2.49–2.39 (m, 5H), 1.94‒1.92 (m, 1H), 1.66 (td, J = 12, 5.4 Hz, 1H), 1.41 (sx, J = 7.2 Hz, 2H), 0.85 (t, J = 7.2 Hz, 3H); [α]D25 = −33.8 (c = 1.0 in CH2Cl2); Anal. Calcd for C23.8H41Cl4N5O3.2S2: C, 43.69; H, 6.32; N, 10.70. Found: C, 43.82; H, 6.62; N, 10.66.
Procedure C. (S)-6-({2-[4-(4-Hydroxy-benzenesulfonyl)-piperazin-1-yl]-ethyl}-propyl-amino)-5,6,7,8-tetrahydro-naphthalen-1-ol (6) (D-660)
To a stirred solution of 4 (0.1 g, 0.2 mmol) in 8 mL of CH2Cl2 was added BBr3 (1.2 mL, 1.2 mmol, 1.0 M solution in CH2Cl2) at −78 °C under N2 atmosphere. The reaction mixture was stirred at −78 °C for 2 h and then at room temperature overnight. The reaction was quenched with a saturated solution of NaHCO3 at 0 °C and the aqueous layer was extracted with CH2Cl2 (3 × 30 mL). The combined organic layer was dried over Na2SO4, and evaporated under reduced pressure. The crude product was purified by silica gel column chromatography (EtOAc:MeOH = 98:2) to yield compound 6 (0.055 g, 58%). 1H NMR (600 MHz, CD3OD): δ 7.56–7.54 (m, 2H), 6.91–6.89 (m, 2H), 6.87 (d, J = 7.8 Hz, 1H), 6.53 (t, J = 8.4 Hz, 2H), 3.35 (s, 1H), 2.98–2.87 (m, 6H), 2.82–2.79 (m, 1H), 2.74–2.70 (m, 3H), 2.59–2.51 (m, 6H), 2.48 (t, J = 7.2 Hz, 4H), 2.05–2.02 (m, 1H), 1.55 (td, J = 12, 5.4 Hz, 1H), 1.49 (sx, J = 7.2 Hz, 2H), 0.88 (t, J = 7.2 Hz, 3H); [α]D25 = −28.6 (c = 1.0 in CH3OH); Anal. Calcd for C25H39Cl2N3O5S: C, 53.19; H, 6.96; N, 7.44. Found: C, 53.07; H, 6.94; N, 7.06.
(S)-4-(4-{2-[(2-Amino-4,5,6,7-tetrahydro-benzo[d]thiazol-6-yl)-propyl-amino]-ethyl}-piperazine-1-sulfonyl)-phenol (7) (D-662)
Compound 5 (0.15 g, 0.3 mmol) in 12 mL of CH2Cl2 was reacted with BBr3 (0.9 mL, 0.9 mmol, 1.0 M solution in CH2Cl2) according to procedure C. The crude product was purified by silica gel column chromatography (CH2Cl2:MeOH = 9:1) to afford 7 (0.09 g, 63%). 1H NMR (600 MHz, CD3OD): δ 7.58–7.56 (m, 2H), 6.92–6.90 (m, 2H), 3.33 (s, 1H), 3.02‒2.93 (m, 5H), 2.68–2.43 (m, 14H), 1.94‒1.92 (m, 1H), 1.67 (td, J = 12, 5.4 Hz, 1H), 1.44 (sx, J = 7.2 Hz, 2H), 0.85 (t, J = 7.2 Hz, 3H); [α]D25 = −40.6 (c = 1.0 in CH3OH); Anal. Calcd for C24H44Cl4N5O4.5S2: C, 42.35; H, 6.52; N, 10.29. Found: C, 42.11; H, 6.38; N, 10.49.
1-[2-(tert-Butyl-dimethyl-silanyloxy)-ethyl]-4-(4-nitro-phenyl)-piperazine (8)
A suspension of 1-(4-nitro-phenyl)-piperazine (2.0 g, 9.65 mmol), potassium carbonate (4.0 g, 28.95 mmol), and (2-bromoethoxy)(tert-butyl)dimethylsilane (2.54 g, 10.62 mmol) in acetonitrile (20 mL) was refluxed under N2 for 15 h. The reaction mixture was filtered off and the filtrate was evaporated under reduced pressure. The residue was then diluted with EtOAc, washed with water, dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (hexane:EtOAc = 2:1) to afford compound 8 as yellow solid (2.75 g, 78%). 1H NMR (600 MHz, CDCl3): δ 8.12 (dd, J = 5.4, 1.8 Hz, 2H), 6.82 (dd, J = 5.4, 1.8 Hz, 2H), 3.80 (t, J = 6.0 Hz, 2H), 3.43 (t, J = 4.8 Hz, 4H), 2.69 (t, J = 4.8 Hz, 4H), 2.60 (t, J = 6.0 Hz, 2H), 0.90 (s, 9H), 0.07 (s, 6H).
2-[4-(4-Nitro-phenyl)-piperazin-1-yl]-ethanol (9)
Into a stirring solution of compound 8 (1.4 g, 3.83 mmol) in THF (18 mL) was added n-tetrabutylammonium fluoride (5.75 mL, 5.75 mmol, 1.0 M solution in THF) at 0 °C. The reaction mixture was then stirred at room temperature for 2 h. THF was evaporated in vacuo, and the residue was diluted with EtOAc (25 mL) and washed with a saturated solution of NaHCO3. The water layer was extracted with EtOAc (3 × 40 mL). The combined organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure. The crude product was purified by silica gel column chromatography (EtOAc:MeOH = 9:1) to give a yellow solid 2 (0.86 g, 89%). 1H NMR (600 MHz, CDCl3): δ 8.12 (dd, J = 5.4, 1.8 Hz, 2H), 6.83 (dd, J = 5.4, 1.8 Hz, 2H), 3.69 (t, J = 5.4 Hz, 2H), 3.45 (t, J = 4.8 Hz, 4H), 2.68 (t, J = 4.8 Hz, 4H), 2.63 (t, J = 5.4 Hz, 2H).
[4-(4-Nitro-phenyl)-piperazin-1-yl]-acetaldehyde (10)
Into a stirring solution of compound 9 (0.4 g, 1.59 mmol) in CH2Cl2 (8 mL) and DMSO (4 mL), was added Et3N (1.55 mL, 11.14 mmol) at 0 °C. The reaction mixture was stirred for 5 min followed by addition of SO3.py complex (1.27 g, 7.96 mmol) at 0 °C. Ice bath was removed and the reaction mixture was stirred at room temperature for 2 h. The reaction mixture was quenched by addition of water and extracted with CH2Cl2 (3 × 30 mL). The combined organic layer was dried using Na2SO4, and the solvent was removed under reduced pressure. The crude product was purified by silica gel column chromatography with EtOAc as the eluent to give aldehyde 10 (0.35 g, 88%). The purified aldehyde was used immediately for next step. 1H NMR (600 MHz, CDCl3): δ 9.74 (s, 1H), 8.13–8.09 (m, 2H), 6.84–6.81 (m, 2H), 3.49 (t, J = 4.8 Hz, 4H), 3.29 (m, 2H), 2.70 (t, J = 4.8 Hz, 4H).
(S)-(5-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-{2-[4-(4-nitro-phenyl)-piperazin-1-yl]-ethyl}-propyl-amine (11)
Aldehyde 10 (0.34 g, 1.36 mmol) in CH2Cl2 (15 mL) was reacted with (S)-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine (0.27 g, 1.23 mmol) and NaBH(OAc)3 (0.58 g, 2.73 mmol) according to procedure B. Crude product was purified by column chromatography with EtOAc as the eluent to afford compound 11 (0.5 g, 81%). 1H NMR (600 MHz, CDCl3): δ 8.12 (dd, J = 5.4, 1.8 Hz, 2H), 7.09 (t, J = 7.8 Hz, 1H), 6.81 (dd, J = 5.4, 1.8 Hz, 2H), 6.71 (d, J = 7.8 Hz, 1H), 6.65 (d, J = 7.8 Hz, 1H), 3.81 (s, 3H), 3.42 (t, J = 4.8 Hz, 4H), 3.02–2.98 (m, 1H), 2.96–2.92 (m, 1H), 2.86–2.83 (m, 1H), 2.78–2.76 (m, 1H), 2.73 (t, J = 7.2 Hz, 2H), 2.66–2.62 (m, 4H), 2.55–2.49 (m, 5H), 2.07–2.05 (m, 1H), 1.57 (td, J = 12, 5.4 Hz, 1H), 1.48 (sx, J = 7.2 Hz, 2H), 0.90 (t, J = 7.2 Hz, 3H); [α]D25 = −15.6 (c = 1.0 in CH2Cl2).
(S)-{2-[4-(4-Amino-phenyl)-piperazin-1-yl]-ethyl}-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine (12) D-413
To a suspension of Pd/C (0.005 g) in anhydrous MeOH (10 mL), compound 11 (0.475 g, 1.05 mmol), dissolved in EtOAc/MeOH, was added under N2 atmosphere. The reaction vessel was degassed by applying vacuum, replaced by H2 gas (50 psi) and then stirred at room temperature overnight. The mixture was filtered through a pad of celite, washed with MeOH and the filtrate thus obtained was evaporated under reduced pressure. The crude product was purified by silica gel column chromatography (CH2Cl2:MeOH = 9:1) to furnish compound 12 (D-413) (0.385 g, 87%). 1H NMR (600 MHz, CDCl3): δ 7.09 (t, J = 7.8 Hz, 1H), 6.81 (d, J = 8.4 Hz, 2H), 6.71 (d, J = 7.2 Hz, 1H), 6.66–6.64 (m, 3H), 3.81 (s, 3H), 3.06 (t, J = 4.8 Hz, 4H), 3.02–2.98 (m, 1H), 2.92–2.88 (m, 1H), 2.82–2.78 (m, 3H), 2.67 (s, 5H), 2.57–2.50 (m, 5H), 2.10 (bs, 1H), 1.64–1.49 (m, 3H), 0.90 (t, J = 7.2 Hz, 3H); [α]D25 = −19.2 (c = 1.0 in CH3OH).
(S)-4-Methoxy-N-[4-(4-{2-[(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amino]-ethyl}-piperazin-1-yl)-phenyl]-benzenesulfonamide (13) (D-669)
Compound 12 (0.18 g, 0.43 mmol) was reacted with Et3N (0.24 mL, 1.7 mmol) and 4-methoxy-benzenesulfonyl chloride (0.106 g, 0.51 mmol) in CH2Cl2 (5 mL) according to procedure A. The crude material thus obtained was purified by silica gel column chromatography (EtOAc:MeOH = 19:1) to give compound 13 (0.22 g, 87%). 1H NMR (600 MHz, CDCl3): δ 7.61 (dd, J = 5.4, 1.8 Hz, 1H), 7.10–7.07 (m, 1H), 6.97 (dd, J = 5.4, 1.8 Hz, 1H), 6.93 (d, J = 9.0 Hz, 2H), 6.86–6.83 (m, 2H), 6.75 (dd, J = 5.4, 1.8 Hz, 2H), 6.70 (d, J = 7.8 Hz, 1H), 6.64 (d, J = 7.8 Hz, 1H), 3.81 (s, 3H), 3.80 (s, 3H), 3.24 (t, J = 4.8 Hz, 1H), 3.13 (t, J = 4.8 Hz, 3H), 3.01–2.97 (m, 1H), 2.93–2.83 (m, 2H), 2.77–2.71 (m, 3H), 2.64–2.57 (m, 4H), 2.53–2.49 (m, 5H), 2.06‒2.04 (m, 1H), 1.56 (td, J = 12, 5.4 Hz, 1H), 1.48 (sx, J = 7.2 Hz, 2H), 0.89 (t, J = 7.2 Hz, 3H); [α]D25 = −22.2 (c = 1.0 in CH2Cl2); Anal. Calcd for C37H57Cl3N4O5S: C, 57.25; H, 7.40; N, 7.22. Found: C, 57.64; H, 7.09; N, 7.39.
(S)-4-Hydroxy-N-[4-(4-{2-[(5-hydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amino]-ethyl}-piperazin-1-yl)-phenyl]-benzenesulfonamide (14) (D-668)
Compound 13 (0.14 g, 0.24 mmol) in 12 mL of CH2Cl2 was reacted with BBr3 (1.42 mL, 1.42 mmol, 1.0 M solution in CH2Cl2) according to procedure C. The crude product was purified by silica gel column chromatography (CH2Cl2:MeOH = 9:1) to afford 14 (0.075 g, 56%). 1H NMR (600 MHz, CD3OD): δ 7.48 (d, J = 8.4 Hz, 2H), 6.94–6.91 (m, 3H), 6.78 (d, J = 9.0 Hz, 2H), 6.76 (d, J = 9.0 Hz, 2H), 6.60 (d, J = 4.8 Hz, 1H), 6.59 (d, J = 4.8 Hz, 1H), 3.53–3.49 (m, 1H), 3.24–3.16 (m, 2H), 3.09 (t, J = 4.8 Hz, 4H), 3.06–3.01 (m, 4H), 2.98–2.93 (m, 1H), 2.77–2.71 (m, 2H), 2.69 (t, J = 4.8 Hz, 4H), 2.62–2.57 (m, 1H), 2.25‒2.22 (m, 1H), 1.79 (td, J = 12, 5.4 Hz, 1H), 1.72 (sx, J = 7.2 Hz, 2H), 0.99 (t, J = 7.2 Hz, 3H); [α]D25 = −26.4 (c = 1.0 in CH3OH); Anal. Calcd for C31H45Cl3N4O5S: C, 53.79; H, 6.55; N, 8.09. Found: C, 53.45; H, 6.63; N, 7.63.
(S)-N-[4-(4-{2-[(5-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amino]-ethyl}-piperazin-1-yl)-phenyl]-benzenesulfonamide (15)
Compound 12 (0.165 g, 0.39 mmol) was reacted with Et3N (0.22 mL, 1.56 mmol) and benzenesulfonyl chloride (60 µL, 0.47 mmol) in CH2Cl2 (5 mL) according to procedure A. The crude material thus obtained was purified by silica gel column chromatography (EtOAc:MeOH = 19:1) to give compound 15 (0.19 g, 87%). 1H NMR (600 MHz, CDCl3): δ 7.94 (dd, J = 7.2, 1.2 Hz, 1H), 7.69–7.63 (m, 2H), 7.53 (t, J = 7.8 Hz, 2H), 7.40 (t, J = 7.8 Hz, 1H), 6.92–6.91 (m, 1H), 6.86–6.84 (m, 1H), 6.79–6.78 (m, 1H), 6.75 (dd, J = 4.8, 1.8 Hz, 1H), 6.72–6.70 (m, 1H), 6.66–6.64 (m, 1H), 3.80 (s, 3H), 3.25 (t, J = 5.4 Hz, 2H), 3.13 (t, J = 5.4 Hz, 2H), 3.02–2.94 (m, 2H), 2.86–2.84 (m, 1H), 2.78–2.71 (m, 3H), 2.64–2.59 (m, 4H), 2.56–2.48 (m, 5H), 2.07‒2.05 (m, 1H), 1.57 (td, J = 12, 5.4 Hz, 1H), 1.49 (sx, J = 7.2 Hz, 2H), 0.91–0.88 (m, 3H); [α]D25 = −17.3 (c = 1.0 in CH2Cl2).
(S)-N-[4-(4-{2-[(5-Hydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amino]-ethyl}-piperazin-1-yl)-phenyl]-benzenesulfonamide (16) (D-672)
Compound 15 (0.12 g, 0.21 mmol) in 10 mL of CH2Cl2 was reacted with BBr3 (0.64 mL, 0.64 mmol, 1.0 M solution in CH2Cl2) according to procedure C. The crude product was purified by silica gel column chromatography (EtOAc:MeOH = 19:1) to afford 16 (0.075 g, 65%). 1H NMR (600 MHz, CDCl3): δ 7.68 (d, J = 7.8 Hz, 2H), 7.54–7.48 (m, 1H), 7.41–7.37 (m, 2H), 6.95–6.91 (m, 3H), 6.75 (d, J = 4.8 Hz, 1H), 6.73 (d, J = 4.8 Hz, 1H), 6.54 (t, J = 7.8 Hz, 1H), 6.49–6.47 (m, 1H), 3.48–3.47 (m, 1H), 3.21–3.12 (m, 3H), 2.93–2.89 (m, 1H), 2.86–2.82 (m, 1H), 2.72–2.61 (m, 7H), 2.54–2.41 (m, 5H), 2.35–2.28 (m, 1H), 1.96 (bs, 1H), 1.48–1.36 (m, 3H), 0.87 (t, J = 7.2 Hz, 3H); [α]D25 = −15.6 (c = 1.0 in CH2Cl2); Anal. Calcd for C31H44Cl3N4O3.5S: C, 55.81; H, 6.65; N, 8.40. Found: C, 55.73; H, 6.72; N, 7.98.
2-Chloro-1-[4-(4-methoxy-phenyl)-piperazin-1-yl]-ethanone (18)
Chloroacetylchloride (6.3 ml, 39.01 mmol) was added drop wise into a solution of 1-(4-Methoxy-phenyl)-piperazine (5 g, 26 mmol) and triethylamine (19.07 ml) in anhydrous methylene chloride at −40 °C under N2 atmosphere and then stirred at room temperature for 30 min. The reaction was diluted with CH2Cl2, washed with water, brine, and the organic layer was dried over Na2SO4, evaporated, purified by column chromatography Hexane/ EtOAc=50:50 to obtain pure product 18 (6.08 g, 89%).
1H NMR (400 MHz, CDCl3) δ: 3.06 (t, J = 5.2 Hz, 2H); 3.12 (t, J = 5.2 Hz, 2H); 3.68 (t, J = 4.8 Hz, 2H); 3.74–3.84 (m, 5H); 4.11 (s, 2H); 6.86 (d, J = 9.2 Hz, 2H); 6.91 (d, J = 9.2 Hz, 2H).
1-[4-(4-Methoxy-phenyl)-piperazin-1-yl]-2-[(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amino]-ethanone (19)
Compound 5-methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine (2 g, 7.819 mmol), compound 18 (3.15 g, 11.73 mmol), K2CO3 (4.58 g, 23.46 mmol) were refluxed in CH3CN (50 ml) for 2.5 hr. The solution was cooled, filtered, and concentrated. The crude material was then partitioned between EtOAc and H2O, and the organic layer was separated, dried (Na2SO4), and concentrated. The crude mixture was purified by column chromatography EtOAc =100 to yield pure compound 19 (4.4 g, 98%).
1H NMR (400 MHz, CDCl3) δ: 0.88 (t, J = 7.2 Hz, 3H); 1.42–1.70 (m, 3H); 1.97–2.12 (m, 1H); 2.46–2.64 (m, 3H); 2.74–3.12 (m, 8H); 3.40–3.52 (m, 2H); 3.61–3.92 (m, 4H); 3.77 (s, 3H); 3.79 (s, 3H); 6.64 (d, J = 8 Hz, 1H); 6.69 (d, J = 7.6 Hz, 1H); 6.84 (d, J = 9.2 Hz, 2H); 6.90 (d, J = 9.2 Hz, 2H); 7.08 (t, J = 8 Hz, 1H).
{2-[4-(4-Methoxy-phenyl)-piperazin-1-yl]-ethyl}-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine (20)
To a suspension of LiAlH4 (0.84 g, 4.42 mmol) in THF (50 ml) in an ice bath was added compound 19 (2 g, 2.21 mmol) in a solution of THF (25 ml). After addition, the mixture was refluxed for 2 h and cooled to 0 °C. 15% NaOH was added dropwise, and the mixture stirred for 20 min, and filtered. The solution was dried (Na2SO4), filtered, and concentrated to give 20 (1.09 g, 58%).
1H NMR (400 MHz, CDCl3) δ: 0.90 (t, J = 7.6 Hz, 3H); 1.41–1.66 (m, 3H); 2.02–2.14 (m, 1H); 2.44–2.62 (m, 5H); 2.66 (t, J = 4.8 Hz, 4H); 2.70–3.06 (m, 6H); 3.10 (t, J = 4.4 Hz, 4H); 3.76 (s, 3H); 3.81 (s, 3H); 6.65 (d, J = 8 Hz, 1H); 6.71 (d, J = 7.6 Hz, 1H); 6.83 (d, J = 9.2 Hz, 2H); 6.90 (d, J = 9.2 Hz, 2H); 7.09 (t, J = 8 Hz, 1H).
6-({2-[4-(4-Hydroxy-phenyl)-piperazin-1-yl]-ethyl}-propyl-amino)-5,6,7,8-tetrahydro-naphthalen-1-ol (21) D-367
A mixture of 30 (1 g, 2.285 mmol) and 10 ml of 48% Aq. HBr was refluxed under N2 atmosphere for 3 h. Reaction mixture was then cooled down, evaporated to dryness. Reaction mixture was then dissolved in sat. NaHCO3 solution and extracted with ethyl acetate. The organic layer was dried (Na2SO4), filtered, and concentrated. Compound was purified over column chromatography using EtOAc/MeOH=80:20 to yield pure compound 21 (D-367) (0.85 g, 91%).
1H NMR (400 MHz, CDCl3) δ: 0.89 (t, J = 7.6 Hz, 3H); 1.32–1.58 (m, 3H); 1.94–2.06 (m, 1H); 2.38–2.60 (m, 6H); 2.60–2.81 (m, 7H); 2.82–3.02 (m, 2H); 3.04–3.22 (m, 4H); 6.54 (d, J = 8 Hz, 1H); 6.62 (d, J = 7.2 Hz, 1H); 6.70–6.78 (m, 2H); 6.78–6.88 (m, 2H); 6.98 (t, J = 7.6 Hz, 1H).
The free base of 23 was converted in to its hydrochloride salt. m.p. decomp. at 215–217 °C Anal. (C25H35N3O2 ·3HCl· 0.5H2O) C, H, N.
DA D2 and D3 receptor assays
Binding potency was monitored by inhibition of [3H]spiroperidol (16.2 Ci/mmole, Perkin-Elmer) binding to dopamine rD2 and rD3 receptors expressed in HEK-293 cells, in a buffer containing 0.9% NaCl under conditions corresponding to our ‘high [radioligand] protocol’ as described by us previously [37]. Observed IC50 values were converted to inhibition constants (Ki) by the Cheng–Prusoff equation (see Ghosh et al. [38]). Functional activity of test compounds in activating dopamine hD2 and hD3 receptors expressed in CHO cells was measured by stimulation of [35S]GTPγS (1250 Ci/mmole, Perkin-Elmer) binding in comparison to stimulation by the full agonist dopamine as described by us previously.
References
Emilien G, Maloteaux J-M, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors-physiological understanding to therapeutic intervention potential. Pharmacol Therapeutics. 1999;84:133–56.
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225.
Das B, Modi G, Dutta A. Dopamine d3 agonists in the treatment of Parkinson’s disease. Curr Top Med Chem. 2015;15:908–26.
Li C, Biswas S, Li X, Dutta AK, Le W. Novel D3 dopamine receptor-preferring agonist D-264: Evidence of neuroprotective property in Parkinson’s disease animal models induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and lactacystin. J Neurosci Res. 2010;88:2513–23. https://doi.org/10.1002/jnr.22405.
Keck TM, John WS, Czoty PW, Nader MA, Newman AH. Identifying Medication Targets for Psychostimulant Addiction: Unraveling the Dopamine D3 Receptor Hypothesis. J Med Chem. 2015;58:5361–80. https://doi.org/10.1021/jm501512b.
Das B, Rajagopalan S, Joshi GS, Xu L, Luo D, Andersen JK, et al. A novel iron (II) preferring dopamine agonist chelator D-607 significantly suppresses alpha-syn- and MPTP-induced toxicities in vivo. Neuropharmacology. 2017;123:88–99. https://doi.org/10.1016/j.neuropharm.2017.05.019.
Shah M, Rajagopalan S, Xu L, Voshavar C, Shurubor Y, Beal F, et al. The high-affinity D2/D3 agonist D512 protects PC12 cells from 6-OHDA-induced apoptotic cell death and rescues dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. J Neurochem. 2014;131:74–85. https://doi.org/10.1111/jnc.12767.
Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–6.
Giros B, Martres MP, Sokoloff P, Schwartz JC. Gene cloning of human dopaminergic D3 receptor and identification of its chromosome. C R Acad Sci III. 1990;311:501–8.
Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–51.
Sunahara RK, Guan HC, O’Dowd BF, Seeman P, Laurier LG, Ng G, et al. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614–9.
Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, et al. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–4.
Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. https://doi.org/10.1016/s0893-133x(98)00066-9.
Sun J, Xu J, Cairns NJ, Perlmutter JS, Mach RH. Dopamine D1, D2, D3 receptors, vesicular monoamine transporter type-2 (VMAT2) and dopamine transporter (DAT) densities in aged human brain. PLoS One.7:e49483. https://doi.org/10.1371/journal.pone.0049483PONE-D-12-23292.
Park BH, Fishburn CS, Carmon S, Accili D, Fuchs S. Structural organization of the murine D3 dopamine receptor gene. J Neurochem. 1995;64:482–6.
Boeckler F, Gmeiner P. The structural evolution of dopamine D3 receptor ligands: structure-activity relationships and selected neuropharmacological aspects. Pharmacol Therapeutics. 2006;112:281–333.
Luedtkea RR, Mach RH. Progress in developing D3 dopamine receptor ligands as potential therapeutic agents for neurological and neuropsychiatric disorders. Curr Pharm Des. 2003;9:643–71.
Moritz AE, Free RB, Sibley DR. Advances and challenges in the search for D(2) and D(3) dopamine receptor-selective compounds. Cell Signal. 2018;41:75–81. https://doi.org/10.1016/j.cellsig.2017.07.003.
Kiss B, Laszlovszky I, Kramos B, Visegrady A, Bobok A, Levay G et al. Neuronal Dopamine D3 Receptors: Translational Implications for Preclinical Research and CNS Disorders. Biomolecules. 2021;11. https://doi.org/10.3390/biom11010104.
Biswas S, Hazeldine S, Ghosh B, Parrington I, Kuzhikandathil E, Reith ME, et al. Bioisosteric heterocyclic versions of 7-{[2-(4-phenyl-piperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphth alen-2-ol: identification of highly potent and selective agonists for dopamine D3 receptor with potent in vivo activity. J Med Chem. 2008;51:3005–19. https://doi.org/10.1021/jm701524h.
Johnson M, Antonio T, Reith ME, Dutta AK. Structure-activity relationship study of N(6)-(2-(4-(1H-Indol-5-yl)piperazin-1-yl)ethyl)-N(6)-propyl-4,5,6,7-tetrahydroben zo[d]thiazole-2,6-diamine analogues: development of highly selective D3 dopamine receptor agonists along with a highly potent D2/D3 agonist and their pharmacological characterization. J Med Chem. 2012;55:5826–40. https://doi.org/10.1021/jm300268s.
Das B, Vedachalam S, Luo D, Antonio T, Reith ME, Dutta AK. Development of a Highly Potent D2/D3 Agonist and a Partial Agonist from Structure-Activity Relationship Study of N(6)-(2-(4-(1H-Indol-5-yl)piperazin-1-yl)ethyl)-N(6)-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine Analogues: Implication in the Treatment of Parkinson’s Disease. J Med Chem. 2015;58:9179–95. https://doi.org/10.1021/acs.jmedchem.5b01031.
Gopishetty B, Zhang S, Kharkar PS, Antonio T, Reith M, Dutta AK. Modification of agonist binding moiety in hybrid derivative 5/7-[2-(4-aryl-piperazin-1-yl)-ethyl]-propyl-amino-5,6,7,8-tetrahydro-naphthalen-1-ol/-2-amino versions: impact on functional activity and selectivity for dopamine D2/D3 receptors. Bioorg Med Chem. 2013;21:3164–74. https://doi.org/10.1016/j.bmc.2013.03.059.
Woodward R, Coley C, Daniell S, Naylor LH, Strange PG. Investigation of the role of conserved serine residues in the long form of the rat D2 dopamine receptor using site-directed mutagenesis. J Neurochem. 1996;66:394–402.
Sartania N, Strange PG. Role of conserved serine residues in the interaction of agonists with D3 dopamine receptors. J Neurochem. 1999;72:2621–4.
Varady J, Wu X, Fang X, Min J, Hu Z, Levant B, et al. Molecular modeling of the three-dimensional structure of dopamine 3 (D3) subtype receptor: discovery of novel and potent D3 ligands through a hybrid pharmacophore- and structure-based database searching approach. J Med Chem. 2003;46:4377–92. https://doi.org/10.1021/jm030085p.
Kortagere S, Cheng SY, Antonio T, Zhen J, Reith ME, Dutta AK. Interaction of novel hybrid compounds with the D3 dopamine receptor: Site-directed mutagenesis and homology modeling studies. Biochem Pharm. 2011;81:157–63. https://doi.org/10.1016/j.bcp.2010.08.026.
Perachon S, Schwartz JC, Sokoloff P. Functional potencies of new antiparkinsonian drugs at recombinant human dopamine D1, D2 and D3 receptors. Eur J Pharm. 1999;366:293–300.
Elsner J, Boeckler F, Heinemann FW, Hubner H, Gmeiner P. Pharmacophore-guided drug discovery investigations leading to bioactive 5-aminotetrahydropyrazolopyridines. Implications for the binding mode of heterocyclic dopamine D3 receptor agonists. J Med Chem. 2005;48:5771–9. https://doi.org/10.1021/jm0503805.
Newman AH, Beuming T, Banala AK, Donthamsetti P, Pongetti K, LaBounty A, et al. Molecular determinants of selectivity and efficacy at the dopamine D3 receptor. J Med Chem. 2012;55:6689–99. https://doi.org/10.1021/jm300482h.
Micheli F, Bernardelli A, Bianchi F, Braggio S, Castelletti L, Cavallini P, et al. 1,2,4-Triazolyl octahydropyrrolo[2,3-b]pyrroles: A new series of potent and selective dopamine D3 receptor antagonists. Bioorg Med Chem. 2016;24:1619–36. https://doi.org/10.1016/j.bmc.2016.02.031.
Dutta AK, Venkataraman SK, Fei XS, Kolhatkar R, Zhang S, Reith ME. Synthesis and biological characterization of novel hybrid 7-[[2-(4-phenyl-piperazin-1-yl)-ethyl]-propyl-amino]-5,6,7,8-tetrahydro-naphthale n-2-ol and their heterocyclic bioisosteric analogues for dopamine D2 and D3 receptors. Bioorg Med Chem. 2004;12:4361–73. https://doi.org/10.1016/j.bmc.2004.06.019.
Ghosh B, Antonio T, Zhen J, Kharkar P, Reith ME, Dutta AK. Development of (S)-N6-(2-(4-(isoquinolin-1-yl)piperazin-1-yl)ethyl)-N6-propyl-4,5,6,7-tet rahydrobenzo[d]-thiazole-2,6-diamine and its analogue as a D3 receptor preferring agonist: potent in vivo activity in Parkinson’s disease animal models. J Med Chem. 2010;53:1023–37. https://doi.org/10.1021/jm901184n.
Ghosh B, Antonio T, Gopishetty B, Reith M, Dutta A. Further delineation of hydrophobic binding sites in dopamine D(2)/D(3) receptors for N-4 substituents on the piperazine ring of the hybrid template 5/7-{[2-(4-aryl-piperazin-1-yl)-ethyl]-propyl-amino}-5,6,7,8-tetrahydro-na phthalen-2-ol. Bioorg Med Chem. 2010;18:5661–74. https://doi.org/10.1016/j.bmc.2010.06.025.
Dutta AK, Armstrong C, Luo D, Das B, Spencer B, Rissman RA. D-685 Reverses Motor Deficits and Reduces Accumulation of Human alpha-Synuclein Protein in Two Different Parkinson’s Disease Animal Models. ACS Chem Neurosci. 2023;14:885–96. https://doi.org/10.1021/acschemneuro.2c00655.
Biswas S, Zhang S, Fernandez F, Ghosh B, Zhen J, Kuzhikandathil E, et al. Further structure-activity relationships study of hybrid 7-{[2-(4-phenylpiperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphtha len-2-ol analogues: identification of a high-affinity D3-preferring agonist with potent in vivo activity with long duration of action. J Med Chem. 2008;51:101–17. https://doi.org/10.1021/jm070860r.
Zhen J, Antonio T, Dutta AK, Reith ME. Concentration of receptor and ligand revisited in a modified receptor binding protocol for high-affinity radioligands: [3H]Spiperone binding to D2 and D3 dopamine receptors. J Neurosci Methods. 2010;188:32–8. https://doi.org/10.1016/j.jneumeth.2010.01.031.
Ghosh B, Antonio T, Reith ME, Dutta AK. Discovery of 4-(4-(2-((5-Hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)ethyl)piperaz in-1-yl)quinolin-8-ol and its analogues as highly potent dopamine D2/D3 agonists and as iron chelator: in vivo activity indicates potential application in symptomatic and neuroprotective therapy for Parkinson’s disease. J Med Chem. 2010;53:2114–25. https://doi.org/10.1021/jm901618d.
Acknowledgements
This work is supported by National Institute of Neurological Disorders and Stroke/ National Institute of Health (NS047198, AKD). We are grateful to Dr. K. Neve, Oregon Health and Science University, Portland, OR, for D2L and D3 expressing HEK cells. We are also grateful to Dr. J. Shine, Garvan Institute for Medical Research, Sydney, Australia, for D2L expressing CHO cells.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dutta, A.K., Das, B., Lote, A. et al. Further exploration of N-4 substituents on the piperazine ring of the hybrid template 5/ 7-{[2-(4-Aryl-piperazin-1-yl)-ethyl]-propyl-amino}-5,6,7,8-tetrahydro-naphthalen-2-ol and its analog: development of an exceptionally potent agonist for D2 & D3 receptors. Med Chem Res (2024). https://doi.org/10.1007/s00044-024-03291-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00044-024-03291-3